Abstract
The zinc oxide (ZnO) thin films were deposited onto the glass substrates by a successive ionic layer adsorption and reaction method, which is based on the alternate dipping of substrate in a zinc sulphate solution complexed with \(\hbox {NH}_{4}\hbox {OH}\) and distilled water heated at \(75\,^{\circ }{\hbox {C}}\). The time duration for which a substrate is dipped in the distilled water, plays an important role and it has been shown in this work that the time period for which a substrate is dipped in distilled water, which we referred as rinsing period, affects significantly on the structural and optical properties. The structural analyses from X-ray diffraction patterns displayed the formation of polycrystalline ZnO with high orientation along c-direction (002). The optical band gap was found to be declined from 3.3 to 3.27 eV. X-ray photoelectron spectroscopy indicates that the ZnO films are free of decomposed precursor residues in the bulk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide (ZnO) semiconductors are of current interest because of their potential applications in light-emitting diodes (Savariumuthu et al. 2007), photodetector (Saito et al. 2002), lasers (Kind et al. 2002; Huang et al. 2001), solar cells (Atsushi et al. 2005), varistors (Pradhan et al. 2007), gas sensors (Ohashi et al. 2003), and memory (Li et al. 2009) because they have distinctive physical properties, such as low dielectric constants, high chemical stabilizations, large band strengths, large exciton binding energies, and good photoelectric and piezoelectric properties (Li et al. 2007; Wang 2009). Different methods have been used to deposit ZnO thin films. These include spray pyrolysis (Sahay et al. 2007), sol-gel (Srinivasan and Kumar 2006), pulsed laser deposition (Bellingeri et al. 2005), chemical bath deposition CBD (Mitra and Khan 2006), electrodeposition (Fahoume et al. 2006), successive ionic layer adsorption and reaction (SILAR) (Raidou et al. 2010, 2011).
Successive ionic layer adsorption and reaction (SILAR) method is one of the solution methods. SILAR method does not require any sophisticated instruments like physical techniques. More details of SILAR method are well documented in literature (Pathan and Lokhande 2004). Due to the scarcity of p-type thin films used for p–n junction solar cells devices, we have reported synthesis of ZnO thin films using SILAR method and characterized for its structural and optical properties.
2 Experimental
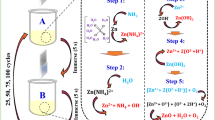
In this present work, ZnO thin films have been deposited onto the glass substrates by alternate immersion of substrate in \(\hbox {Zn}^{2+}\) source complexed with \(\hbox {NH}_{4}\hbox {OH}\). Aqueous zinc sulphate solution was used as source of zinc ion. The glass substrate \(75\times 25\times 1.5\,\hbox {mm}^{3}\) was cleaned by acetone and with distilled water. First, the cleaned glass substrate was immersed in zinc sulphate solution so to get zinc complex adsorbed onto the substrate, and in the hot distilled water. The second bath served the additional purpose of removal of loosely bound particles from the substrate, and formation of ZnO particles. The period for which the substrate was kept in zinc bath was referred as adsorption period and that for which the substrate was kept in distilled water is named rinsing period. The rinsing period was varied in such manner that ZnO films of different grain sizes could be deposited. We fixed adsorption period at 30 s, and we varied rinsing period 20, 30, and 40 s.
The crystalline quality of the ZnO thin films is investigated by the X-ray diffraction (XRD). The XRD patterns were recorded using the XPERT-PRO PW-3064 X-ray diffractometer using Cu \(\hbox {K}\upalpha \) radiation with wavelength \(\lambda \) = 1.540598  . Optical measurements were realized using Perkin Elmert Instrument lambda 900 UV/VIS/NIR spectrophotometer.
. Optical measurements were realized using Perkin Elmert Instrument lambda 900 UV/VIS/NIR spectrophotometer.
