Abstract
Lead lithium borate glass samples composition 50Li2B4O7–(50−x)Pb3O4–x CuO, where x = 0–35 mol% were prepared by melt quenching method. The density of the prepared samples was measured and molar volume was calculated. IR spectra were measured for the prepared samples at room temperature to investigate the glass structure. The IR spectra were deconvoluted using curves of Gaussian shape at approximately the same frequencies. The deconvoluted data were used to study the effect of CuO content on all the structural borate and water groups. The optical band gap obtained directly from absorption coefficient, refractive index and extinction coefficient, also by using the Tauc model. The type of transition is determined by the simple and accurate method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of glass structure is critical for the investigation of their properties before the interpretation of the results of studies samples; we will examine some information about borate glasses. Pure borate glass is consists of a boroxol ring (Rao et al. 1984). The addition of Lithium oxide to B2O3 change the coordination number of the boron atom from 3 to 4, foming (BO4) tetrahedral in the pentaborate, triborate and diborate structural units. Lithium tetraborate glasses, (Li2O–2B2O3) consist of different structural groupings (boroxol units, pentaborate units, triborate units, diborate units, metaborate units, pyroborate units, orthoborate units or loose tetraborate units). These structural units are constructed from tetrahedral BO4 and triangle BO3 vibration units and the ratio between lithium (Li) and boron (B) is constant for all samples (Li/B = 1/2). The lithium tetraborate glass is a completely disordered mixture of the previously mentioned configurations characterized by an order distance of triangles and/or an order distance of tetrahedral borate units (Kamitsos et al. 1987; Rao et al. 1985; Soliman et al. 2009; Leventhal and Bray 1965).
In lead glasses, lead atoms play the role of former and modifier and described as a glass former at high lead oxide content and as network modifier at low lead oxide content. Such differences in the structural role of Pb2+ ions would imply significant modifications of their local environments and of their coordination. Earlier studies (Leventhal and Bray 1965; Mydlar et al. 1970; Dupree et al. 1987) consider that the glass forming groups are the [PbO4] units while recent studies (Singh et al. 2008; Coelho et al. 2012) claim that PbO3 units or a mixture of the two structural units (PbO4, PbO3) are the basic building units in high PbO content glasses.
In (Li2B4O7-Pb3O4) glass, the substitution of lithium tetraborate by Pb3O4 from 0 to 70 mol%, the composition of Pb3O4 increased by step 10 mol% in expense of Li2B4O7. lead oxide plays the dual role in the glass network, where the major part of Pb is acting as a modifier at Pb3O4 ≤ 30 mol% and the remaining of Pb enters as a network former and reversed behaviors at 70 ≥ Pb3O4 > 30 content the major part of PbO act as a network former (Kashif et al. 2012).
The reasons for choosing the glass system PbO-B2O3 are that it displays a low rate of crystallization, humidity resistant, stable, transparent, high refractive index and high density.
Lead oxide (PbO) plays a different role in the structure of glasses (Wang et al. 1991), acting as a network modifier and as a glass network former. In three-dimensional structures, the PbO6 structural units if Pb–O is ionic, and act as a glass network modifier. And the PbO4 structural units are Pb–O covalent bond which acts as a glass network former. B2O3 acts as glass network forming oxide and the boron atoms can coordinate with oxygen to form BO4 tetrahedral units and also BO3 triangular units (Wang et al. 1991; Cormier et al. 1994; Musi et al. 1994; Raghavaiah et al. 2004; Massot et al. 1995; Wen et al. 2016).
Some glasses absorbed OH, the absorption of OH in the glass samples causes an increase in the optical lessening and decrease the effectiveness of the exited level of the rare earth ions also caused luminescence quenching which decrease in the applications in glass system. The absorption of OH is found to be low for the samples containing high content of PbO, which makes them promising as a laser host material (Pisarski et al. 2005; Othman et al. 2016).
