Abstract
Stochastic resonance and energy consumption are significant for information processing and transmission in the neural system. In this paper, we constructed an excitatory–inhibitory cortical neuronal network to investigate the response of the system to weak signals and the corresponding energy consumption. The findings indicate that the excitability of neurons modulates the performance of signal response. Furthermore, the performance of signal response exhibits a bell-shaped dependence on ion channel noise, which is a typical manifestation of the stochastic resonance phenomenon. Stochastic resonance also exists in the network with increasing noise at different excitatory coupling strengths and inhibitory coupling strengths. Furthermore, it is found that the neuronal system obtains optimal transmission of the weak signal at a lower energy consumption. It illustrates that there is a certain economy and efficiency in the signal transmission. At weak inhibitory coupling strength, an optimal excitatory coupling strength exists to allow the neuronal network to make the optimal transmission of the weak signal. However, the phenomenon of double resonant peaks occurs at strong inhibitory coupling strength, which is due to the balance of excitatory and inhibitory synaptic currents. Finally, we demonstrated the robustness of the results to network topology and initial conditions. The results of this paper may contribute to the understanding of signal transmission and its energy consumption in cortical networks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Stochastic resonance (SR) is a phenomenon that improves the response of a nonlinear system to the weak signal by adding a random perturbation [1,2,3,4,5], and this random perturbation generally refers to noise. A number of biological experiments have demonstrated the widespread existence of SR in the neural system. For example, the mechanoreceptors of crayfish [6], the cricket cercal sensory system [7], and the human proprioceptive system [8]. Moreover, SR is extensively investigated theoretically in individual neurons and neural networks [9,10,11].
These numerous studies have shown that there are many types of noise that can induce SR, such as Gaussian white noise [10, 12,13,14], color noise [15,16,17], ion channel noise [9, 18], etc. In particular, ion channel noise is the main source of noise in neuronal systems. It is an internal source of noise due to the random opening and closing of individual ion channels [18, 19], random permeability, and differences in ion concentrations inside and outside the cell. Therefore, it is relevant to consider ion channel noise in the investigation of SR in the neural system. Researchers studied ion channel noise-induced SR phenomena in various network topologies. Ozer et al. demonstrated in a small-world network [20] and feedforward network [21] that the existence of optimal intensity of ion channel noise makes the optimization of signal transmission for neuronal networks. Yilmaz et al. showed that the best temporal coherence in a scale-free Hodgkin-Huxley (H-H) neuronal network is obtained with a certain degree of intrinsic noise [22].
With the deepening of the study, some experimental studies have revealed that SR can also be generated in cortical networks. Funke et al. discovered a similar phenomenon to SR by adding weak, sub-threshold, and peri-threshold visual stimuli to the cat primary visual cortex and superimposing noise with variable jitter amplitude [23]. Van der Groen and Wenderoth demonstrated that cortical networks that process visual stimuli can exhibit the SR phenomenon [24]. It is shown that SR has an important impact on the transmission of weak signal and information processing in the neural system. There are two major kinds of neurons in the mammalian cerebral cortex, the excitatory pyramidal neurons and the inhibitory interneurons [25, 26]. Moreover, the ratio of excitatory neurons to inhibitory neurons was found to be 4:1 in the visual cortex of rats [27]. Yu et al. constructed a cortical network based on this [28]. They demonstrated that time delay and electrical-chemical synapses have an important role in SR and determine the capacity of the neural system to enhance information transmission. Enlightened by this, We constructed an excitatory-inhibitory cortical neuronal network using the H-H neuron as the basic unit in this paper.
The normal operation of the brain is inextricably connected to energy. It is known that the human brain accounts for only 2% of body weight but consumes 20% of human metabolic energy [29]. Furthermore, the energy required for the processing and transmission of information accounts for a large portion of the energy consumption of the brain [30]. Currently, there are three methods used to study the energy of neuronal systems. The methods used to calculate energy consumption are the ion counting method [31,32,33] and the equivalent circuit method [34,35,36]. The Hamilton energy function based on Helmholtz’s theorem is mainly used to study the energy storage, conversion and energy balance of the system [37]. The ion counting method limits the accurate assessment of the metabolic cost of ionic currents in action potential conduction along the axon [38]. Therefore the equivalent circuit method is more accurate compared to the ion counting method, so the energy consumption has been calculated using the equivalent circuit method in this paper. The consumption of energy during information transmission in the neural system has been studied in recent years based on this method. For example, it is reported that information processing capacity and energy efficiency can be maximized by regulating the number of active ion channels [39]. Yu et al. showed that neuronal systems can obtain reliable logic operations with low energy consumption in logical stochastic resonance [40, 41]. SR is related to information transmission [23, 24], thus it is significant to research its energy consumption.
Despite the extensive studies on SR in the neural system, so far as we know that energy consumption during the transmission of weak signals by the neural system has not been considered. Therefore, in this paper, the response performance and energy consumption of a single H–H neuron and an excitatory-inhibitory cortical neuronal network to the weak signal are investigated by using the equivalent circuit method.
The paper is organized as follows: in Sect. 2, mathematical models of the single neuron and the excitatory-inhibitory cortical neuronal network are introduced, and equations for energy consumption are derived by the equivalent circuit method. In Sects. 3.1 and 3.2, the SR and energy consumption of the single neuron and neuronal network are discussed, respectively. In Sect. 3.3, we verify the robustness of the results. Finally, a summary of the results is given in Sect. 4.
2 Model and methods
In this paper, the classical H-H model [42] is used to investigate the capability of neurons to respond weak signals and the energy consumption of neuronal action potentials.
