Abstract
Ternary compound films of ZnO–In2O3 system were prepared by spray pyrolysis method. The films were deposited on heated glass substrates by pyrolytic decomposition of zinc chloride and indium chloride in distilled water with different ratio x of [Zn] to [Zn + In] (x = [Zn]/[Zn + In]). The phase change, chemical composition, electrical and optical properties of the Zn–In oxides were investigated over wide range of ratio x. Quasi-amorphous sub-oxide films with thickness of 200–400 nm were first obtained, the films were then annealed in Argon atmosphere at 550 °C for 1 h. To optimize the physical properties of sprayed ZnO–In2O3 thin films, we carry out on a systematic study of their microstructural characterization and transport properties. Continuous structural variation from In2O3 to ZnO through Zn2In2O5 and Zn3In2O6 was observed on the deposited films with increasing the ratio x. Maximum of conductivity of 1.47 × 103 Ω−1 cm−1 was reached for x = 0.5 (at.%). We also concluded that the transport properties of the films are greatly governed by their microstructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
New transparent conducting oxide (TCO) films with enhanced physical properties and lower cost have been synthesized and studied for their potential applications in optoelectronics and photonic industry, including solar cells and electro chromic devices (Kulyk et al. 2009; Kulyk et al. 2010; Abed et al. 2011; Wang et al. 1996; Ueda et al. 1992).
However only a few investigations were performed on ternary compound films of ZnO–In2O3. It’s well known that TCO films consisting of a binary compound such as SnO2, In2O3, ZnO doped with an impurity are widely used, but they are often limited in their application, because of chemical and thermal instability when used in various environments for example. Most of their problems are caused by their intrinsic properties and the preparation method. On the other hand, ITO (Indium Tin Oxide) which is the representative material of transparent and conductive oxides (because it’s innocuous material manifesting high transparency (90 %) and resistivity of the order of 10−4 Ω cm) is composed mainly with Indium which is rare and expensive that alternative materials are required (Wang et al. 1996; Ueda et al. 1992; Minami et al. 1995a, b, c; El Hichou et al. 2004; Kawazoe et al. 1997). To obtain a TCO film with high performances and low cost, new materials consisting of ternary compounds and/or multi-component oxides have recently attracted much attention (Minami et al. 1995a, b, c, 1996a, b). The use of ternary compound allows an improvement in the attainable properties. In addition, multi-component oxides may have properties suitable for specialized applications, as a result of changes in the physical properties obtained by controlling the composition of materials composed of binary and/or ternary compounds. Numerous studies on various combinations of indium-, tin-, or zinc- oxide with gallium-, magnesium-, cadmium-, and aluminium-oxide have been reported (Wang et al. 1996; Ueda et al. 1992; Minami et al. 1995a, b, c, 1996a, b; El Hichou et al. 2004; Kawazoe et al. 1997; Bartolomé et al. 2013; Buchholz and Proffit 2012, 4–13). In particular, the indium-zinc oxide system has attracted considerable attention as promising materials for transparent conducting films. Indium-zinc oxide films have been prepared using sputtering (Minami et al. 1996a, b), Metal Organic Chemical Vapour Deposition (Wang et al. 1998) and Laser Deposition (Phillips et al. 1995; Zheng and Kwok 1993), but rarely, multi-compound oxides have been prepared by simples methods like spray pyrolysis which is low cost, and offer the possibility to produce large area layers desirable for solar and electrochromic applications. Another advantage of this technique is the facility to prepare homogeneous thin films when doping is required.
In this paper, we describe the preparation of highly transparent and conductive In2O3–ZnO films by spray pyrolysis method. The ternary Zn2In2O5 and Zn3In2O6 thin films are for the first time obtained by this technique. Structural, electrical and optical properties under a wide change in the supply ratio of [Zn]/[Zn + In] during the deposition are investigated. We also study the correlation between structural and transport properties of these films.