Rare earth doped glasses have been an increasing attention in the region of photonic application such as photoluminescence, up conversion, laser emission and amplification. The glass host is very important. The lithium tetraborate glass system containing lead and copper oxides is very important and promises in the optical property area. Lithium tetraborate glass samples containing Chromium and Neodymium were prepared. The estimated Judd–Ofelt parameters Ωλ (λ = 2, 4, 6) found that the J–O parameters mainly depend on the host composition, symmetry of the rare-earth site and the surrounding ions of the rare earth and Nd2O3 and Cr2O3 concentration. Lithium tetraborate glass samples containing Nb2O5 and Pr6O11were studied. And found that the physical properties depend on the increase of Nb2O5 on expense of Li2B4O7 and the transition of Pr3+ from the 3H4 ground state to 1D2 have been found to be the most probable laser transitions in the visible region. The influence of neodymium oxide ions on some physical properties and structural changes in bismuth lead borate glasses has been investigated. The optical absorption results are indicating the higher covalency of the Nd–O bond for glass containing 2 mol% of Nd2O3. In addition, a lowest covalency is observed in glass with 1 mol% Nd2O3. In addition, it is considered necessary in the construction of compact and efficient laser source. Copper-doped Lead lithium borate glass samples were studied and found that the glass samples containing copper are used as green light long pass filter and absorption glass (Kashif et al. 2015; Kashif and Ratep 2015; Kashif et al. 2017; Abdel-Khalek et al. 2016; Farouk et al. 2015; Kashif et al. 2014).
From pervious the glass system containing lead oxide and lithium tetraborate, they found that the sample with 1:1 ratio has higher content of BO4 group. Lead oxide is very important in glass host sample for decreases the water content in glass. Moreover increases the optical properties of glass sample. Also the addition of copper ions increases the optical properties. In this study, we study the effect of replacement of Pb3O4 with CuO on BO4, H2O groups and optical properties of lithium tetraborate glass samples.
2 Experimental work
Samples of 50Li2B4O7–(50−x)Pb3O4–x CuO (where x = 5, 10, 15, 20, 30 and 35 mol%) were prepared. All the chemicals were weighed accurately using an electrical balance; the used raw materials were all of chemically pure grade. The chemical was melted in a porcelain crucible at 1100 °C in an electrically programmable heated furnace, type-VAF15/10 lenton thermal designs, equipped with an automatic temperature controller. The molted materials quenched in air and poured at room temperature. The samples were examined by using Philips Analytical X-Ray diffraction system, type PW3710 based with Cu tube anode. The density was measured by the Archimedes method using toluene as the immersion liquid. The FTIR absorption spectra of the prepared samples were measured at room temperature in the range 4000–400 cm−1 by an infrared spectrometer (type JASCO FT/IR-4100, Japan) using the KBr disc technique. The transmission spectra of the samples were measured in a wide range of wavelength: UV, visible and NIR. The transmission spectra near the absorption edge of glasses were measured using V570JASCO spectrophotometer.
3 Results and discussion
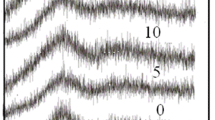
The infrared (IR) spectra of the prepared samples are shown in Fig. 1. The spectra are characterized by several absorption bands. A broadband expands over the range of frequencies (850–1120) cm−1. This band between 850 and 1100 cm−1 may be due to B–O stretching vibrations of different BO4 units present in diborate (B4O9 2−), triborate (B3O7 −), tetraborate (B8O16 2−) and Penta borate (B5O10 −) groups(Kaur et al. 2016; Bhogi et al. 2015). Another broadband expands over the range of frequencies from 1200 to 1600 cm−1. This band shows B–O bond stretching vibrations of trigonal BO3 unit existence in different meta-, pyro- and ortho- borate groups (Kaur et al. 2016; Obayes et al. 2016; Sasi Kumar et al. 2016). An absorption peak centered at about 3400 cm−1is assigned to OH group (Gautam et al. 2012). The absorption bands in the far infrared region around 460 cm−1 are related to Pb–O vibrations (Musi et al. 1994). And its intensity decreases with increasing PbO content. The absorption peak at 700 cm−1 is attributed to the bending vibrations of B–O–B linkage in borate network (Raghavaiah et al. 2004). It is observed that by increasing CuO content, this band shifts to higher wavenumbers since the electrostatic field of strongly polarizing Pb2+ ions serves to increase the energy of B–O–B bending vibrations (Massot et al. 1995). The two broad absorption bands in the mid IR region around 950 and 1350 cm−1 are assigned to BO4 and BO3 units respectively (Wen et al. 2016).