2.1 Single neuron
The dynamics of the membrane potential of a neuron can be described as follows:
where
where Cm = 1 µF/cm2 is the neuronal cell membrane capacitance, V denotes the membrane potential of the neuron. iNa, iK, and iL are sodium ion current, potassium ion current, and leakage current, respectively. gNa = 120 mS/cm2, gK = 36 mS/cm2 and gL = 0.3 mS/cm2 are the maximum values of the conductance of each ion channel. ENa = 50 mV, EK = − 77 mV, EL = − 54.4 mV are the Nernst potentials for iNa, iK, and iL. To consider the effect of ion channel noise on neuronal dynamics, the Langevin equation with the addition of ion channel noise to the three gating variables, n, m, and h, is given as follows [43]:
Where αX (t) and βX (t) are rate functions of the gating variables, described as follows:
In Eq. (3), ξX (t) is independent Gaussian white noise with zero means, and its statistical characteristics are described as follows:
where NK = ρKS and NNa = ρNaS indicate the number of potassium and sodium ion channels, respectively, and the number of ion channels affects the level of ion channel noise. When the ion channel density is homogenous with ρK = 18 µm−2 and ρNa = 60 µm−2, the number of ion channels depends on the total cell membrane area S [19]. The larger S, the greater NK and NNa, and therefore the smaller the ion channel noise intensity.
In Eq. (1), the I0 refers to the bias current, which regulates the level of excitability in the neuron [44]. Iext = Acos(ωt) is the external input weak signal, where the amplitude A = 1.03 µA/cm2 and the angular frequency ω = 0.3 rad/ms. The sub-threshold (resting state) and supra-threshold (firing state) states in the amplitude-period parameter plane are illustrated in Fig. 1, so the weak signal we apply to the neuron is sub-threshold. In this section, the Euler algorithm is used, the time step is set as 0.01 ms, and the initial values of (V, n, m, h) are set as (0.0, 0.0, 0.0, 0.0).
The response of a neuron to an input weak signal is evaluated by calculating the Fourier coefficient Q [45], defined as follows:
T0 = 50 ms is set to eliminate the effect of model initialization on the simulation calculation, T = 2nπ/ω indicates the total duration of the calculation, and the number of the period n = 500 is set in the numerical simulation of a single neuron. The results are obtained by averaging 40 independent runs. It is well known that information transmission during neuronal firing primarily relies on large spikes rather than sub-threshold oscillations, so we are primary interested in the frequency of the spikes [46]. Therefore, we set the threshold VS = 0 mV, if V < VS, then replace V with the fixed point Vf = − 1 mV; otherwise, V remains the same [47]. The larger Q indicates the higher level of synchronization between neuronal output activity and weak signal, resulting in a better response of neurons to the weak signal.
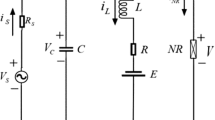
In this paper, the equivalent circuit method is used to calculate the metabolic energy of neuronal action potentials [34, 48, 49]. Considering the H-H neuron model as an electronic circuit, circuit elements include membrane capacitance, maximum variable conductance of sodium, potassium, and leakage ions, and the corresponding Nernst potentials of the ions as batteries. The equivalent circuit of the H-H neuron is shown in Fig. 2. The total energy consumption by the circuit at a given moment is [34]:
the first term on the right is the electrical energy accumulated in the membrane capacitance, and the other three terms are the energy consumption of each battery. It is known that the rate of the electrical energy supplied by the battery to the circuit is equal to the battery potential multiplied by the current through the battery. Thus, the total energy consumption rate is the derivative of Eq. (7) for time:
Substituting Eq. (1) into Eq. (8), we obtain:
the first term on the right side of Eq. (9) represents the electrical energy provided to the neuron by the external input, and the other three terms represent the energy consumed per second by the ion pump. Thus, the average energy consumption Ec of a neuron when it is working can be expressed as:
The phenomenon of SR is important for the transmission of information [28], and the response of neurons to weak signals is also the transmission of signals. To investigate the energy efficiency of the signal transmission process, the energy efficiency η is defined as the ratio of Q to Ec:
2.2 Excitatory–inhibitory cortical neuronal network
To further investigate the collective behavior of the neuronal system, and considering the experimentally observed small-world nature of cortical connectivity [50,51,52]. So an excitatory-inhibitory cortical neuronal network consisting of the same H-H neurons and implemented on the Watts-Strogatz (WS) small-world model is used. The network is composed of N = 60 identical neurons with a 4:1 ratio of excitatory to inhibitory neurons [27]. The rewiring probability is denoted by P. The degree of each node is denoted by K. Unless otherwise specified, P = 0.4, K = 12 [53]. The dynamical equations of the network are expressed as follows:
where Vi is the membrane potential of neuron i. Iext,i = Acos(ωt) is the weak signal applied to neuron i. We apply the weak signal to each neuron. Isyn,i is the synaptic current received by neuron i. Neurons are coupled to each other by excitatory or inhibitory chemical synapses, so Isyn,i consists of the excitatory synaptic current IsynE,i and the inhibitory synaptic current IsynI,i:
gE and gI are excitatory coupling strength and inhibitory coupling strength, respectively. For excitatory coupling, the synaptic reversal potential is set to VrevE = 0 mV; for inhibitory coupling, the synaptic reversal potential is set to VrevI = − 80 mV. Aij denotes the connection matrix. If neuron i is connected to neuron j, then Aij = 1, otherwise Aij = 0. Other parameters are set as λ = 30, θs = 0. In the network, we randomly and uniformly assign distinct initial conditions to each neuron from a predefined region in the four-dimensional state space (V, n, m, h).
To describe the transmission of weak signals in the network, the average Fourier coefficient \(\left\langle Q \right\rangle\) is calculated as the average of each value of Qi as follow:
where
the number of the period n = 500 is set in the numerical simulation of the network. The average energy consumption \(\left\langle {E_{c} } \right\rangle\) of the neurons in the network is the average of the energy consumption Eci of each neuron as follow:
The average energy efficiency \(\left\langle \eta \right\rangle\) in a network is defined as the ratio of \(\left\langle Q \right\rangle\) to \(\left\langle {E_{c} } \right\rangle\) as follows:
We measure the mean firing rate \(\left\langle r \right\rangle\) of neurons in the network:
where nspike is the number of spikes occurring in time length T.