3 Results and discussion
3.1 ZnO film formation
The mechanism of ZnO film formation by SILAR method can be elucidated as follow. The 0.08 M \(\hbox {ZnSO}_{4}\cdot 7\hbox {H}_{2}\hbox {O}\) was used as a source of zinc and to make it complex, ammonium hydroxide solution was added constantly stirring. Initially when ammonium hydroxide solution was added in zinc sulphate solution, the ionic product of \(\hbox {Zn(OH)}_{2}\) exceeds the solubility product and the solution becomes milky turbid due to formation of \(\hbox {Zn(OH)}_{2}\). This can be explained by the following reaction
However, further addition of excess ammonium hydroxide reduces \(\hbox {Zn}^{2+}\) ion by producing the complex ions of the type \(\hbox {Zn}(\hbox {NH}_3 )_\mathrm{n}^{2+}\) (n = 1–4) and n = 4 the most stable coordination number (Sillen and Martell 1971), which avoids precipitation makes solution clear and transparent. This can be explained by the following reaction
When substrate is immersed in the above solution, these Zinc complex ions get adsorbed on the substrate due to attractive force between ions in the solution and surface of the substrate. These forces may be cohesive forces or van der waals or chemical attractive forces (Pathan and Lokhande 2004). Then, the glass substrate is immersed in hot distilled water, and the \([\hbox {Zn}(\hbox {NH}_3 )_4 ]^{2+}\) complex decomposes, forming \(\hbox {Zn(OH)}_2 \):
And finally, the as-deposited \(\hbox {Zn(OH)}_2\) will transform to ZnO
3.2 Structural analysis
Figure 1 shows the XRD patterns of our three samples deposited on glass substrates by SILAR technique at 20, 30 and 40 s of rinsing period. All the peaks in the XRD pattern could be indexed to hexagonal wurtzite structure of ZnO, which are good agreement with the ASTM card [PDF No. 79–207, a = 3.2648  and c = 5.2194
and c = 5.2194  ]. No diffraction peaks of the impurities were detected in the three cases, indicating the high purity and single phase nature of the obtained films. From Fig. 1a, it is clear that ZnO film is highly oriented along (002). Other orientations corresponding to (100) and (101) are present with low relative intensities (65.41 and 47.8 % respectively) as compared to that of (002) plane. And we note, the presence of Zn(OH)\(_{2 }\) has not been detected by XRD pattern. This may be because the hydroxide may be present in small extent and accumulated along grain boundaries of the crystallites constituting the film or Zn(OH)\(_{2}\) may be amorphous (Shinde et al. 2005).
]. No diffraction peaks of the impurities were detected in the three cases, indicating the high purity and single phase nature of the obtained films. From Fig. 1a, it is clear that ZnO film is highly oriented along (002). Other orientations corresponding to (100) and (101) are present with low relative intensities (65.41 and 47.8 % respectively) as compared to that of (002) plane. And we note, the presence of Zn(OH)\(_{2 }\) has not been detected by XRD pattern. This may be because the hydroxide may be present in small extent and accumulated along grain boundaries of the crystallites constituting the film or Zn(OH)\(_{2}\) may be amorphous (Shinde et al. 2005).
The XRD pattern of ZnO film deposited at 30 s of rinsing period is shown in Fig. 1b. As seen from this figure, the orientation along (002) has been remained the same, but other two orientations (100) and (101) have relativity decreased in their relative intensities to 25.94 and 20.52 %.
And it is observed in Fig. 1c, the XRD pattern of ZnO film deposited at 40 s of rinsing period, the absence of (100) and (101) diffraction peaks, with the increase in intensity of (002) diffraction peak, i.e., the c-axis orientation of the ZnO films increases with an increase in rinsing period. It was determined that the quality of the ZnO thin films and the crystallization were influenced by rinsing period.
The decrease in intensity of the peaks relative to the background noise with increasing of rinsing period, demonstrates high purity of the hexagonal ZnO and good crystallinity of samples obtained at 40 s.
Increasing the rinsing period provided more thermal energy for the decomposition of the intermediate \(\hbox {Zn(OH)}_{2}\) into ZnO and water.
3.3 Optical properties
The variation of absorbance and transmittance of the ZnO thin films, of three samples with wavelength \(\lambda \) are shown in Fig. 2a, b respectively. The spectrum of absorbance reveals that ZnO films have low absorbance in the visible region, which is characteristic of ZnO. The transmittance has decreased with increase of rinsing period. This may be due to decrease in the hydroxide accumulation along grain boundaries and morphological changes.
The theory of optical absorption gives the relationship between the absorption coefficient \(\alpha \) and the photon energy \((h\nu )\) for direct allowed transition as
This equation gives the band gap \((E_g )\), when straight portion of \((\alpha h\nu )^{2}\) against plot is extrapolated to the point \(\alpha =0\).