With the addition of copper and decrease of lead oxide it can be observed that the intensity of a band around 1249 cm−1 decrease and combine with the band between 1200 and 1500 cm−1 to form abroad band of BO3 group. And the band at 1029 cm−1 combines with the band 941 as the addition of copper on glass samples and formed a band that represent the BO4 structure. The two bands that indicate the presence of lead as the former one at 563 cm−1 become broader and disappear and the second at 647 cm−1 shifted to 682 cm−1 and to 709 cm−1 that indicate the increase of the band that represent the BO4 bending. The band at 439 cm−1 that represent the Li–O shifted to 451 cm−1 it may be due to the specific vibrations of Cu–O in CuO6 units (Kashif and Ratep 2015).
The dependence of the change in the structural groups on CuO content can be deduced from the deconvoluted data. The relative intensity of these two broad peaks is represented in Fig. 2. It is obvious from this figure that with increasing CuO content, BO4 structural units, decreases in the glass structure.
The absorption peak around 3400 cm−1 is assigned to the OH group (Farouk et al. 2015). The intensity of this peak decreases with increasing CuO content, see Fig. 3. It is known that the absorption of OH causes a luminescence quenching (Pisarski et al. 2005).
In oxide glasses, study of density D and molar volume Vm becomes important as both are reputed to provide very good insight about the network forming and modifying units. The variations of Vm and density are shown in Fig. 4 and 5. It is quite visible from this figure that molar volume and density are decreasing. The decrease in molar volume may be due to the conversion of non-bridging oxygen atoms into bridging oxygen atoms and change BO4 to BO3 and this results agreement with infrared results. These results are also supported by the density variations. But a sharp decrease in molar volume of samples reveals that the structure is having compactness. And the decrease in density for samples is due the replacement Pb3O4 have higher molecular weight with CuO which have lighter molecular weight.
The concentration of ions Cu per cm3 is given by (Kumar and Sankarappa 2008):
where Vm is the glass molar volume. From the calculated values of N, the polaron radius (rp) can be determined using the relation:
Therefore the short separation distances, r, between identical Cu ions are given by:
All the calculated parameters of N, rp and R are shown in Table 1.
From these results, it can be concluded that the separation between Cu–Cu (R) is greater than polaron radius (rp) for all samples. And the values of rp are smaller than R and greater than the ionic radius of copper (ionic radius of Cu ion is 0.74E−12 cm), which indicates that electrons are present in normal behavior (Kashif and Ratep 2015; Kumar and Sankarappa 2008).
The dielectric constant can be calculated according to the relations (Nadeem and Ahmed 2000)
and
where n is the refractive index and k is the extinction coefficient and calculated according Eq. (4)
[α is the absorption coefficient and λ is the wavelength].
Figure 6 shows the relation between ɛ’ and ɛ as a function of wavelength. As shown in Fig. 13a, b, the electrooptical phenomena at the same wavelength (start and end bandpass) can be observed in samples containing CuO up to 20 mol% (Kashif et al. 2015). Lithium lead borate glass samples containing copper oxide are used to remove a region of the near infrared spectrum from sunlight and to produce glasses which transmit in the green region.
Figure 7 shows the optical transmission of the glass samples containing different content of CuO. The all glass samples cut the UV, but the glass sample containing 15 and 20 mol% CuO cuts the infrared. From Fig. 7 it can be observed that the glass samples containing 15 and 20 mol% CuO can be used as a narrow band color filter, the all parameters determined and tabulated in Table 2. The two samples containing 30 and 35 mol% CuO cut off the wavelength from 190 up to 1090 and 1290 respectively.
The energy band gap of glasses is very important properties which can provide information about the structural changes and the nature of chemical bonds in the glass network. The localized states in the band gap can also be determined from the analysis of the optical absorption spectra(Davis and Mott 1970).
Two types of transitions can occur in the absorption of amorphous materials; direct transitions and indirect transitions. Both types of transition involve the interaction of an electromagnetic wave with an electron in the valence band, which is raised across the fundamental gap to the conduction band (Gnutzmann and Clausecker 1974).
For the direct optical transitions, it is essential that the wave vector for an electron remains the same as it absorbs a photon. On the other hand, the indirect transitions will also involve simultaneous interaction between the electron and lattice vibrations (Madhuri et al. 2015).