In order to systematically measure the coherence and degree of regularity of neuronal firing, a statistic is introduced, the coefficient of variation CV [54],
where
where ISIi represents the inter-spike interval of each neuron and M is the number of ISI. The lower the value of CV, the more regular the firing of neurons.
In addition, a synchronization factor SF that can measure the collective synchronous dynamical behavior of neurons in the network is introduced with the following expression [55]:
the average symbol in this equation represents the average of physical quantities over time, and SF close to 1 means the network is completely synchronized.
3 Numerical results and discussion
3.1 Single neuron response to the weak signal and energy consumption
The effect of ion channel noise on the response of a single neuron to the weak signal is investigated in Fig. 3a. It is found that Q exhibits a bell-shaped dependence on with S, there is an optimal S to enable the best response to the weak signal. Furthermore, the temporal evolution of the membrane potential of a neuron and the corresponding energy consumption rate are plotted in Fig. 3b-d for different S. To facilitate comparison, the weak signal is marked with the red line. When S = 1 µm2 (Fig. 3b), the noise intensity is large enough to induce the neuron to firing in the absence of weak signal. It can lead to a destruction of the correlation between the neuronal firing activity and the weak signal. In Fig. 3c, a reduction in noise intensity results in the neuron’s optimal response to the weak signal, at which the weak signal is enhanced. As S increases further (Fig. 3d), the neuron still responds to the weak signal, but the response becomes worse because the noise is reduced and the neuron firing rate decreases. Furthermore, it can be observed that the ion pump energy consumption rate changes with the neuronal action potential, and its peak and oscillation frequency strictly relate to neuronal firing.
a The dependence of Q on S. Temporal evolution of membrane potential and ion pump energy consumption at different S: b S = 1 µm2; c S = 10 µm2; d S = 100 µm2. A part of the time series is shown for clear observation. There exists an optimal S such that the neuron has the best response to the weak signal. Fixed parameter: I0 = 0 µA/cm2
To more clearly illustrate the response of a neuron to the weak signal in Fig. 3, the inter-spike interval histogram (ISIH) is further calculated for 1000 ISIs, as in Fig. 4. In this paper, the angular frequency of the weak signal added is ω = 0.3 rad/ms, so its period is about 21 ms. It is marked by a black dashed line at ISI = 21 ms in the figure. When S is small (Fig. 4a), there is a sharp peak in ISIH. However, the frequency corresponding to the sharp peak is not coherent with the frequency of the weak signal, so the Q value is small at a large noise intensity. For S = 10 µm2 in Fig. 4b, the appropriate noise intensity causes the peak of ISIH to appear at the period of the weak signal. Because the time scale of the intrinsic oscillations caused by the weak signal matches the time scale of the noise-influenced neuronal dynamics [56], and therefore a better Q value can be obtained. As S increases further, ISIH exhibits a sharp peak at the weak signal period and some small sharp peaks at the harmonics of the period. The peak at S = 100 µm2 is smaller than that at S = 10 µm2, so the Q value is relatively reduced. It is because the noise intensity is diminished and the neurons sometimes do not firing even with the addition of the weak signal.
The dependence of Q and Ec on the S at different I0 are investigated in Fig. 5. As shown in Fig. 5a, it can be seen that the ion channel noise-induced SR phenomenon is present in neurons at different I0. As I0 increases, the optimal S gradually increases. In other words, the larger I0, the smaller the noise intensity is required for the neuron to obtain the best response to the weak signal. Furthermore, the peak of Q has a nonlinear effect with increasing I0, i.e., increasing first and then decreasing. In Fig. 5b, Ec decreases and finally plateaus with increasing S. The reason is that the higher the noise, the higher the firing rate of the neuron and thus the more energy is consumed. The larger I0 leads to an increase in energy consumption when subjected to the same level of noise [34, 41]. Thus the efficiency is shown in Fig. 5c, and it can be seen that noise maximizes the energy efficiency of the neuron. Comparing with Fig. 5a, the neuron still keeps the weak signal response high enough under the noise that maximizes energy efficiency. Moreover, the maximum value of Q has a nonlinear effect, while the maximum value of η is monotonic. The SR phenomenon is robust for different I0, and the best signal transmission efficiency is obtained at I0 = 0 µA/cm2. Therefore, in the following investigation, we set the bias current to I0 = 0 µA/cm2.
3.2 Excitatory–inhibitory cortical neuronal network transmission of weak signals and energy consumption
We have studied SR and its energy consumption of a single neuron in Sect. 3.1, but the human brain consists of hundreds of billions of neurons [56]. Therefore, further research the signal transmission and its energy consumption in the network level is essential. The small-world network model has been widely used to study SR [20, 57, 58] and cortical connections have been experimentally observed to have small-world properties [50,51,52]. The network schematic is shown in Fig. 6. Therefore, we constructed an excitatory-inhibitory cortical neuronal network based on the small-world network model.
First, the effects of excitatory coupling strength gE and inhibitory coupling strength gI in the network are investigated. In Fig. 7a, the dependence of \(\left\langle Q \right\rangle\) on gE is calculated for different gI. The increase of gE results in the SR phenomenon, where the existence of an optimal gE allows the best transmission of the weak signal, and the increase of gI decreases the peak of \(\left\langle Q \right\rangle\) The \(\left\langle {E_{c} } \right\rangle\) initially rises as gE increases and stabilizes at a relatively constant level once it reaches the optimal gE value. This is because a larger gE leads to an increased firing rate of neurons, which in turn results in higher energy consumption. Additionally, when the energy efficiency is maximized, it corresponds to a better signal transmission performance.
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on gE for different gI in the excitatory-inhibitory cortical neuronal network. \(\left\langle Q \right\rangle\) has a bell-shaped dependence on gE and when energy efficiency is maximized, the network corresponds to a better signal transmission performance. Fixed parameter: S = 30 µm2
The effects of gE and gI are further analyzed globally, as shown in Fig. 8. It can be seen that the increase of gE can induce the SR phenomenon, but gI cannot, which can only make \(\left\langle Q \right\rangle\) gradually decrease. Furthermore, when gI is small, the results are consistent with those shown in Fig. 7. However, there are double resonant peaks in \(\left\langle Q \right\rangle\) with increasing gE when gI increases to a certain level. The balance between excitatory and inhibitory synaptic currents induces an energy-efficient mode in the cortical network [59,60,61,62,63,64,65].
