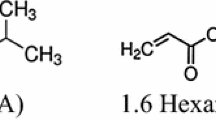Abstract
In this work, nanoparticles of nonlinear optical organic molecular cocrystal DAST (4-dimethylamino-N-methylstilbazolium p-toluenesulfonate) were obtained by pulsed laser ablation in liquid monomer isodecyl acrylate (IDA). Sizes of obtained nanoparticles were determined by scanning electron microscope and were about 200 nm in width. Laser ablation does not lead to photocuring or photodegradation of liquid monomer. Ablation processes was similar to the processes in classical liquids (water, organic solvents). Since in this case the nanoparticles were synthesized directly in the liquid medium of the surfactant monomer, so they are stabilized in it. Nanocomposites films were synthesized on the basis of these stable colloid solution by photocuring. For aliphatic polymer IDA long molecules cross-linking 1,6-Hexandiol diacrylate was used (DIOL). Optical properties of colloid solution and nanocomposites were investigated. It was found that obtained nanoparticles are amorphous form of DAST cocrystal and have a rod-like shape. Also dodecane and polyphenelene ether were tried in this work as liquids with different viscosity to find its effect on size and shape of nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Methods of organic nanocrystals synthesis are in particular interest in the area of nanotechnology as one of the main directions of last decade science and technology development. Thus, molecular crystals such as trans-4′-(dimethylamino)-N-methyl-4-stilbazolium tosylate) (DAST) are materials with the highest value of the second order nonlinear optical coefficient (Ruiz et al. 2008). The difficulty of growing large single crystals limits their practical application, so it is promising to study methods of these crystals nanoparticle synthesis and their incorporation into the polymer matrix.
One advantage of nanoparticles, as polymer additives appear to have is that compared to traditional additives, loading requirements are quite low. Microsized particles used as additive scatter light, thus reducing light transmittance and optical clarity. Efficient nanoparticle dispersion combined with good polymer–particle interfacial adhesion eliminates scattering and allows the exciting possibility of developing strong yet transparent films and coatings. A large body of literature exists on the inorganic particle embedded into polymeric matrix included non-linear nanoparticles. However, little is known about polymer–nanoparticle systems based on organic nanoparticles in polymers and especially on molecular nanocrystals having strong non-linear proprieties. Investigation of synthesis method of molecular nanocrystals having strong nonlinear proprieties was the aim of this work.
Currently, there are several methods of various materials nanoparticles synthesis based on carrying out chemical reactions and, therefore, associated with the need to conduct and control the reaction between the specific substances, which is not always feasible. Laser ablation method in liquid in some cases allows to avoid chemical synthesis reactions, the method is universal and has no contamination of reaction products in resulting nanoparticles. It is an effective method for the preparation of a number of nonlinear optical materials such as colloids and nanolayers. Laser ablation of solid target leads to formation of materials composed by atoms, ions (electrons) and clusters. These atoms and clusters tend to aggregate during the laser pulse, or shortly thereafter, which leads to the formation of larger clusters or nanoparticles (Ganeev et al. 2011). Synthesis of metal and dye nanoparticles by laser ablation in liquids was discussed, for example, in (Kassavetis et al. 2005; Bagga et al. 2005; Tamaki et al. 2002; Li et al. 2003; Elaboudi et al. 2007; Barcikowski et al. 2008).
It is a promising direction to synthesize nanocomposite materials based on photopolymers containing metal and organic nanoparticles. However, it may be difficult to incorporate nanoparticles in polymer matrix or monomers. Such procedures are associated with the use of solvents for storing particles in a colloidal solution. The evaporation of the solvent leads to a nanoparticles agglomeration and formation of micron-sized structures. In this connection, it becomes an urgent task obtaining nanoparticles directly in the liquid monomer.
In our work, we combine processes of nanoparticles synthesis and their incorporation in polymer matrices by usage of physical way—the method of laser ablation in liquid monomer. This paper presents the first results of DAST cocrystals laser ablation in the liquid process research. Optical and structural properties of obtained nanoparticles were studied in stable colloids and in nanocomposites based on these colloids.
2 Materials and method
The method of laser ablation in liquid consists of instantaneous material removing from target surface and form nanoparticles in liquid volume. Furthermore, this method carrying out in liquid monomer, it allows monomer adsorption on the surface of the newly formed nanoparticles and the formation of stable colloidal solutions. Obtained colloidal solution of nanoparticles in the monomer can be used to form solid films of polymer nanocomposites by photocuring.
