Abstract
Polymer–ceramic piezoelectric composites are promising materials for sensor and actuator, because of large piezoelectric response properties. Thin films composites of poly(vinylidene fluoride) (PVDF) and piezoceramics potassium sodium niobate (KNN) have been prepared by cast method on indium–tin-oxide-coated glass substrates. The β-phase in PVDF is responsible for piezoelectric property. KNN with different concentrations was used as filler in composites. Corona poling method is used to the polarized the thin films of PVDF/KNN composites. The morphology of thin films determines using SEM and the crystalline structure investigates by XRD and FTIR techniques. SEM shows the rough surface contained spherulitic structural with less porosity and the size of the spherulitic range between 1 and 3 μm in diameter. XRD demonstrated that the structure of pure PVDF film is mix of α and β-phase and converted to the pure β-phase after filler by KNN. FTIR results confirmed the XRD. The fraction β in PVDF films alter from 77.5 to 80.3 % after poling, and enhance to the 86.0 % by filler of KNN in PVDF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Piezoelectric materials are technologically important because of their application in various kinds of devices especially in the sensors and actuators (Qiu and Ji 2010; Uchino 2009). In the recent years, piezoelectric composites of polymers and ceramic gained increasing attention among the materials research community. Polymer–ceramic piezoelectric composites have been usually made in a variety of devices such as sensor and actuator. Polyvinylidene fluoride (PVDF) is one of the most promising smart polymer materials for the piezoelectric and pyroelectric effects. The PVDF has low thermal conductivity, low permittivity, high flexibility and low in cost. They have good mechanical rigidity and piezoelectricity when manufactured as composites. Considering the toxicity of lead and its compounds, there is a general awareness for the development of environmental friendly lead-free materials (Jia et al. 2009; Panda 2009), therefore, research on ceramic materials is mainly focused on lead-free potassium sodium niobate (KNN), because of large piezoelectric response and strong ferroelectricity properties and high Curie temperature (TC > 400 °C) (Baker et al. 2009; Wu et al. 2008) and suitable when a low-cost and light device is required. The piezoelectric composites combined with polymeric phase and piezoelectric ceramic phase are multi-functional composites, and they take important part in smart materials and structural systems.
A particular class of composites consists of a polymer matrix in which randomly distributed particles ceramic are embedded without being connected to each other’s. These composites are usually referred to as 0–3 composites (Newnham et al. 1978). The behavior of polymer–ceramic composites, as a potential material group, is suitable for preparing demanding and functional packages that combine the chemical stability, mechanical flexibility and processing options of polymers with the electrical properties of ceramics. The very important thing to get the required properties is the connectivity between the phases in the composite materials (Sebastian and Jantunen 2010).
Most of the attentions are given on incorporation of lead zirconate titanate ceramic (PZT) and barium titanate (BaTiO3) into PVDF matrix. To the best of our knowledge, there is no available report on the use of KNN to prepare PVDF composites, especially in the form of thin films. In present work, we employed a polymeric material, namely PVDF and a ceramic material, namely KNN for the fabrication of composite thin films by casting method using N,N-dimethylformamide (DMF) as a solvent. Albeit exhibiting different material properties, both PVDF and KNN are known for their excellent dielectric properties. Therefore, our current study aims to enhance the piezoelectricity of the PVDF, which is crucial for the application of pressure sensor.
2 Materials and methods
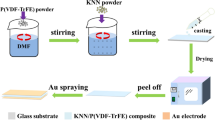
The PVDF/KNN composite films were fabricated via solution cast method. Firstly the PVDF (in powder form) received from Sigma-Aldrich was dissolved in N,N-dimethylformamide (DMF 99.5 %) to form a PVDF solution with a concentration of 15 wt%. The solution was continuously stirred using a magnetic stirrer for 1 h at 400 rpm at 60 °C. The KNN with different concentrations namely 1, 3, 4 and 5 wt% were added to the mixed solution of PVDF/DMF. The resulting solution was placed in an ultrasonic water bath for 30 min at 30 °C. After that, the solution was continuously stirred using a magnetic stirrer for 24 h at 400 rpm at room temperature. Approximately 0.1 ml of the PVDF/KNN solution was cast on the clean ITO coated glass substrates (2.5 × 2 cm2) to form a thin film and directly move in the oven at 60 °C for 24 h. By using corona poling, the PVDF thin films pure and composites polarized by subjected it to the poling treatment at 80 °C by corona charging from a point about 1 cm apart from the surface of the films; at a voltage of (2–4 kV) and duration at 30 min.