There are two methods which have been given in the literature about the determined the band gap by Urbach, by Tauc et al. and by Davis and Mott (Davis and Mott 1970; Urbach 1953; Tauc et al. 1966). Analyzing the behavior of the optical absorption coefficient (α), near the fundamental absorption edge is a standard method for investigating optically induced electronic transitions in many materials. The experimental optical band gap can be obtained directly by using the relation between absorption coefficient and extinction coefficient, which is given by (Lim et al. 2002);
By extrapolating the linear region of the extinction coefficient to zero, the experimental optical band gaps can be obtained. The extinction coefficient is plotted as a function of energy, hv. By extrapolating the linear region of the extinction coefficient to zero, as shown in Fig. 8.
Besides, Urbach reports that the absorption coefficient α(v) may depend exponentially on the photon energy (hv) of the materials. The Urbach rule is given by (Gnutzmann and Clausecker 1974; Lim et al. 2002):
where \(\nu\) is the angular frequency of the radiation, B is a constant and σ/KT gives a measure of the steepness of the absorption edge and it is sometimes interpreted as the width of the tail of localized state in the band gap (Al-Ani et al. 1985). The origin of the exponential part in Eq. (5) is not very clear and then Tauc et al. has suggested that the origin is arising from electronic transitions between the localized states where the density of the state are totally depends on the energy (Tauc et al. 1966) and the optical band gap determined from Fig. 9.
Davis and Mott modified and simplify the rule and recommended the new expression of direct transition can be written as:
where \(\beta\) is a constant, \(E_{opt}\) is the optical band gap, and n is the index determined by the nature of the electronic transitions during the process of absorption. The optical band gap in amorphous materials proposed by Davis and Mott can be explained as the width of the localized states near the mobility edge which is turn depends on the degree of disorder and defects present in the amorphous structure. For direct transitions, the value of n = 1/2 or 3/2 depending on whether the transition is allowed or forbidden and for indirect transitions, the value of n = 2or 3 depending on whether the transition is allowed or forbidden, respectively. The type of transition can be obtained from the value of n. In order to determine the unique value of n, the differential method has been used as the following equation:
The differential curve has a discontinuity at a particular energy value (hv = Eopt) which gives the optical band gap as shown in Fig. 10.
In order to find the type of transition, the Eopt values are calculated by extrapolation of the linear parts of (αhv)1/n versus hv curves to (αhv)1/n = 0 for different values of transition(Zaid et al. 2016) were calculated and tabulated in Table 1.
From table can be observed the all \(E_{opt}\) determine directly from draw the relation between α or n or k and hv are equal and also equal the Direct allowed transition n = ½, but can determine the weather transition (Table 3).
From equation
Take log of every side, then
Draw the relation between ln(αhυ) and ln(hυ- \({\text{E}}_{\text{opt}}\)) where \({\text{E}}_{\text{opt}}\) obtained from table (exp α)
Then calculate n from the slope line which gives the weather of transition and constant B.
Table 4 show (n and B).
From table can be observed the amount of n indicate the transition is Direct allowed transition.
The obtained values of the optical energy gap were decreased with the increasing of CuO concentration. The addition of CuO to lithium tetraborate glass network has caused the breakdown of a continuous BO4 network, whereby a significant shift of the absorption edge to longer wavelengths was observed. The shifting of the optical band gap were more likely related to structural rearrangements of the glass and relative concentrations of various fundamental units. The movement of absorption band to a lower energy was due NBO’s, whereby the electrons were loosely bonded to NBO’s than BO’s. The decreased in the optical energy gap as the CuO content was increased, whereby breaking of lithium tetraborate glass structure happened due to the increment of NBO’s ion content (Cormack and Cao 1996; Rosmawati et al. 2008).
4 Conclusion
Transparent glasses of the composition 50Li2B4O7–(50−x)Pb3O4–x CuO with x = 1, 2, 3 and 4 mol% were prepared via melt quenching technique and characterized using X-ray powder diffraction, Optical, density and FTIR measurements. The replacement Pb3O4 has a higher molecular weight with CuO which have lighter molecular weight caused the decrease of density, increasing CuO content, BO4 structural units, decreases in the glass structure. This caused the molar volume decrease. Then, as the concentration of CuO increases some of BO4 structural units are converted to BO3 structural units causing a decrease in the molar volume. The absorption of OH is found to be of low intensity for the samples containing high CuO concentrations, which makes them promising as a laser host material. The optical band gap can be determined directly from absorption coefficient, extinction coefficient and refractive index. The weather of transition calculated directly with logarithm of tuck’s equation.