2 Experimental procedure
The samples used in these researches are pseudo binary oxides system (ZnO–In2O3) deposited by spray pyrolysis technique. The deposition apparatus consisted of a spray chamber, hot plate, spray nozzle (with diameter of 500 μm) and input gas valve. The experimental set-up used for the preparation of pyrolytically spray deposited films is described elsewhere in Ref. Addou et al. (1999). The substrates were chemically cleaned and placed on the surface of hot plate heated to attain the required substrate temperature (500 °C) which was measured by a Ni–Fe–Ni thermocouple.
The preparation of crystallised transparent conductive thin films was carried out in two steps. The first one consisted of spraying solution of indium chloride (InCl3) and zinc chloride (ZnCl2, 5H2O) with right weight proportion, on heated glass substrates using compressed air as the carrier gas at atmospheric pressure. We added systematically a few drops of chloride acid to increase the clarity of the starting solution by dissolving precipitates. The solution with concentration of 0.05 M was sprayed during 15 min with 5 ml/min as solution spray rate and 6 l/min as gas flow. The starting composition was ranged from pure InCl3 to pure ZnCl2, 5H2O. In the second step, the as deposited films (quasi-amorphous) are annealed in argon atmosphere at 550 °C for 1 h.
The thickness, about 400 nm, of the films was measured by a SEM (Scanning Electron Microscope) apparatus. The composition of the films was investigated by electron probe microanalysis (EPMA). Structural characterizations were performed using Philips X-ray diffractometer (XRD) with Cu Kα radiation (λ = 0.1542 nm). The optical measurements in the visible region were carried out at room temperature with a Shimadzu 3101PC UV–VIS–NIR spectrophotometer. The room temperature electrical conductivity and Hall coefficient were determined using the Van der Paw technique (Hawson et al. 1979; Huang et al. 1987). Data acquisition and analysis were performed using a personal computer.
3 Results and discussion
3.1 Composition
Table 1 summarizes the composition of deposited films of ZnO–In2O3 system for different ratio x, with x = [Zn]/[Zn + In] in the solution.
As shown in Table 1 and Fig. 1, the y value follows that of x, which means that relative atomic concentration [Zn]/[Zn + In] of the film is controlled by the precursor solution composition. Therefore, this linear variation shows one of the superior spray pyrolysis technique. The [Zn] composition in the solution is reflected directly toward that in the prepared films.
3.2 Optical properties
The optical transparency of ZnO–In2O3 thin films for various Zn-concentrations is shown in Fig. 2. The average optical transmittance of samples in the visible range (400–800 nm) is in the 80–85 % range. Optical band gap Eg values were calculated according to a direct allowed transition from the general relation \(\alpha h\nu \propto (h\nu - E_{g} )^{1/2}\). The values of Eg are presented in Fig. 3 versus Zn content. All obtained values are all higher than 2.9 eV, the value obtained by Minami et al. (1995a, b, c). It should be noted that band gap energy of at least 3.5 eV is needed for TCO in most anticipated application.
3.3 Structural characterization
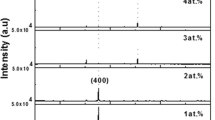
The variation of the structure as function of Zn content is investigated. Figure 4 shows the diffraction patterns for the as-deposited films before annealing as function of ratio x. It’s quite clear from the diffraction patterns that less than x = 0.25 (Fig. 4a), the films represent polycrystalline structure of In2O3 with preferential (400) and (222) orientations. Increasing x to 0.30 had the effect to induce some (222) alignment (not shown here), as was previously reported by Moriga et al. (1998) for the ZnO–In2O3 deposited by the simultaneous sputtering method ZnO and In2O3 targets. The structure change to amorphous—like phase, for 0.30 < x < 0.60 with some weak picks, due certainly to homologous phases ZnkIn2Ok+2 and to ZnO phase which may exist in an amorphous state, but with very small crystallites. Effectively, the signals at x = 0.50 (Fig. 4b) and x = 0.60 (Fig. 4c) are assigned to homologous Zn2In2O5 (JCPDS card n°20-1442) and Zn3In2O6 (JCPDS card n°20-1439), respectively.