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on gE and gI in the excitatory-inhibitory cortical neuronal network. The figure shows that gE induces the SR phenomenon while gI cannot, and \(\left\langle Q \right\rangle\) shows double resonant peaks at the larger gI. Fixed parameter: S = 30 µm2
A comprehensive explanation is provided in Fig. 9. The results are obtained by averaging over 20 independent runs, where each run has different initial values and random noise, but the network topology remains the same. The robustness of the results to the network topology is demonstrated in Sect. 3.3.
a The dependence of \(\left\langle Q \right\rangle\) on gE in the excitatory-inhibitory cortical neuronal network. b The dependence of \(\left\langle r \right\rangle\) multiplied by 2π on gE. b1-b4 The PDH of r (ω = 2πr) of 60 neurons in the network at gE = 0.074 mV/cm2, gE = 0.144 mV/cm2, gE = 0.34 mV/cm2, and gE = 1.174 mV/cm2. a1-a4 correspond to (b1)-(b4). The ω is divided equally into 20 parts. c The dependence of CV on gE. d The dependence of SF on gE. The figure shows that the network has better signal transmission performance only when the firing period of most neurons matches the period of the weak signal. Fixed parameter: S = 30 µm2, gI = 2.0 mV/cm2
When gI = 2.0 mV/cm2, there is a peak of \(\left\langle Q \right\rangle\) at small and large gE in Fig. 9a, respectively. The four points (a1)-(a4) are selected to further investigate the dynamics mechanism, corresponding to gE of 0.074 mV/cm2, 0.144 mV/cm2, 0.34 mV/cm2, and 1.174 mV/cm2, respectively. To better correspond with the angular frequency of the signal, the \(\left\langle r \right\rangle\) in the vertical axis in Fig. 9b is multiplied by 2π to convert it into mean angular frequency \(\left\langle \omega \right\rangle\). Moreover, the probability distribution histogram (PDH) of the firing rate of 60 neurons in the network at the corresponding point is plotted respectively in Fig. 9b1-b4. We infer that the network should have the best signal transmission when \(\left\langle \omega \right\rangle\) = 0.3 rad/ms. In contrast to our expectations, point (a1) corresponds to the first peak of \(\left\langle Q \right\rangle\), but with \(\left\langle \omega \right\rangle\) < 0.3 rad/ms; the \(\left\langle Q \right\rangle\) at point (a2) is smaller than (a1), while \(\left\langle \omega \right\rangle\) = 0.3 rad/ms. As in Fig. 9b1, most neurons have ω = 0.3 rad/ms so \(\left\langle Q \right\rangle\) is high, but the ω of each neuron after overall averaging has \(\left\langle \omega \right\rangle\) < 0.3 rad/ms. However, for Fig. 9b2, most neurons have ω > 0.3 rad/ms so \(\left\langle Q \right\rangle\) is low and \(\left\langle \omega \right\rangle\) = 0.3 rad/ms. The ω of the neuron deviates further by 0.3 rad/ms in Fig. 9b3, resulting in a decrease of \(\left\langle Q \right\rangle\) to a minimum and \(\left\langle \omega \right\rangle\) reaches a maximum. It is not until ω returns to 0.3 rad/ms for essentially all neurons \(\left\langle Q \right\rangle\) reaches its peak again in Fig. 9b4. Therefore, the network has better signal transmission performance only if the firing period of most neurons matches the period of the weak signal.
Such a phenomenon is mainly related to the different mechanisms of action of excitatory and inhibitory synaptic currents on the network. The presence of strong inhibitory synaptic currents (large gI) leads to increased disorder in each neuron within the network [66], resulting in a higher CV (Fig. 9c). Additionally, it also leads to reduced synchrony between neurons, indicated by a smaller SF (Fig. 9d). This collective asynchrony introduces the influence of synaptic noise [66, 67], resulting in worse signal transmission within the network, \(\left\langle Q \right\rangle\) decreasing. The effect of excitatory synaptic currents is indeed opposite, they enhance the firing regularity and synchrony of the neuronal network [41]. When there is a balance between the excitatory and inhibitory components in the network, the firing of neurons becomes more orderly, resulting in a decrease in CV and an increase in SF. This enhances the signal transmission in the network. Our results indicate that the signal transmission performance in the cortical network strongly relies on the balance between excitatory and inhibitory synaptic currents.
In addition, the weak signal transmission of the network and \(\left\langle {E_{c} } \right\rangle\) under the influence of ion channel noise at different gE are plotted in Fig. 10. Regardless of the strength of gE, ion channel noise induces the SR phenomenon in the network, and the optimal weak signal transmission can be obtained at moderate noise levels. It is just that at small gE, the network has a poor transmission for weak signals and the corresponding \(\left\langle {E_{c} } \right\rangle\) is small. Within the S range where \(\left\langle Q \right\rangle\) exhibits better values, a plateau in \(\left\langle {E_{c} } \right\rangle\) is observed, indicating a level of moderate energy consumption. These results suggest that information transmission in cortical networks also depends on the noise intensity and that better signal transmission is obtained at lower energy consumption.
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on for different gE in the excitatory-inhibitory cortical neuronal network. It can be seen that better \(\left\langle Q \right\rangle\) can be obtained at moderate noise levels and better signal transmission can be obtained at lower energy consumption. Fixed parameter: gI = 0.5 mV/cm2
The global contour plots of gE and S are shown in Fig. 11. There is an island (red-shaded region) in Fig. 11a, and the presence of optimal gE and S in this region allows the network to best transmit weak signals. Therefore, when gI is small, the effect of gE on weak signal transmission is similar to that of ion channel noise. In Fig. 11b, it can be seen that the region with optimal \(\left\langle Q \right\rangle\) corresponds to a lower \(\left\langle {E_{c} } \right\rangle\), which indicates the economy of signal transmission. The region with the best \(\left\langle \eta \right\rangle\) in Fig. 11c is essentially consistent with the region with the optimal \(\left\langle Q \right\rangle\) in Fig. 11a, which illustrates the efficiency of signal transmission.