Figure 3 shows the plot of \((\alpha h\nu )^{2}\) versus \((h\nu )\) for ZnO films. From this figure, the band gap was found to 3.29 and 3.33 eV for 20 and 30 s respectively, of rinsing period. The higher band gaps may be due to the presence of zinc hydroxide. The band gap was found to be decreased to 3.27 eV for 40 s of rinsing period, can be attributed to the lowest thickness, which due to the removal of \(\hbox {Zn(OH)}_{2}\) particles from surface of the film and/or removal of defect levels. Removal of stacking faults result in orientation of individual crystallites, and the occurrence of defect free grain boundaries.
3.4 Chemical characterization (XPS)
X-ray photoelectron spectroscopy (XPS) was used to monitor changes in the stoichiometry and oxidation state of ZnO thin films. Figure 4 shows XPS spectra wide scan (a), and core level regions \(\hbox {Zn}_{\mathrm{2p}}\) (b) and \(\hbox {O}_{\mathrm{1s}}\) (c) of ZnO film. Charging effects due to the low conductivity of the glass slides used as substrates have been minimized by calibrating the binding energy (BE) scale using the \(\hbox {O}_{\mathrm{1s}}\) XPS peaks of ZnO (531.2 eV) (Battistoni et al. 1981).
Our sample show XPS peaks at \(\sim \)1,022.08 and \(\sim \)1,045 eV (Fig. 4b), corresponding to the \(\hbox {2p}_{3/2}\) and \(\hbox {2p}_{1/2}\) core levels of Zn in ZnO. The BE difference between \(\hbox {Zn}_{\mathrm{2p3/2}}\) and \(\hbox {Zn}_{\mathrm{2p1/2}}\) is \(\sim \)23 eV which is a characteristic value of ZnO.
Figure 4c shows a clear photoelectron peak at \(\sim \)532 eV, corresponding to the \(\hbox {O}_{\mathrm{1s}}\) core level in ZnO. This BE can be attributed to \(\hbox {O}_{\mathrm{1s}}\) in \(\hbox {SiO}_{2}\) (Paparazzo 1987), or to oxide species located at the surface of ZnO film (shifted +1.8 eV with respect to \(\hbox {O}_{\mathrm{1s}}\) in bulk like ZnO) (Säuberlich et al. 2003), as well as to hydroxide species (Säuberlich et al. 2003). We note, no XPS signal from the \(\hbox {SiO}_{2}\) substrate is expected. However, since this signal, we can attribute the shoulder in our \(\hbox {O}_{\mathrm{1s}}\) spectra to surface oxygen species on ZnO surface.
4 Conclusion
Zinc oxide (ZnO) thin films were deposited on glass substrates with a precursor of zinc-ammonia complex using a simple SILAR method under different rinsing period. For defining the optimal rinsing period, the structural and optical properties of the films were studied as a function of rinsing period. The XRD studies showed that films have a polycrystalline structure with no preferred orientation, and the crystalline of these thin films is improved with increasing rinsing period. The band gap energy values decreased with increasing rinsing period. The band gap values reduced from 3.33 to 3.27 eV. XPS investigation indicates that the ZnO films are free of decomposed precursor residues in the bulk, with a good crystallinity.