Theses phases are effectively confirmed after annealing. Figure 5 shows the evolution of the X-ray diffraction pattern of the same samples annealed in argon atmosphere at 550 °C for 1 h. For x < 0.25 (Fig. 5a), all the peaks could be assigned to the bixbiyte-type In2O3 (JCPDS card n°6-0416), the films crystallized in the cubic phase of the In2O3 lattice. But, for x in the range from 0.30 to 0.40, a mixture of In2O3 and amorphous-like phase seems to exist (not shown here). Under substantial Zn supply for x > 0.40, the diffraction spectra showed crystallized phases corresponding to homologous phases of ZnkIn2Ok+3. Signal appearing at 0.40 < x < 0.50 were ascribed to homologous compound Zn2In2O5 (Fig. 5b), whereas at x = 0.60, a single (0015) diffraction peak corresponding to Zn3In2O6, was observed (Fig. 5c). At above x > 0.80, as shown in Fig. 5d, the films are in highly oriented ZnO phase.
3.4 Electrical properties
In our last work (El Rhaleb et al. 2004), conductivity of about 10+3 Ω−1 cm−1 was obtained for the sprayed films and we have suggested that the improvement of the conductivity can be achieved by adequate post treatment. So we present here the study of annealed samples in Argon atmosphere at 550 °C for 1 h. The transport properties, resistivity (ρ), carrier concentration (n) and Hall mobility (μ) of all the annealed films are shown in Fig. 6 as a function of the [Zn]/[Zn + In] content.
We can deduce from the sign of the Hall Effect deviation that all the films are n-type semiconductors. Furthermore, considering the obtained carrier concentration (n > 1020 cm−3), these films are also degenerated. This behaviour mainly results from oxygen stoechiometric deviation. Conduction electrons in these films originate from donor sites associated with oxygen vacancies, according to Eq. (1):
The electrical measurements show that with the increase Zn-content up to 20 at.%, resistivity of film increases (not shown here). The same behaviour has been observed by Minami et al. for ZnO–In2O3 films prepared at room temperature by dc magnetron sputtering deposition (Minami et al. 1998). However, with more increasing Zn-content as shown in Fig. 6, resistivity decreases sharply and reaches a minimum at x = 0.50. The structural characterization has shown that for this composition, the annealed films are highly textured with only one strong Bragg reflection assigned to the (008) reflection of Zn2In2O5. The increase in conductivity may be achieved by increasing either the carrier concentration or the carrier mobility, or both. In our case, the carrier concentration n is mainly influenced by oxygen vacancies while the mobility may be influenced by change in texture and structure of the films. It can be seen in Fig. 6 that n is nearly constant, while μ increases with the Zn content in the film and then decreases. Indeed, for x < 0.20, films are granular (Fig. 7a) in polyhedral In2O3 bixbyite-type structure. The insertion of zinc oxide in the In2O3 host induces a drastic decrease in the crystallite size which induces more grain boundaries leading to a decrease in mobility. Furthermore, the addition of zinc oxide to In2O3, which can be considered as a p-type doping, will lead to an expected decrease in the carrier concentration for x < 0.2. But for intermediaries concentrations (0.20 < x < 0.70), where the films present a fibber texture (Fig. 7b), with a layered ZnkIn2Ok+3-type structure as was shown by Dupont et al. (2001), which facilitates electron mobility (Moriga et al. (1999), conductivity arises and reaches maximum at the homologous phase Zn2In2O5. We can note that the best conductivity and mobility are reached for the sample with x = 0.50. As the zinc ratio keeps on increasing, the mobility continuously decreases. For zinc rich films, the microscopy measurements revealed that the films have a hexagonal-type texture (Fig. 7c) with a wurtzite type structure.
