Abstract
Alpha2-adrenoreceptor (α2-AR) is a noradrenergic receptor that is frequently studied for modulation of seizure activity. However, the precise role of this receptor agonists in regulating seizure activity is still unclear. Our aim in this study was to investigate the effects of α2-AR agonist dexmedetomidine (DEX) and atipamezole (α2-AR antagonist, ATI) on seziures in rats. In the study, 32 adult male Wistar Albino rats (weighing 220–260 g) were used. To induce seizures in rats, pentylenetetrazole (PTZ, 35 mg/kg) was injected intraperitoneally (i.p.) and seizure stages were determined according to the Racine scale. After induction of seizures, DEX (0.1 mg/kg, i.p.) and ATI (1 mg/kg, i.p.) were administered to rats and their effects determined on seizures. GABA levels of the brain hippocampal tissue sample were measured using an ELISA kit and c-Fos positive cells of the dentate gyrus and hippocampal regions were quantitatively analyzed with Image J software. The results showed that DEX decreased the seizure stages according to the Racine scale, significantly prolonged the onset time of first myoclonic jerk (FMJ) and reduced the number of spikes and percentage seizure duration (p < 0.05). In contrast, ATI increased the seizure stage, the number of spikes and percentage seizure duration. The hippocampal GABA level was significantly decreased in rats with only PTZ injection (p < 0.05). In addition, DEX reduced the number of c-Fos positive cells in dentate gyrus and the hippocampal CA1 and CA3 regions. In conclusion, our findings showed that α2-AR agonist DEX had a reducing activity on PTZ-induced seizure, while α2-AR antagonist ATI facilitated seizure formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a common neurological disorder that is characterized by recurrent seizures and affects more than 75 million people worldwide [1, 2]. Seizures indicate a disorder in the neuronal excitatory system as well as inhibitory systems that prevent hyperexcitability [3, 4]. The etiology of epileptic disorders is complex and can be of genetic, developmental or acquired origin. Although specific causes vary, ionotropic and metabotropic receptors of glutamate appear to be an important factor in the activity of some seizure types [4,5,6]. Seizures often are terminated with depletion of ions, energy substrates and neurotransmitters necessary for seizure activity or activation of endogenous inhibitory mechanisms. Therefore, investigating the mechanisms of epilepsy is a promising way to develop drugs for the treatment of epilepsy [7].
There is numerous clinical and experimental evidence suggesting that monoamines play an important role in the regulation of epileptogenesis and convulsions [8]. It has been reported that the noradrenergic system is a significant regulator in reducing epileptic seizures [2]. The alpha-2 adrenoceptor (α2-AR) is frequently investigated for its regulation of seizure activity. However, due to the complexity of multiple α2-AR subtypes and their differential distribution, the role of this receptor on seizure activity is not completely understood [9]. The complex α2-adrenoceptors consist of three different subtypes: α2A-, α2B- and α2C-adrenoceptors [10, 11]. The α2A subtype is located in the brain at a high density (about 90% central) and participates in the regulation of seizure activity [12].
α2-Adrenoceptors can differentially affect the susceptibility to different types of seizures. The α2-AR agonist clonidine showed anticonvulsant effects against seizures induced of pentylenetetrazole (PTZ) by relatively high doses (0.5 mg/kg) [13], whereas lower dose clonidine was not able to alter seizure susceptibility in this model [14]. In addition, clonidine indicated anticonvulsant effects in the amygdala kindling model in rats [15]. Likewise, yohimbine, a α2-AR antagonist, exerts anticonvulsant effects on handling-induced seizures in mice [16], and at smaller doses of yohimbine has a proconvulsant activity on audiogenic seizures in rats [17]. Dexmedetomidine (DEX), a highly specific α2-AR agonist, is a drug that is frequently used clinically for analgesia and sedation [18]. DEX has been reported to increase the convulsive threshold in rats injected with cocaine intraperitoneally (i.p.) and attenuate ropivacaine-induced seizures depending on the dose [19, 20]. However, it remains unclear whether DEX is neuroprotective in epilepsy.
The effects of the α2-adrenergic agonist DEX and antagonist atipamezole (ATI) on seizures are not yet fully understood. In the light of all this information, our purpose in this study was to investigate the effects of DEX and ATI on PTZ-induced seizure in rats.