References
Abdel-Khalek, E.K., Mohamed, E.A., Ratep, A., Salem, S.M., Kashif, I.: Structural, optical and dielectric characterization of niobium lithium tetraborate glasses doped praseodymium. J. Non-Cryst. Solids 441, 58–65 (2016)
Al-Ani, S.K.J., Hogarth, C.A., El-Mallawany, R.A.: A study of optical absorption in tellurite and tungsten-tellurite glasses. J. Mater. Sci. 20, 661–667 (1985)
Bhogi, A., Kumar, R.V., Kistaiah, P.: Effect of alkaline earths on spectroscopic and structural properties of Cu2+ ions-doped lithium borate glasses. J. Non Cryst. Solids 426, 47–54 (2015)
Coelho, J., Freire, C., Hussain, N.S.: Structural studies of lead lithium borate glasses doped with silver oxide. Spectrochim Acta Part A Mol Biomol Spectrosc 86, 392–398 (2012)
Cormack, A.N., Cao, Y.: Molecular dynamics simulation of silicate glasses. Mol. Eng. 6, 183–227 (1996)
Cormier, G., Capobianco, J.A., Monteil, A.: Molecular dynamics simulation of lead metaphosphate Pb (PO3)2 glass. J. Non-cryst. Solids 168, 115–124 (1994)
Davis, E.A., Mott, N.: Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22, 0903–0922 (1970)
Dupree, R., Ford, N., Holland, D.: An examination of the 29 Si environment in the PbO-SiO2 system by magic angle spinning nuclear magnetic resonance. Phys. Chem. Glasses 28, 78–84 (1987)
Farouk, M., Abd El-Maboud, A., Ibrahim, M., Ratep, A., Kashif, I.: Optical properties of Lead bismuth borate glasses doped with neodymium oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 149, 338–342 (2015)
Gautam, C., Yadav, A.K., Singh, A.S.: A review on infrared spectroscopy of borate glasses with effects of different additives. ISRN Ceram. 2012, 428497 (2012)
Gnutzmann, U., Clausecker, K.: Theory of direct optical transitions in an optical indirect semiconductor with a superlattice structure. Appl. Phys. 3, 9–14 (1974)
Kamitsos, E.I., Karakassides, M.A., Chryssikos, G.D.: A vibrational study of lithium borate glasses with high Li2O content. Phys. Chem. Glasses 28, 203–209 (1987)
Kashif, I., Ratep, A.: Effect of copper oxide on structure and physical properties of lithium lead borate glasses. Appl. Phys. A 120, 1427–1434 (2015a)
Kashif, I., Ratep, A.: Role of copper metal or oxide on physical properties of lithium borate glass. J. Mol. Struct. 1102, 1–5 (2015b)
Kashif, I., Abd El-Maboud, A., El-said, R., Sakr, E.M., Soliman, A.A.: The role of lead oxide on structural and physical properties of lithium diborate glasses. J. Alloys Compd 539, 124–128 (2012)
Kashif, I., Abd El-Maboud, A., Ratep, A.: Effect of Nd2 O3 addition on structure and characterization of lead bismuth borate glass. Result Phys. 4, 1–5 (2014)
Kashif, I., Ratep, A., Sanad, A.M.: Optical properties of lithium lead borate glass containing copper oxide for color filter and absorption glass. Opt. Quantum Electron. 47, 673–684 (2015)
Kashif, I., Ratep, A., El-Mahy, S.K.: Structural and optical properties of lithium tetraborate glasses containing chromium and neodymium oxide. Mater. Res. Bull. 89, 273–279 (2017)
Kaur, S., Kaur, P., Pal Singh, G., Arora, D., Kumar, S., Singh, D.P.: White light emission of Ce3+ sensitized Sm3+ doped lead alumino borate glasses. J. Luminescence 180, 190–197 (2016)
Kumar, M.P., Sankarappa, T.: Conductivity studies in mixed alkali vanadotellurite glasses. Mater. Sci Poland 26(3), 647–657 (2008)
Leventha, M., Bray, P.J.: Nuclear magnetic resonance investigations of compounds and glasses in systems PbO-B2O3 and PbO-SiO2. Phys. Chem. Glasses 6, 113–125 (1965)
Lim, S.G., Kriventsov, S., Jackson, T.N., Haeni, J.H., Schlom, D.G., Balbashov, A.M., Uecker, R., Reiche, P., Freeouf, J.L., Lucovsky, G.: Dielectric functions and optical bandgaps of high-K dielectrics for metal-oxide-semiconductor field-effect transistors by far ultraviolet spectroscopic ellipsometry. J. Appl. Phys. 91, 4500–4505 (2002)