References
Atsushi, T., Akira, O., Takeyoshi, O., Makoto, O., Takayuki, M.: Repeated temperature modulation epitaxy for p-type doping and light-emitting diode based on ZnO [J]. Nat. Mater. 4, 42–46 (2005)
Battistoni, C., Dormann, J.L., Fiorani, D., Paparazzo, E., Viticoli, S.: An XPS and Mössbauer study of the electronic properties of \(\text{ ZnCr }_{\rm x} \text{ Ga }_{{\rm 2-x}}\text{ O }_{4}\) spinel solid solutions. Solid State Commun. 39, 581–581 (1981)
Bellingeri, E., Marre, D., Pallecchi, I., Pellegrino, L., Canu, G., Siri, A.S.: Deposition of ZnO thin films on \(\text{ SrTiO }_{3}\) single-crystal substrates and field effect experiments. Thin Solid Films 486, 186–190 (2005)
Fahoume, M., Maghfoul, O., Aggour, M., Hartiti, B., Chraibi, F., Ennaoui, A.: Growth and characterization of ZnO thin films prepared by electrodeposition technique. Sol. Energy Mater. Sol. Cells 90, 1437–1444 (2006)
Huang, M.H., Mao, S., Feick, H., Yan, H., Wu, Y., Kind, H., Weber, E., Russo, R., Yang, P.: Room-temperature ultraviolet nanowire nanolasers. Science 292(5523), 1897–1899 (2001)
Kind, H., Yan, H., Messer, B., Law, M., Yang, P.: Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 14, 158–160 (2002)
Li, C.C., Du, Z.F., Li, L.M., Yu, H.C., Wan, Q., Wang, T.H.: Surface-depletion controlled gas sensing of ZnO nanorods grown at room temperature. Appl. Phys. Lett. 91, 032101 (2007). doi: 10.1063/1.2752541
Li, F., Cho, S.H., Son, D.I., Kim, T.W., Lee, S.K., Cho, Y.H., Jin, S.: UV photovoltaic cells based on conjugated ZnO quantum dot/multiwalled carbon nanotube heterostructures. Appl. Phys. Lett. 94, 111906 (2009). doi: 10.1063/1.3098400
Mitra, P., Khan, J.: Chemical deposition of ZnO films from ammonium zincate bath. Mater. Chem. Phys, 98, 279–284 (2006)
Ohashi, N., Kataoka, K., Ohgaki, T., Miyagi, T., Haneda, H., Morinaga, K.: Synthesis of zinc oxide varistors with a breakdown voltage of three volts using an intergranular glass phase in the bismuth-boron-oxide system. Appl. Phys. Lett. 83, 4857–4859 (2003)
Paparazzo, E.: X-ray photoemission and Auger spectra of damage induced by Ar\(^{+}\)-ion etching at SiO\(_{2}\) surfaces. J. Phys. D: Appl. Phys. 20, 1091–1094 (1987)
Pathan, H.M., Lokhande, C.D.: Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci. 27, 85–111 (2004)
Pradhan, B., Batabyal, S.K., Pal, A.J.: Vertically aligned ZnO nanowire arrays in Rose Bengal-based dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 91, 769–773 (2007)
Raidou, A., Aggour, M., Qachaou, A., Laanab, L., Fahoume, M.: Preparation and characterisation of Zno thin films deposited by SILAR method. J. Condens. Matter 12(2), 125–130 (2010)
Raidou, A., Aggour, M., Qachaou, A., Laanab, L., Fahoume, M.: J. Optoelectron. Adv. Mater. SYMPOSIA 3(3), 147–150 (2011)
Sahay, P.P., Tewari, S., Nath, R.K.: Optical and electrical studies on spray deposited ZnO thin films. Cryst. Res. Technol. 42, 723–729 (2007)
Saito, N., Haneda, H., Sekiguchi, T., Ohashi, N., Sakagushi, I., Koumoto, K.: Low-temperature fabrication af light-emitiing zinc oxide micropatterns using selfassemled monolayers. Adv. Mater. 14, 418–421 (2002)
Säuberlich, F., Fritsche, J., Hunger, R., Klein, A.: Properties of sputtered ZnO films and its interfaces with CdS. Thin Solid Fillms 431(432), 378–381 (2003)
Savariumuthu, P.A., Jeong, I.L., Jin, K.K.: Tuning optical band gap of vertically aligned ZnO nanowire arrays grown by homoepitaxial electrodeposition Appl. Phys. Lett. 90, 103107 (2007). doi: 10.1063/1.2711419
Shinde, V.R., Lokhande, C.D., Mane, R.S., Han, S.-H.: Hydrophobic and textured ZnO films deposited by chemical bath deposition: annealing effect. Appl. Surf. Sci. 245, 407–413 (2005)
Sillen, L.G., Martell, A.F. (eds.). Stability Constants of Metal Ion Complexes, Suppl. 1, Special Publication No. 25. The Chemical Society, London, (1971)
Srinivasan, G., Kumar, J.: Optical and structural characterisation of zinc oxide thin films prepared by sol-gel process. Cryst. Res. Technol 41, 893–896 (2006)
Wang, Z.L.: ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R 64, 33–71 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raidou, A., Benmalek, F., Sall, T. et al. The influence of rinsing period on the structural and optical properties of ZnO thin films. Opt Quant Electron 46, 171–178 (2014). https://doi.org/10.1007/s11082-013-9737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11082-013-9737-6