2.1 Corona poling
Figure 1 shows the electrical circuit of the corona poling. It consist of the several needles (referred as corona needles) which is connect to high power supply (4 K) voltages, and suspended above the sample which is placed on a hot plate at 80 °C. The bottom surface of sample with electrode connected to the ground. When the corona discharge occurs by applying high voltage, the ionized particles are accelerated towards the ground and are deposited on the sample’s top surface. The charges remain on the surface generating a poling electric field between the top surface and the ground. To ensure that charged ions are evenly distributed and to control the potential at the surface of the film, a conducting grid is placed at the distance of 3–4 mm from the PVDF and held at a constant voltage.
2.2 Characterization
The surface morphology of the films was determined using scanning electron microscopy (SEM) model ZEISS EVO MA10. The samples were gold plated before SEM characterization. The crystalline structure and phases of the KNN powder, pure PVDF and PVDF/KNN composite samples were subsequently determined using a Bruker D8 Advance diffractometer which operated at 40 kV and 40 mA, using a CuKα1 wavelength of 0.15406 nm. The peaks were recorded in the 2θ range of 10°–60°. Fourier transformation infrared spectroscopy (FTIR) was used to confirm the phase of all samples. The FTIR spectra of the samples were obtained in a Perkin Elmer spectrum, with a resolution of 4.00 cm−1 over a range of 650–4000 cm−1. Corona poling was used to polarized the thin films.
3 Results and discussion
3.1 Scanning electron microscopy (SEM)
SEM was performed in order to evaluate the composites microstructure and the dispersion of the ceramic powder within the polymer matrix. Figure 2 shows the SEM micrographs with different magnification of the KNN powder, pure PVDF film and PVDF/KNN composite films with different concentration of KNN namely, 1, 3, 4 and 5 wt%. For powder KNN, dense agglomerate and non-homogeneous grains are observed with an average grain size of 1.3 μm. PVDF, pure and composites films, exhibited the surface of all samples was rough and dens of spherulite structure, while the sizes and distribution of spherulites were not homogeneous. In addition, randomly distributed some pores at spherulite boundaries were also observed on samples. For pure PVDF, the size of spherulite is 2.7 μm in diameter. However, the spherulite sizes of composite PVDF/KNN are 3.1, 3.5, 2.4 and 1.6 μm for 1, 3, 4 and 5 wt%, respectively. The distribution of KNN particles are homogenous and agglomerate in some region to filling the some pores in PVDF polymer. The spherulite size reflects the crystallization kinetics of the polymer. It is determined both by the nucleation ability of the fillers and by the growth rate of the spherulites. During the crystallization process of PVDF, spherulites grow and expand absorbing the liquid polymer phase that is in the region between the crystallized PVDF. At the end of crystallization, no liquid phase remains, and as a consequence pore is generated between the crystallized spherulites. The presence of the KNN in PVDF matrix affect both in the nucleation process and in the growth kinetic of the spherulites. It promotes the crystallization of the β-phase of the PVDF matrix, indicating that the ceramic filler act as a nucleating agent for this phase.
The concentration of the KNN is effected to the nucleation of PVDF in addition to the size of spherulites. At low concentrations of the KNN, the amount of KNN mostly is affected on the nucleation process and the spherulites growth. So the size of the spherulites is larger in the composites compare by the pure PVDF. For higher concentrations of KNN particles, on the other hand, the amount of KNN particles increase, and the ceramic aggregates are formed due to the occupation of the free space within the pores. This aggregates will affect to the nucleation of spherulitic structure and hindering of the spherulite growth due to the presence of the KNN particles. So the spherulites have a small size.