4 Conclusion
In this paper, ZnO–In2O3 transparent conducting multicomponent films have been deposited by spray pyrolysis technique utilising a solution containing InCl3 and ZnCl2. Quasi amorphous-like films for intermediate values of x, were first obtained with good transparency (85 %) in the visible wavelength range. We annealed the samples in argon atmosphere at 550 °C for 1 h and carried out systematic study of their micro-structural and transport properties. Three composition ranges of the ZnO–In2O3 phase diagram over which well-defined microstructure were identified: For indium rich part (x < 0.30 at) the diffraction patterns indicate that the films are polycrystalline In2O3 with polyhedral grains revealed by SEM micrographs. At the middle composition range (0.40 < x < 0.70) the films present a fibber texture and two homologous phases; Zn2In2O5 and Zn3In2O6 were observed for 0.40 < x < 0.50 and x = 0.60 respectively. For zinc rich part, the films present a columnar type texture with a wurtzite-type structure. The maximum carrier mobility and minimum resistivity were obtained for the film deposited at x = 0.50 which was identified as Zn2In2O5. The present work highlights Zn2In2O5 as the best TCO in the ZnkIn2O3+k series obtained by spray pyrolysis method for the first time.
References
Abed, S., Aida, M.S., Bouchouit, K., Arbaoui, A., Iliopoulos, K., Sahraoui, B.: Non-linear optical and electrical properties of ZnO doped Ni thin films obtained using spray ultrasonic technique. Opt. Mater. 33, 968–972 (2011)
Addou, M., Moumin, A., Elidrissi, B., Regragui, M., Bougrine, A., Kachouane, A., Monty, C.: Structural, optical and electrical properties of undoped and indium doped zinc oxide prepared by spray pyrolysis. J. Chim. Phys. 96, 232–234 (1999)
Bartolomé, J., Maestre, D., Amati, M., Cremade, A., Piqueras, J.: Two-dimensional ZnkIn2Ok+3 nanostructures: synthesis, growth mechanism, self-assembly, and luminescence. J. Nanopart. Res. 15(10), 1–8 (2013)
Buchholz, D.B., Proffit, D.E., Wisser, M.D., Mason, T.O., Chang, R.P.H.: Electrical and band-gap properties of amorphous zinc–indium–tin oxide thin films. Prog. Nat. Sci. Materi. Int. 22, 1–6 (2012)
Dupont, L., Maugy, C., Naghavi, N., Guéry, C., Tarascon, J.M.: Structures and textures of transparent conducting pulsed laser deposited In2O3–ZnO thin films revealed by transmission electron microscopy. J. Solid State Chem. 158(2), 119–133 (2001)
El Hichou, A., Addou, M., Bougrine, A., Dounia, R., Ebothé, J., Troyon, M., Amrani, M.: Cathodoluminescence properties of undoped and Al-doped ZnO thin films deposited on glass substrate by spray pyrolysis. Mater. Chem. Phys. 83, 43–47 (2004)
El Rhaleb, H., En Naciri, A., Dounia, R., Jhann, L., Hakam, A., Addou, M.: Spectroscopic ellipsometry characterisation of ZnO–In2O3 systems. Thin Solid Films 455, 384–387 (2004)
Hawson, R.P., Avaristsiatis, J.N., Ridge, M.I., Bishop, C.A.:Properties of conducting transparent oxide films produced by ion plating onto room-temperature substrate. Appl. Phys. Lett. 35, 161 (1979). doi:10.1063/1.91065
Huang, K.F., Uen, T.M., Gou, Y.S., Huang, C.R.: Temperature dependence of transport properties of evaporated indium tin oxide films. Thin Solid Films 148, 7–15 (1987)
Kawazoe, H., Yasukawa, M., Hyodo, H., Kurita, M., Yanagi, H., Hosono, H.: P-type electrical conduction in transparent thin films of CuAlO2. Nature 389, 939–942 (1997)