In this work nanoparticles of nonlinear optical organic cocrystal DAST trans-4′-(dimethylamino)-N-methyl-4-stilbazolium tosylate) (Sigma-Aldrich 514160) were obtained by laser ablation in liquid aliphatic monomer IDA (isodecyl acrylate, Sigma-Aldrich 408956). Also, liquids with different viscosity were tried as media for ablation–dodecane and polyphenelene ether.
For laser ablation Nd: YAG laser was used (Sol instruments LF117, 355 nm) with a pulse repetition rate of 10 Hz, a pulse energy of 30 mJ, and 10 ns pulse width.
The experimental setup for the laser ablation is shown in Fig. 1. The target was placed at the bottom of the quartz cell filled with liquid. The laser beam was focused on the target through the liquid layer of 2–5 mm thick. Irradiation was carried out for 5 min and was accompanied by a homogeneous liquid staining in a characteristic red-brown color.
Obtained colloidal solution have been investigated by UV–visible spectroscopy with UV-spectrometer Shimadzu UV-1800.
Further, the obtained colloidal solution was placed on a silicon substrate and heated to a temperature of the monomer evaporation to isolate the nanoparticles. Size and shape of nanoparticles obtained by laser ablation were estimated with a scanning electron microscope (SEM) Carl Zeiss Merlin.
Stable colloid prepared by laser ablation of DAST in IDA was used for organic nanocomposites synthesis. IDA is a photocurable monomer; however, to produce solid films on its base diacrylates should be used for IDA molecules cross-linking. In this study following diacrylates were tried: UDMA (Diurethane dimethacrylate, Sigma-Aldrich 436909), DIOL (1,6-Hexandiol diacrylate, Sigma-Aldrich 246816) and TEGDA (Tetra (ethylene glycol) diacrylate, Sigma-Aldrich 398802).
The usage of different diacrylates gave different physical properties to polymeric materials. For example, the most flexible patterns were obtained with UDMA, but they were too soft and even low pressure of micro hardness measurements made significant defects on their surface, opposite patterns were obtained for TEGDA, which has shown a highest hardness, but the most fragility. Patterns with DIOL seem to have a significant hardness and flexibility. These quality estimations were used just for picking the most applicable and “comfortable” composition for experiment. In this study DIOL has been chosen as cross-linked diacrylate.
Mixtures of colloidal solutions with DIOL were prepared by intensive stirring. Polymerization of colloidal solution in IDA and DIOL occurred by using a polymerization initiator 2,2-dimethoxy-2-fenilatsetofenol (0.5 wt%). The initiator was injected into the monomer mixture as a methylene chloride solution. Then composition was mixed thoroughly using an ultrasonic disperser. Further colloidal solution was placed on the surface of polyester between spacers height of 1 mm to form a film of a certain thickness, and cover the top in the same polyester for oxygen limitation that prevents photocuring. The composition was irradiated with UV lamp (365 nm) for 10–15 min. The surface of the obtained films was treated with isopropyl alcohol to remove residual monomer and put in thermostat for 12 h with temperature 50 °C to complete polymerization.
The refractive indices of the polymeric matrices and polymerized nanocomposites were also measured for comparison by an Abbe refractometer.
3 Results and discussion
It was found by SEM that obtained nanoparticles of DAST (4-dimethylamino-N-methylstilbazolium p-toluenesulfonate) have a rod-like shape with sizes vary from 200 nm in width and reach almost 500 nm or more in length. These nanoparticles agglomerate in structures with micrometer sizes (Fig. 2a, b). Reason of this agglomeration was not investigated, since it is not clear in which moment this process occurs—in colloid state or after monomer evaporation.
Laser ablation does not lead to photocuring or photodegradation of liquid monomer, so it was possible to form a stable colloid and use it in further polymerization process.
Nanocomposites synthesized on the basis of this stable colloid solution by photocuring were transparent and homogeneous. Refractive index for pure polymer matrix was about 1.47, and for DAST based organic nanocomposite—1.5.
Optical properties of colloid solution and nanocomposites were investigated. It was found that obtained nanoparticles are amorphous form of DAST cocrystal. Figure 2c demonstrates absorption spectra of colloid solution of obtained DAST nanoparticles in IDA. Absorbance peak at 450–470 nm corresponds to amorphous DAST (Burunkova et al. 2014). The same absorption peak was observed for DAST nanoparticles based organic nanocomposites.