Madhuri, V., Kumar, J.S., Rao, M.S., Cole, S., Phys, J.: Investigations on spectral features of tungsten ions in sodium lead alumino borate glass system. Chem. Solids 78, 70–77 (2015)
Massot, M., Souto, S., Balkanski, M.: Short and medium range order in ternary borate glasses. J. Non-cryst. Solids 182, 49–58 (1995)
Musić, S., Gotić, M., Popović, S., Furić, K., Mohaček, V.: Structural properties of lead vanadate glasses containing La3+ or Fe3+ ions. J. Mater. Sci. 29, 1227–1232 (1994)
Mydlar, M.F., Kreidl, N.J., Hendren, J.K., Clayton, G.T.: X-ray diffraction study of lead silicate glasses. Phys. Chem. Glasses 11, 196–201 (1970)
Nadeem, M.Y., Ahmed, W.: Optical properties of ZnS thin films. Turk. J. Phys. 24, 651–659 (2000)
Obayes, H.K., Wagiran, H., Hussin, R., Saeed, M.A.: Structural and optical properties of strontium/copper co-doped lithium borate glass system. Mater. Des. 94, 121–131 (2016)
Othman, H.A., Elkholy, H.S., Hager, I.Z.: FTIR of binary lead borate glass: structural investigation. J. Mol. Struct. 1106, 286–290 (2016)
Pisarski, W.A., Goryczka, T., Wodecka-Dus, B., Plonska, M., Pisarska, J.: Structure and properties of rare earth-doped lead borate glasses. Mater. Sci. Eng. B 122(2), 94–99 (2005)
Raghavaiah, B.V., Laxmikanth, C., Veeraiah, N.: Spectroscopic studies of titanium ions in PbO–Sb2 O3–As2 O3 glass system. Opt. Commun. 235, 341–349 (2004)
Rao, B.G., Sundar, H.K., Rao, K.J.: Investigations of glasses in the system PbO—PbF 2. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 80, 3491–3501 (1984)
Rao, K.J., Rao, B.G., Elliott, S.R.: Glass formation in the system PbO-PbCl2. J. Mater. Sci. 20, 1678–1682 (1985)
Rosmawati, S., Sidek, H.A.A., Zainal, A.T., Zobir, H.M.: Effect of zinc on the physical properties of tellurite glass. J. Appl. Sci. 8, 1956–1961 (2008)
Sasi Kumar, M.V., Reddy, B.R., Babu, S., Balakrishna, A., Ratnakaram, YC.: Thermal, structural and spectroscopic properties of Pr3+-doped lead zinc borate glasses modified by alkali metal ions. J. Taibah Univ. Sci. (2016) (in press)
Singh, K.J., Singh, N., Kaundal, R.S., Singh, K.: Gamma-ray shielding and structural properties of PbO–SiO2 glasses. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 266, 944–948 (2008)
Soliman, A.A., Sakr, E.M., Kashif, I.: The investigation of the influence of lead oxide on the formation and on the structure of lithium diborate glasses. Mater. Sci. Eng. B 158, 30–34 (2009)
Tauc, J., Grigorovici, R., Vancu, A.: Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 15, 627–637 (1966)
Urbach, F.: The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92, 1324–1326 (1953)
Wang, B., Szu, S.P., Greenblatt, M.: Structure and ionic conductivity in lithium–lead–borate glasses. J. Non-cryst. Solids 134, 249–258 (1991)
Wen, H., Tannerb, P.A., Cheng, B.-M.: Optical properties of 3d N transition metal ion-doped lead borate glasses. Mater. Res. Bull. 83, 400–407 (2016)
Zaid, M.H.M., Matori, K.A., Ab Aziz, S.H., Kamari, H.M., Wahab, Z.A., Effendy, N., Alibe, I.M.: Comprehensive study on compositional dependence of optical band gap in zinc soda lime silica glass system for optoelectronic applications. J. Non-Cryst. Solids 449, 107–112 (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashif, I., Ratep, A. Effect of copper addition on BO4, H2O groups and optical properties of lithium lead borate glass. Opt Quant Electron 49, 231 (2017). https://doi.org/10.1007/s11082-017-1067-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-017-1067-7