3.2 X-ray diffraction (XRD)
The crystalline structures of the KNN powder, PVDF pure and composite films are characterized by XRD spectrums and shown in Fig. 3. For KNN powder (Fig. 3a), its reveal a single phase of perovskite structure with orthorhombic symmetry and no secondary phases can be detected. The orthorhombic phase is characterized by 45.2° (002) and 46.0° (200) peaks splitting (Lusiola et al. 2015), where the peak at 45.2° is high intensity compared by the peak at 46.0°. The crystalline structures of PVDF powder represent the α-phase as studied in previous work (Abdullah et al. 2014, 2015). For pure PVDF without poling (Fig. 3b), a sharp diffraction peak at 2θ = 20.1°, reflection the α-phase (110) and the β-phase (200/110) (Huang et al. 2008). In addition, two small peaks appears at 30.2° and 35.2° belongs to the ITO substrate. In the case of poling samples (Fig. 3c), the crystalline peak appeared at 20.3° (110/200), reflection the β-phase (Ostaševičius et al. 2010). Another diffraction peak at 2θ = 20.8° (110), reflection the β-phase (Chen et al. 2007). Figure 3 shows also the XRD pattern of the PVDF/KNN composite films without and with poling. The intensities of the PVDF/KNN composites films are higher than the intensity of pure PVDF film, this indicate that the increasing in the crystalline structure of PVDF/KNN composites, so the composites films are more crystalline compare by the pure PVDF film. All the samples reveal the same structure of KNN, where a single phase of perovskite structure with tetragonal symmetry is characterized by splitting the peaks at 45.2° (002) and 46.0° (200) (Hussain et al. 2015), whereas the peak at 45.2° is lower intensity compared by the peak at 46.0°. Also, its show the peaks at 38.2° and 22.5° became lower intensity and the peak at 22.2° developed to high intensity compared by the KNN powder, this suggest that a large fraction of grains are aligned parallel to the tape casting direction (Li et al. 2015). It conclude that filler PVDF with KNN ceramic enhances the crystalline structure of composites and transfer the structure of KNN from orthorhombic to the tetragonal structure.
The piezoelectricity in PVDF formed due to dipole created by fluorine and carbon bonded in its structure. When the PVDF was in the β-form structure, the dipoles are stronger and hence increasing in piezoelectricity. In the β-phase, all the dipoles alignment throughout the polymer chain creating polarization that extends to the boundaries of the PVDF. The polarization increase during the poling process which applied the high electric filed on the PVDF films. The molecular interaction between the PVDF and solvent is consider important parameters to enhance the crystallization β-phase in PVDF. In the case of DMF as a solvent, the molecular interaction include the dipolar interactions between C=O and CH2–CF2 in DMF and PVDF, respectively, and the presence of weak hydrogen bonding C=O···H–C, both of which disrupt the inter-chain forces of solid PVDF and finally dissolve the PVDF (Benz et al. 2001).
The presence of KNN ceramic within PVDF matrix nucleate the β-phase. According to the previous works, this effect depend on several factors including the size and filler content as well as the interfacial bonding between the filler and polymer matrix (Sebastian and Jantunen 2010; Ramajo et al. 2007; Dang et al. 2008; Venkatragavaraj et al. 2001). Since the connection between the polymer and the ceramic is 0–3, therefore, the nucleation of the piezoelectric phase caused by the interface interactions between the local electric field of the filler and PVDF dipoles (Martins et al. 2013). These local field–dipole interactions can have different nature such as ion–dipole and dipole–dipole, among others (Zhong et al. 2011).
3.3 Fourier transform infrared (FTIR)
In order to distinguish the crystalline phase for PVDF films and evaluate the β fraction in each samples, Fourier transform infrared (FTIR) was used. Figure 4 shows the FTIR spectra of pure PVDF and composites films with and without poling. For pure PVDF, Fig. 4a, it’s appeared that the spectrum of two cases have the same peaks position but different in the intensity of the peaks, whereas, the spectra of poled films is high intensity. The bands at 836 (CH2 rocking), 875 and 1070 (CC skeletal) and 1402 cm−1 (CF2 wagging) characterized the β-phase, while, the bands identified the α-phase is 983 cm−1 (CH2 twisting). So the crystalline phases of pure PVDF represented mixed of α and β phases. Figure 4b, c also shows the FTIR spectra of the PVDF/KNN composites films with and without poling for different percent of KNN. All vibration peaks appearing belong to the polymer PVDF. It’s obvious that all peaks appeared in this figure characterized the β-phase. By compared the results of pure PVDF films with PVDF/KNN composites films, it show the disappearance of the peaks at 746 and 983 cm−1 which belong to the α-phase and appeared new the peak at 1276 cm−1 which distinguished the β-phase. So add KNN as a filler modified the phase of PVDF and convert it from mix of α and β-phase to the pure β-phase. To evaluate the fraction of β in each samples, FTIR technique was used. The following formula was used to calculate it (Cardoso et al. 2008; Gregorio and de Souza Nociti 1995).