Kulyk, B., Sahraoui, B., Figà, V., Turko, B., Rudyk, V., Kapustianyk, V.: Influence of Ag, Cu dopants on the second and third harmonic response of ZnO films. J. Alloys Compd. 481(1–2), 819–825 (2009)
Kulyk, B., Kapustianyk, V., Tsybulsky, V., Krupka, O., Sahraoui, B.: Optical properties of ZnO/PMMA nanocomposite films. J. Alloys Compd. 502, 24–27 (2010)
Minami, T., Tanaka, S., Kakumu, T., Takata, S.: Highly transparent and conductive Zn2In2O5 thin films prepared by RF magnetron sputtering. Jpn. J. Appl. Phys. 34, L971–L974 (1995a)
Minami, T., Takata, S., Kakumu, T., Sonohara, H.: New transparent conducting MgIn2O4–Zn2In2O5 thin films prepared by magnetron sputtering. Thin Solid Films 270, 22–26 (1995b)
Minami, T., Sonohara, H., Kakumu, T., Takata, S.: Highly transparent and conductive Zn2In2O5 thin films prepared by RF magnetron sputtering. Jpn. J. Appl. Phys. Part 2 Lett. 34, L971–L974 (1995c)
Minami, T., Kakumu, T., Takata, S.: Preparation of transparent and conductive In2O3–ZnO films by radio frequency magnetron sputtering. J. Vac. Sci. Technol. A14, 1704–1708 (1996a)
Minami, T., Kakumu, T., Takata, S.: New multicomponent transparent conducting oxide films for transparent electrodes of flat panel displays. J. Vac. Sci. Technol. A 14, 1689–1693 (1996b)
Minami, T., Kakumu, T., Takeda, Y., Takata, S.: New transparent conducting ZnO–In2O3–SnO2 thin films prepared by magnetron sputtering. Thin Solid Films 317, 318–321 (1998)
Moriga, T., Edwards, D.D., Mason, T.O., Palmer, G.B., Poeppelmeier, K.R., Schndler, J.L., Kannewurt, C.R., Nakabayashi, I.: Phase relationships and physical properties of homologous compounds in the zinc oxide–indium oxide system. J. Am. Ceram. Soc. 81, 1310–1316 (1998)
Moriga, T., Sakamoto, T., Sato, Y., Khalid, A.H., Suenari, R., Nakabayashi, I.: Crystal structures and electrical and optical properties of MgIn2− xGaxO4 solid solutions. J. Solid State Chem. 142, 206–213 (1999)
Phillips, J.M., Cava, R.J., Tomas, G.A., et al.: Zinc–indium–oxide: a high conductivity transparent conducting oxide. Appl. Phys. Lett. 67, 2246–2248 (1995)
Ueda, N., Omata, T., Hikuma, N., Ueda, K., Mizogushi, H., Hashimoto, T., Kawazoe, H.: New oxide phase with wide band gap and high electroconductivity, MgIn2O4. Appl. Phys. Lett. 61, 1954–1955 (1992)
Wang, R., King, L.H., Sleight, A.W.: Highly conducting transparent thin films based on zinc oxide. J. Mater. Res. 11, 1659–1664 (1996)
Wang, A., Dai, J., Cheng, J.: Charge transport, optical transparency, microstructure, and processing relationships in transparent conductive indium–zinc oxide films grown by low-pressure metal-organic chemical vapor deposition. Appl. Phys. Lett. 73, 327–329 (1998)
Zheng, J.P., Kwok, H.S.: Preparation of indium tin oxide films at room temperature by pulsed laser deposition. Thin Solid Films 232, 99–104 (1993).
Acknowledgments
Thanks are due to Drs H. Bougharraf, A. Kachouane and A. Bougrine for their fruitful cooperation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Advanced Materials for Photonics and Electronics.
Guest Edited by Bouchta Sahraoui, Yahia Boughaleb, Kariem Arof, Anna Zawadzka.
Rights and permissions
About this article
Cite this article
Dounia, R., Migalska-Zalas, A., Addou, M. et al. Preparation and characterization of highly transparent and conductive indium-zinc oxide thin films deposited by pyrolysis spray technique. Opt Quant Electron 48, 339 (2016). https://doi.org/10.1007/s11082-016-0596-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0596-9