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on gE and S in the excitatory-inhibitory cortical neuronal network. It can be seen that an island (red-shaded region) exists in a, which indicates high signal transmission performance. Furthermore, it corresponds to a moderate \(\left\langle {E_{c} } \right\rangle\) region (yellow region) and a high \(\left\langle \eta \right\rangle\) region (red-shaded region). Fixed parameter: gI = 0.5 mV/cm2
The effect of ion channel noise at different gI is also investigated, as shown in Fig. 12. Moreover, contour plots of gI and S are shown in Fig. 13. It can be found that the SR phenomenon also occurs at different gI, but the increase of gI can gradually inhibit the transmission of the network to weak signals. Similarly, the network transmits optimally to weak signals at moderate noise intensity. The region in Fig. 13a where an optimal signal transmission (red-shaded region) corresponds to the moderate \(\left\langle {E_{c} } \right\rangle\) (yellow region) in Fig. 13b. In addition, it also corresponds to the region in Fig. 13c where the \(\left\langle \eta \right\rangle\) is highest (red-shaded region). It demonstrates that the economy and efficiency of signal transmission still exist. Finally, the network can improve the transmission of weak signals compared to that of a single neuron for the weak signal.
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on S for different gI in the excitatory-inhibitory cortical neuronal network. It can be seen that S can also induce SR phenomenon for different gI and better signal transmission can be obtained at lower energy consumption. Fixed parameter: gE = 0.5 mV/cm2
a-c The dependence of \(\left\langle Q \right\rangle\), \(\left\langle {E_{c} } \right\rangle\), and \(\left\langle \eta \right\rangle\) on gI and S in the excitatory-inhibitory cortical neuronal network. It can be seen that the high \(\left\langle Q \right\rangle\) region (red-shaded region) corresponds to the moderate \(\left\langle {E_{c} } \right\rangle\) region (yellow region) and the high \(\left\langle \eta \right\rangle\) region (red-shaded region). Fixed parameter: gE = 0.5 mV/cm2
3.3 Robustness of network topology on results
In this section, to demonstrate that our results do not rely on a certain fixed network connectivity matrix, on fixed noise and initial value settings, or even on the characteristic parameters of network topology. The results of all our calculations in this section are obtained by averaging 20 independent runs, each of which changes the initial value random number, the noise random number, and the network connectivity matrix. The error bars for each curve are also plotted.
In Fig. 14, it is found that changing the connection matrix can still reproduce the previously obtained phenomena. It indicates that the phenomena and analysis we obtained are robust and not characteristic of a particular network connectivity matrix. In Figs. 15 and 16, the robustness of N, K, and P on the results are investigated for weak gI = 0.1 mV/cm2 and strong gI = 2.0 mV/cm2, respectively. In Fig. 15, it is found that changes in N and P have almost no effect on the curve of \(\left\langle Q \right\rangle\) with gE, and only the increase in K causes a decrease in gE corresponding to the peak of the curve. This is because K affects the synaptic currents [41], and the excitatory and inhibitory synaptic currents in the network to maintain balance, so the gE corresponding to the peak of \(\left\langle Q \right\rangle\) changes. In general, however, changes in these three parameters still result in similar phenomena, which indicates that our phenomena are robust. In Fig. 16 it is shown that \(\left\langle Q \right\rangle\) still appears double resonant peaks with increasing gE by changing N, K, and P, respectively. It means that the bimodal phenomenon is not accidental but robust. Furthermore, when N and K are fixed, the bimodal phenomenon gradually decreases by increasing P.
Robustness of the phenomenon. a The dependence of \(\left\langle Q \right\rangle\) on gE for different gI in the excitatory-inhibitory cortical neuronal network (S = 30 µm2). b The dependence of \(\left\langle Q \right\rangle\) on S for different gE (gI = 0.5 mV/cm2). c The dependence of \(\left\langle Q \right\rangle\) on S for different gI (gE = 0.5 mV/cm2). The figure shows that by changing the connectivity matrix, the phenomena and results obtained are robust
a The dependence of \(\left\langle Q \right\rangle\) on gE for different N in the excitatory-inhibitory cortical neuronal network (K = 12, P = 0.4). b The dependence of \(\left\langle Q \right\rangle\) on gE for different K of each node (N = 60, P = 0.4). c The dependence of \(\left\langle Q \right\rangle\) on gE for different P (N = 60, K = 12). At weak gI, the phenomena and results obtained are robust to different characterization parameters of the network topology. Fixed parameter: gI = 0.1 mV/cm2
a The dependence of \(\left\langle Q \right\rangle\) on gE for different N in the excitatory-inhibitory cortical neuronal network (K = 12, P = 0.4). b The dependence of \(\left\langle Q \right\rangle\) on gE for different K of each node (N = 60, P = 0.4). c The dependence of \(\left\langle Q \right\rangle\) on gE for different P (N = 60, K = 12). At strong gI, the double resonance peak phenomenon is robust to the characteristic parameters of different network topologies. Fixed parameter: gI = 2.0 mV/cm2
4 Conclusions
Previous studies of SR have mostly focused on excitatory neuronal networks [11, 12, 58, 68, 69]. However, there are fewer studies of SR based on excitatory-inhibitory cortical neuronal networks. For example, Yu et al. showed that multiple input signals are transmitted independently through a feedforward neural network via SR, and that the E-I relation of local cortical circuits alters the transmission of information between adjacent cortical areas [70]. Lopes et al. investigated the response of neural networks to periodic stimuli plus noise by using a model of cortical circuits [71]. However, these studies have lacked consideration of the energy consumption of SR. Furthermore, resonance is important for the signal transmission in the neuronal system. Baysal et al. in their study of SR in single autapse-coupled neuron hypothesized that autapse can facilitate signal detection in the beta frequency band of brain rhythms [72]. They also discovered that chaotic activity can affect the information processing ability of Type-I and Type-II neurons, producing chaotic resonance [73].Therefore, in this paper, the SR phenomenon and its energy consumption in the single H–H neuron and excitatory-inhibitory cortical neuronal network are investigated.