where Aα and Aβ are the absorption fraction at 763 and 840 of α and β-phase, respectively. Figure 4d, e shows the β-phase content of the PVDF pure and composites thin films without and with poling respectively. It appears that the β content in the pure PVDF films are 77.5 and 80.3 % without and with poling respectively. In the case of PVDF/KNN composites thin films, the maximum contained of β-phase for the samples without poling is 84.6 % at the 4 wt%. By poling the samples, the contained of β-phase increase and have the high value at 4 wt% to became 86.0 %. Consequently, the KNN filler to PVDF enhancing the piezoelectricity in polymer and modified by poling process.
4 Conclusions
PVDF/KNN composite thin films are prepared using solvent cast method. SEM demonstrates that the surface of all samples was rough and spherulite structure, but their sizes and distribution of spherulites were not homogeneous. The distribution of KNN particles is homogenous and agglomerate in some region to filling the some pores in PVDF matrix. The connection between KNN as a filler and PVDF matrix is 0–3. The presence of the KNN in PVDF matrix affect both in the nucleation process and in the growth kinetic of the spherulites and act as a nucleating agent for β-phase. XRD analysis shows that the structure of KNN powder was single phase of perovskite structure with orthorhombic symmetry and no secondary phases can be detected. All PVDF/KNN composites films reveal the same structure, where a single phase of perovskite structure with tetragonal symmetry. The crystalline structures of pure PVDF consist of α and β-phase and PVDF/KNN composites were β-phase and increasing the alignment of the dipolar C-H molecular chain after poled by corona poling. FTIR confirm that the crystalline phases of PVDF/KNN composite are β-phase. The fraction of F(β) in PVDF films alter from 77.5 to 80.3 % after poling, and enhance to the 86.0 % by filler of KNN in PVDF.
References
Abdullah, I.Y., Yahaya, M., Haji Jumali, M.H., Shanshool, H.M.: Effect of annealing process on the phase formation in poly(vinylidene fluoride) thin films. In: AIP Conference Proceedings, vol. 1614, pp. 147–151 (2014). doi:10.1063/1.4895187
Abdullah, I.Y., Haji Jumali, M.H., Yahaya, M., Shanshool, H.M.: Facile formation of β poly(vinylidene fluoride) films using the short time annealing process. Adv. Environ. Biol. 9(20), 20–27 (2015)
Baker, D.W., Thomas, P.A., Zhang, N., Glazer, A.M.: A comprehensive study of the phase diagram of KxNa1–xNbO3. Appl. Phys. Lett. 95(9), 1903-1–1903-3 (2009). doi:10.1063/1.3212861
Benz, M., Euler, W.B., Gregory, O.J.: The influence of preparation conditions on the surface morphology of poly(vinylidene fluoride) films. Langmuir 17(1), 239–243 (2001)
Cardoso, V.F., Rocha, J.G., Nunes, J.S., Lanceros-Mendez, S., Minas, G.: Piezoelectric β-PVDF polymer films as fluid acoustic microagitator. In: IEEE International Symposium on Industrial Electronics. ISIE 2008, pp. 2028–2033. IEEE (2008)
Chen, S., Yao, K., Tay, F.E.H., Liow, C.L.: Ferroelectric poly(vinylidene fluoride) thin films on Si substrate with the beta phase promoted by hydrated magnesium nitrate. J. Appl. Phys. 102(10), 04108-1–104108-7 (2007)
Dang, Z.M., Zheng, Y., Xu, H.P.: Effect of the ceramic particle size on the microstructure and dielectric properties of barium titanate/polystyrene composites. J. Appl. Polym. Sci. 110(6), 3473–3479 (2008)