Laser ablation of DAST cocrystal was also carried out in dodecane. This solvent has a less viscosity than IDA, which probably gave an opportunity for ablated structures to form bigger clusters. Particles of DAST obtained in dodecane had a sizes about few micrometers. Laser ablation in most viscous liquid in this study—polyphenelene ether—showed no DAST nanoparticles synthesis. There was no staining in this liquid during ablation.
One of possible way to obtain crystal form of DAST is thermal annealing of its amorphous patterns. It was shown previosly (Burunkova et al. 2014), that 2 h 180 °C annealing leads to amorphous DAST crystallization in PMMA polymer matrix. Thermostability of IDA + DIOL polymer matrix is not well investigated and this characteristic will be studied at next part of current research, which consists of DAST nanocrystals synthesis in polymer matrix by thermal annealing.
4 Conclusion
The paper studied the conditions for the production of organic polymer composites based on nanoparticles of DAST (4-dimethylamino-N-methylstilbazolium p-toluenesulfonate) by means of laser ablation in liquid monomer isodecyl acrylate (IDA). Stable colloids in monomer were obtained and solid film nanocomposites were prepared by photocuring of these colloids. The formation of nanoparticles in the amorphous form is confirmed by the SEM and absorption spectra. Sizes of obtained rod-like shaped nanoparticles of DAST were less than 200 nm in width. DAST nanoparticles size distribution and possibility of their synthesis in different viscosity liquids were estimated. It was found that laser ablation of DAST in dodecane does not lead to nanoparticles synthesis, size of obtained particles were about few microns. In polyphenelene ether laser ablation of DAST was not observed, just cocrystal burning.
References
Bagga, K., McCann, R., Wang, M., Stalcup, A., Vázquez, M., Brabazon, D.: Laser assisted synthesis of carbon nanoparticles with controlled viscosities for printing applications. J. Colloid Interface Sci. 447, 263–268 (2005)
Barcikowski, S., Hustedt, M., Chichkov, B.: Nanocomposite manufacturing using ultrashort-pulsed laser ablation in solvents and monomers. Polimery 53(9), 657–662 (2008)
Burunkova, J.A., Denisyuk, IYu., Fokina, M.I.: Polymer composite based on DAST submicron crystals: technology and properties. Mol. Cryst. Liq. Cryst. 589, 178–182 (2014)
Elaboudi, I., Lazare, S., Belin, C., Bruneel, J.L., Servant, L.: Organic nanoparticles suspensions preparation by underwater excimer laser ablation of polycarbonate. Appl. Surf. Sci. 253, 7835–7839 (2007)
Ganeev, R.A., Zakirov, A.S., Boltaev, G.S., Tugushev, R.I., Usmanov, T., et al.: Structural, optical, and nonlinear optical absorption/refraction studies of the manganese nanoparticles prepared by laser ablation in ethanol. Opt. Mater. 33, 419–423 (2011)
Kassavetis, S., Kaziannis, S., Pliatsikas, N., Avgeropoulos, A., Karantzalis, A.E., Kosmidis, C., Lidorikis, E., Patsalas, P.: Formation of plasmonic colloidal silver for flexible and printed electronics using laser ablation. Appl. Surf. Sci. 336, 262–266 (2005)
Li, B., Kawakami, T., Hiramatsu, M.: Enhancement of organic nanoparticle preparation by laser ablation in aqueous solution using surfactants. Appl. Surf. Sci. 210, 171–176 (2003)
Ruiz, B., Jazbinsek, M., Gunter, P.: Crystal growth of DAST. Cryst. Growth Des. 8(11), 4173–4184 (2008)
Tamaki, Y., Asahi, T., Masuhara, H.J.: Nanoparticle formation of vanadyl phthalocyanine by laser ablation of its crystalline powder in a poor solvent. Phys. Chem. A 106, 2135–2139 (2002)
Acknowledgments
The authors thank RFBR according to the research project No. 16-32-00448 and President Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Fundamentals of Laser Assisted Micro- & Nanotechnologies.
Guest edited by Eugene Avrutin, Vadim Veiko, Tigran Vartanyan and Andrey Belikov.
Rights and permissions
About this article
Cite this article
Zulina, N.A., Achor, S.U. & Denisyuk, I.Y. Nanoparticles of organic nonlinear optical molecular crystals synthesized by laser ablation in liquid. Opt Quant Electron 48, 489 (2016). https://doi.org/10.1007/s11082-016-0757-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0757-x