It is found that moderate noise levels result in the optimal transmission to weak signals. Also, the network can relatively improve signal transmission performance compared to the single neuron. It can be found that changing the excitatory coupling strength can induce the SR phenomenon at weak inhibitory coupling strength. However, at strong inhibitory coupling strength, there is a double resonance peak phenomenon. This is because there is a balance between excitatory and inhibitory synaptic currents in the network. The strong inhibitory synaptic currents cause the firing of neurons in the network to become disordered and the neurons are asynchrony with each other. It leads to synaptic noise effects that cause worse signal transmission performance. The signal transmission is recovered until the excitatory synaptic currents increase to the level where the effect of strong inhibitory synaptic currents can be inhibited. In addition, it is shown that a high level of signal transmission is obtained with a low energy consumption, which shows the economy and efficiency of the signal transmission. It provides some theoretical basis for further understanding of the dependence of information processing and energy consumption in the brain. Eventually, we demonstrate the robustness of our results by changing the network topology.
In this paper, we have extended SR and its energy consumption to the excitatory-inhibitory cortical network for study, but the number of neurons in the numerical simulation is still relatively small compared to the real cerebral cortex. Therefore, in future work, the SR phenomenon and its energy consumption can continue to be studied in a large-scale network model of the cerebral cortex [74] or in real neuronal models like thermosensitive neurons [10], photosensitive neurons [75, 76], piezoelectric neuron [77], so that the work is more biologically justified.
Data availability
The manuscript has no associated data.
References
Collins, J.J., Imhoff, T.T., Grigg, P.: Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J. Neurophysiol. 76(1), 642–645 (1996)
Benzi, R., Parisi, G., Sutera, A., Vulpiani, A.: Stochastic resonance in climatic change. Tellu 34(1), 10–16 (1982)
Yu, D., Zhou, X.Y., Wang, G.W., Ding, Q.M., Li, T.Y., Jia, Y.: Effects of chaotic activity and time delay on signal transmission in FitzHugh-Nagumo neuronal system. Cogn. Neurodyn. 16(4), 887–897 (2021)
Yao, Y.G., Yang, L.J., Wang, C.J., Liu, Q., Gui, R., Xiong, J., Yi, M.: Subthreshold periodic signal detection by bounded noise-induced resonance in the FitzHugh-Nagumo neuron. Complexity 5632650 (2018)
Xiao, F.L., Fu, Z.Y., Jia, Y., Yang, L.J.: Resonance effects in neuronal-astrocyte model with ion channel blockage. Chaos Soliton Fract. 166, 112969 (2023)
Douglass, J.K., Wilkens, L., Pantazelou, E., Moss, F.: Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365, 337–340 (1993)
Levin, J.E., Miller, J.P.: Broadband neural encoding in the cricket cereal sensory system enhanced by stochastic resonance. Nature 380, 165–168 (1996)
Cordo, P., Inglis, J., Verschueren, S., Collins, J.J., Merfeld, D.M., Rosenblum, S., Buckley, S., Moss, F.: Noise in human muscle spindles. Nature 383, 769–770 (1996)
Yilmaz, E., Ozer, M.: Delayed feedback and detection of weak periodic signals in a stochastic Hodgkin-Huxley neuron. Physica A 421, 455–462 (2015)
Xu, Y., Guo, Y.Y., Ren, G.D., Ma, J.: Dynamics and stochastic resonance in a thermosensitive neuron. Appl. Math. Comput. 385, 125427 (2020)
Yu, D., Wang, G.W., Ding, Q.M., Li, T.Y., Jia, Y.: Effects of bounded noise and time delay on signal transmission in excitable neural networks. Chaos Soliton Fract. 157, 111929 (2022)
Yilmaz, E., Uzuntarla, M., Ozer, M., Perc, M.: Stochastic resonance in hybrid scale-free neuronal networks. Physica A 392(22), 5735–5741 (2013)
Palabas, T., Torres, J.J., Perc, M., Uzuntarla, M.: Double stochastic resonance in neuronal dynamics due to astrocytes. Chaos Soliton Fract. 168, 113140 (2023)
Jin, Y.F., Ma, Z.M., Xiao, S.M.: Coherence and stochastic resonance in a periodic potential driven by multiplicative dichotomous and additive white noise. Chaos Soliton Fract. 103, 470–475 (2017)
Xu, B.H., Li, J.L., Duan, F.B., Zheng, J.Y.: Effects of colored noise on multi-frequency signal processing via stochastic resonance with tuning system parameters. Chaos Soliton Fract. 16(1), 93–106 (2003)
Nozaki, D., Mar, D.J., Grigg, P., Collins, J.J.: Effects of colored noise on stochastic resonance in sensory neurons. Phys. Rev. Lett. 82(11), 2402–2405 (1999)
Nozaki, D., Yamamoto, Y.: Enhancement of stochastic resonance in a FitzHugh-Nagumo neuronal model driven by colored noise. Phys. Lett. A 243(5–6), 281–287 (1998)
Yu, H.T., Galán, R.F., Wang, J., Cao, Y.B., Liu, J.: Stochastic resonance, coherence resonance, and spike timing reliability of Hodgkin-Huxley neurons with ion-channel noise. Physica A 471, 263–275 (2017)
White, J.A., Rubinstein, J.T., Kay, A.R.: Channel noise in neurons. Trends Neurosci. 23(3), 131–137 (2000)