Gregorio Jr, R., de Souza Nociti, N.C.P.: Effect of PMMA addition on the solution crystallization of the alpha and beta phases of poly(vinylidene fluoride) (PVDF). J. Phys. D Appl. Phys. 28(2), 432–436 (1995)
Huang, F., Wei, Q., Wang, J., Cai, Y., Huang, Y.: Effect of temperature on structure, morphology and crystallinity of PVDF nanofibers via electrospinning. e-Polymers 8(1), 1758–1765 (2008)
Hussain, A., Maqbool, A., Kim, J.S., Song, T.K., Kim, M.H., Kim, W.J., Kim, S.S.: Sodium excess Ta-modified (K0.5Na0.5) NbO3 ceramics prepared by reactive template grain growth method. Int. J. Appl. Ceram. Technol. 12(1), 228–234 (2015)
Jia, Q., Shen, B., Hao, X., Song, S., Zhai, J.: Anomalous dielectric properties of Ba1–xCaxTiO3 thin films near the solubility limit. Mater. Lett. 63(3), 464–466 (2009)
Li, L., Bai, W., Zhang, Y., Shen, B., Zhai, J.: The preparation and piezoelectric property of textured KNN-based ceramics with plate-like NaNbO3 powders as template. J. Alloys Compd. 622, 137–142 (2015)
Lusiola, T., Ali, H., Kim, M.H., Graule, T., & Clemens, F.: Ferroelectric KNNT fibers by thermoplastic extrusion process: microstructure and electromechanical characterization. In: Actuators, vol. 4, no. 2, pp. 99–113. Multidisciplinary Digital Publishing Institute (2015)
Martins, P., Costa, C.M., Benelmekki, M., Botelho, G., Lanceros-Méndez, S.: Interface characterization and thermal degradation of ferrite/poly(vinylidene fluoride) multiferroic nanocomposites. J. Mater. Sci. 48(6), 2681–2689 (2013)
Newnham, R.E., Skinner, D.P., Cross, L.E.: Connectivity and piezoelectric–pyroelectric composites. Mater. Res. Bull. 13(5), 525–536 (1978)
Ostaševičius, V., Milašauskaitė, I., Daukševičius, R., Baltrušaitis, V., Grigaliūnas, V., Prosyčevas, I.: Experimental characterization of material structure of piezoelectric PVDF polymer. Mechanika 6(86), 78–82 (2010)
Panda, P.K.: Review: environmental friendly lead-free piezoelectric materials. J. Mater. Sci. 44(19), 5049–5062 (2009)
Qiu, J., Ji, H.: The application of piezoelectric materials in smart structures in China. Int. J. Aeronaut. Space Sci. 11(4), 266–284 (2010)
Ramajo, L., Castro, M.S., Reboredo, M.M.: Effect of silane as coupling agent on the dielectric properties of BaTiO3-epoxy composites. Compos. A Appl. Sci. Manuf. 38(8), 1852–1859 (2007)
Sebastian, M.T., Jantunen, H.: Polymer–ceramic composites of 0–3 connectivity for circuits in electronics: a review. Int. J. Appl. Ceram. Technol. 7(4), 415–434 (2010). doi:10.1111/j.1744-7402.2009.02482.x
Uchino, K.: Ferroelectric Devices, 2nd edn. CRC Press, London (2009)
Venkatragavaraj, E., Satish, B., Vinod, P.R., Vijaya, M.S.: Piezoelectric properties of ferroelectric PZT–polymer composites. J. Phys. D Appl. Phys. 34(4), 487 (2001)
Wu, L., Zhang, J.L., Wang, C.L., Li, J.C.: Influence of compositional ratio K/Na on physical properties in (KxNa1–x) NbO3 ceramics. J. Appl. Phys. 103(8), 84116-1–084116-5 (2008)
Zhong, G., Zhang, L., Su, R., Wang, K., Fong, H., Zhu, L.: Understanding polymorphism formation in electrospun fibers of immiscible poly(vinylidene fluoride) blends. Polymer 52(10), 2228–2237 (2011). doi:10.1016/j.polymer.2011.03.024
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdullah, I.Y., Yahaya, M., Jumali, M.H.H. et al. Enhancement piezoelectricity in poly(vinylidene fluoride) by filler piezoceramics lead-free potassium sodium niobate (KNN). Opt Quant Electron 48, 149 (2016). https://doi.org/10.1007/s11082-016-0433-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0433-1