Ozer, M., Perc, M., Uzuntarla, M.: Stochastic resonance on Newman-Watts networks of Hodgkin-Huxley neurons with local periodic driving. Phys. Lett. A 373(10), 964–968 (2009)
Ozer, M., Perc, M., Uzuntarla, M., Koklukaya, E.: Weak signal propagation through noisy feedforward neuronal networks. NeuroReport 21(5), 338–343 (2010)
Yilmaz, E., Ozer, M.: Collective firing regularity of a scale-free Hodgkin-Huxley neuronal network in response to a subthreshold signal. Phys. Lett. A 377(18), 1301–1307 (2013)
Funke, K., Kerscher, N.J., Worgotter, F.: Noise-improved signal detection in cat primary visual cortex via a well-balanced stochastic resonance-like procedure. Eur. J. Neurosci. 26(5), 1322–1332 (2007)
Van der Groen, O., Wenderoth, N.: Transcranial Random Noise stimulation of visual cortex: stochastic resonance enhances central mechanisms of perception. J. Neurosci. 36(19), 5289–5298 (2016)
Udakis, M., Pedrosa, V., Chamberlain, S.E.L., Clopath, C., Mellor, J.R.: Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat. Commun. 11(1), 4395 (2020)
del Molino, L.C.G., Yang, G.R., Mejias, J.F., Wang, X.J.: Paradoxical response reversal of top-down modulation in cortical circuits with three interneuron types. Elife 6, e29742 (2017)
Meinecke, D.L., Peters, A.: GABA immunoreactive neurons in rat visual cortex. J. Comp. Neurol. 261(3), 388–404 (1987)
Yu, H.T., Li, K., Guo, X., Wang, J., Deng, B., Liu, C.: Firing rate oscillation and stochastic resonance in cortical networks with electrical-chemical synapses and time delay. IEEE Trans. Fuzzy Syst. 28(1), 5–13 (2020)
Clarke, D.D., Sokoloff, L.: Basic neurochemistry: molecular, cellular and medical aspects. In Siegel, G.J., et al. (eds.), pp. 637–669. Lippincott-Raven, Philadelphia (1999)
Torrealdea, F.J., d’Anjou, A., Graña, M., Sarasola, C.: Energy aspects of the synchronization of model neurons. Phys. Rev. E 74(1), 011905 (2006)
Yu, L.C., Yu, Y.G.: Energy-efficient neural information processing in individual neurons and neuronal networks. J. Neurosci. Res. 95(11), 2253–2266 (2017)
Sengupta, B., Faisal, A.A., Laughlin, S.B., Niven, J.E.: The effect of cell size and channel density on neuronal information encoding and energy efficiency. J. Cereb. Blood Flow Metab. 33(9), 1465–1473 (2013)
Wang, Y.H., Wang, R.B., Xu, X.Y.: Neural energy supply-consumption properties based on Hodgkin-Huxley Model. Neural Plast. 2017, 6207141 (2017)
Moujahid, A., d’anjou, A., Torrealdea, F.J., Torrealdea, F.: Energy and information in Hodgkin-Huxley neurons. Phys. Rev. E 83(3), 031912 (2011)
Wang, Y.H., Xu, X.Y., Zhu, Y.T., Wang, R.B.: Neural energy mechanism and neurodynamics of memory transformation. Nonlinear Dyn. 97(1), 697–714 (2019)
Wang, Y.H., Xu, X.Y., Wang, R.B.: The place cell activity is information-efficient constrained by energy. Neural Netw. 116, 110–118 (2019)
Jia, J.E., Yang, F.F., Ma, J.: A bimembrane neuron for computational neuroscience. Chaos Soliton Fract. 173, 113689 (2023)
Ju, H.W., Hines, M.L., Yu, Y.G.: Cable energy function of cortical axons. Sci. Rep. 6, 29686 (2016)
Liu, Y.J., Yue, Y., Yu, Y.G., Liu, L.W., Yu, L.C.: Effects of channel blocking on information transmission and energy efficiency in squid giant axons. J. Comput. Neurosci. 44(2), 219–231 (2018)
Yu, D., Zhan, X., Yang, L.J., Jia, Y.: Theoretical description of logical stochastic resonance and its enhancement: fast Fourier transform filtering method. Phys. Rev. E 108(1), 014205 (2023)
Yu, D., Yang, L.J., Zhan, X., Fu, Z.Y., Jia, Y.: Logical stochastic resonance and energy consumption in stochastic Hodgkin-Huxley neuron system. Nonlinear Dyn. 111(7), 6757–6772 (2023)
Hodgkin, A.L., Huxley, A.F.: The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 116(4), 497–506 (1952)
Fox, R.F.: Stochastic versions of the Hodgkin-Huxley equations. Biophys. J. 72(5), 2068–2074 (1997)
Yu, D., Wang, G., Li, T., Ding, Q.M., Jia, Y.: Filtering properties of Hodgkin-Huxley neuron to different time-scale signals. Commun Nonlinear Sci. 117, 106894 (2023)
Li, T.Y., Wu, Y., Yang, L.J., Fu, Z.Y., Jia, Y.: Neuronal morphology and network properties modulate signal propagation in multi-layer feedforward network. Chaos Soliton Fract. 172, 113554 (2023)
Deng, B., Wang, J., Wei, X.L., Tsang, K.M., Chan, W.L.: Vibrational resonance in neuron populations. Chaos 20(1), 013113 (2010)
Volkov, E.I., Ullner, E., Zaikin, A.A., J, Kurths, J.: Frequency-dependent stochastic resonance in inhibitory coupled excitable systems. Phys. Rev. E 68, 061112 (2003)
Lu, L.L., Yi, M., Gao, Z.H., Zhao, X.: Critical state of energy-efficient firing patterns with different bursting kinetics in temperature-sensitive Chay neuron. Nonlinear Dyn. 111(17), 16557–16567 (2023)
Ding, Q.M., Wu, Y., Li, T., Yu, D., Jia, Y.: Metabolic energy consumption and information transmission of a two-compartment neuron model and its cortical network. Chaos Soliton Fract. 171, 113464 (2023)
Song, S., Sjöström, P.J., Reigl, M., Nelson, S., Chklovskii, D.B.: Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3(10), 1838–1838 (2005)
Humphries, M.D., Gurney, K., Prescott, T.J.: The brainstem reticular formation is a small-world, not scale-free, network. Proc. Biol. Sci. 273(1585), 503–511 (2006)
Iturria-Medina, Y., Sotero, R.C., Canales-Rodriguez, E.J., Aleman-Gomez, Y., Melie-Garcia, L.: Studying the human brain anatomical network via diffusion-weighted MRI and graph theory. Neuroimage 40(3), 1064–1076 (2008)
Watts, D.J., Strogatz, S.H.: Collective dynamics of ‘small-world’ networks. Nature 393(6684), 440–442 (1998)
Schmid, G., Goychuk, I., Hanggi, P.: Stochastic resonance as a collective property of ion channel assemblies. Europhys. Lett. 56(1), 22–28 (2001)
Wu, Y., Ding, Q., Li, T.Y., Yu, D., Jia, Y.: Effect of temperature on synchronization of scale-free neuronal network. Nonlinear Dyn. 111(3), 2693–2710 (2023)
Baysal, V., Sarac, Z., Yilmaz, E.: Chaotic resonance in Hodgkin-Huxley neuron. Nonlinear Dyn. 97(2), 1275–1285 (2019)
Yu, D., Wu, Y., Yang, L.J., Zhao, Y.J., Jia, Y.: Effect of topology on delay-induced multiple resonances in locally driven systems. Physica A 609, 128330 (2023)
Yu, H.T., Guo, X.M., Wang, J.: Stochastic resonance enhancement of small-world neural networks by hybrid synapses and time delay. Commun. Nonlinear Sci. Numer. Simul. 42, 532–544 (2017)
Shew, W.L., Yang, H., Yu, S., Roy, R., Plenz, D.: Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J. Neurosci. 31(1), 55–63 (2011)
Sengupta, B., Laughlin, S.B., Niven, J.E.: Balanced excitatory and inhibitory synaptic currents promote efficient coding and metabolic efficiency. PLoS Comput. Biol. 9(10), e1003263 (2013)
Mariño, J., Schummers, J., Lyon, D.C., Schwabe, L., Beck, O., Wiesing, P., Obermayer, K., Sur, M.: Invariant computations in local cortical networks with balanced excitation and inhibition. Nat. Neurosci. 8(2), 194–201 (2005)
Dorrn, A.L., Yuan, K., Barker, A.J., Schreiner, C.E., Froemke, R.C.: Developmental sensory experience balances cortical excitation and inhibition. Nature 465(7300), 932–936 (2010)
Litwin-Kumar, A., Doiron, B.: Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat. Neurosci. 15(11), 1498–1505 (2012)
Landau, I.D., Egger, R., Dercksen, V.J., Oberlaender, M., Sompolinsky, H.: The impact of structural heterogeneity on excitation-inhibition balance in cortical networks. Neuron 92(5), 1106–1121 (2016)
Lu, L.L., Gao, Z.H., Wei, Z., Yi, M.: Working memory depends on the excitatory-inhibitory balance in neuron-astrocyte network. Chaos 33(1), 013127 (2023)
Yu, D., Wu, Y., Ye, Z.Q., Xiao, F.L., Jia, Y.: Inverse chaotic resonance in Hodgkin-Huxley neuronal system. Eur. Phys. J. Spec. Top 231(22–23), 4097–4107 (2022)
Uzuntarla, M., Barreto, E., Torres, J.J.: Inverse stochastic resonance in networks of spiking neurons. PLoS Comput. Biol. 13(7), e1005646 (2017)
Perc, M.: Stochastic resonance on excitable small-world networks via a pacemaker. Phys. Rev. E 76(6), 066203 (2007)
Zhao, J., Qin, Y., Che, Y.Q., Ran, H.Y.Q., Li, J.W.: Effects of network topologies on stochastic resonance in feedforward neural network. Cogn. Neurodyn. 14(3), 399–409 (2020)
Yu, H.T., Li, K., Guo, X.M., Wang, J.: Resonance transmission of multiple independent signals in cortical networks. Neurocomputing 377, 130–144 (2019)
Lopes, M.A., Goltsev, A.V., Lee, K.-E., Mendes, J.F.F.: Stochastic resonance as an emergent property of neural networks. AIP Conf. Proc. 1510, 202–206 (2013)
Baysal, V., Calim, A.: Stochastic resonance in a single autapse-coupled neuron. Chaos Soliton Fract. 175, 114059 (2023)
Baysal, V., Solmaz, R., Ma, J.: Investigation of chaotic resonance in Type-I and Type-II Morris-Lecar neurons. Appl. Math. Comput. 448, 127940 (2023)
Mejias, J.F., Murray, J.D., Kennedy, H., Wang, X.J.: Feedforward and feedback frequency-dependent interactions in a large-scale laminar network of the primate cortex. Sci. Adv. 2(11), e1601335 (2016)
Yang, F.F., Ma, J.: A controllable photosensitive neuron model and its application. Opt. Laser Technol. 163, 109335 (2023)
Liu, Y., Xu, W., Ma, J., Alzahrani, F., Hobiny, A.: A new photosensitive neuron model and its dynamics. Front Inform Tech El. 21(9), 1387–1396 (2020)
Guo, Y.T., Zhou, P., Yao, Z., Ma, J.: Biophysical mechanism of signal encoding in an auditory neuron. Nonlinear Dyn. 105(4), 3603–3614 (2021)
Acknowledgements
This work is supported by National Natural Science Foundation of China under Grant 12175080, and also supported by the Fundamental Research Funds for the Central Universities under CCNU22JC009.
Funding
This research was funded by National Natural Science Foundation of China, (Grant no: 12175080).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Yu, D., Li, T. et al. Signal transmission and energy consumption in excitatory–inhibitory cortical neuronal network. Nonlinear Dyn 112, 2933–2948 (2024). https://doi.org/10.1007/s11071-023-09181-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-09181-4