Materials and Methods
Animals
Thirty-two adult male 8–13 weeks old Wistar Albino rats (weighing 220–260 g) were used in this study. All experiments performed were carried out according to the rules listed in the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health (USA). Permission was obtained from the Animal Ethics Committee of Cumhuriyet University before the experiment (Ethics No: 2016/95). The rats were kept in quiet rooms with 12-hlight—dark cycles (light from 07.30 to 19.30) and an ambient temperature of 22 ± 1 °C. The rats were given standard laboratory food and tap water ad libitum. Experimental studies were performed between 09.00–15.00 and the light and sound level stability of the experimental environment was provided.
Drugs
Before experimental application, dexmedetomidine (DEX, α2-AR agonist) and atipamezole (ATI, α2-AR antagonist) were dissolved in dimethyl sulfoxide (DMSO) solution. Physiological saline was used to dissolve pentylenetetrazole (PTZ). All research drugs were purchased from Sigma-Aldrich (Co., St Louis, MO).
Experimental Design
Rats were randomly divided into 4 groups (n = 7–8 for each group) for behavioral and histological examination. The chemical doses administered to rats in the groups were as follows: Control (CTRL) (saline solution 1 mg/kg, i.p.), epileptic control (PTZ), DEX (0.1 mg/kg, i.p.) and ATI (1 mg/kg, i.p.) group. Drug doses were determined based on previous studies [21,22,23]. PTZ (35 mg/kg, i.p.) was injected into the rats to induce seizures (Fig. 1). Rats with seizure stage 4 or 5 after three consecutive PTZ injections were identified as seizure-induced. The rats were placed with electrodes to obtain electrocorticography (ECoG) recordings and rested for one week recovery period. Seizure activities of the rats were also confirmed by performing control tests in the postoperative 1 week period. It was observed that the seizure activity of the fully kindling rats continued in the controls. After the experimental process was completed, all experimental animals were sacrificed by decapitation. The samples of brain tissue were removed and centrifuged after homogenization for gamma-aminobutyric acid (GABA) analysis in each sample and frozen at − 80 °C. Tissue samples reserved for histological examination were stored in neutral buffered formaldehyde.
PTZ Kindling
Kindling model epilepsy in rats was performed according to a previously described method [24]. The rats were induced seizures by administering PTZ injections 1 h before with or without drugs 15 times every other day. After the seizure induction, rat behavior was observed by taking image recordings for a period of 30 min. The seizure activity of the rats was scored using the revised Racine scale [25]: Stage 0: No response, Stage 1: Ear and facial twitching, Stage 2: Myoclonic body jerks without upright position, Stage 3: Myoclonic jerks, upright position with clonic fore limb convulsions, Stage 4: Tonic–clonic seizures, Stage 5: Generalized tonic–clonic seizures, loss of postural control. Seizure duration was measured in rats with seizure stage 4 or 5. Seizure stages 4 or 5 after three consecutive injections of PTZ in rats are defined as completely kindling [26].
Stereotaxic Surgery
The rats were anesthetized and sedated with ketamine (90 mg/kg, i.p.) and xylasine (10 mg/kg, i.p.). After the depth of anesthesia was controlled by cornea and paw reflexes, the rats were placed on the stereotaxic instrument with the bregma and lambda points in the same plane. After the head of the rat was fixed to the stereotaxic instrument, the skin and subcutaneous tissue on the bone tissue were cleaned. The locations of the screws where the electrode will be placed were calculated using the rat brain atlas of Paxinos and Watson [27] and the bregma was taken as the “0” reference point. Three different small burr holes were made with a drill (diameter 1 mm) at the points determined in the skull of the rat. A stainless steel screw was placed in these holes in contact with the brain membranes for electrocorticogram (ECoG) recording. Tripolar electrodes were placed over the motor cortex.
Electrode coordinates: First electrode 4 mm anterior to the bregma and 3 mm right lateral to the midline (frontal cortex); the second electrode 4 mm posterior to the bregma and 3 mm right lateral to the midline (occipital cortex), and the reference electrode 4 mm posterior to the bregma and 3 mm left lateral to the midline). All of the electrodes (0.12 mm diameter, Plastic One, Roanoke, VA, USA) were fixed to the skull with two layers of dental acrylic and linked by insulated wires to a connector for the ECoG recordings. After this procedure, the animals were injected with 50 mg/kg sultamicillin (i.p.) twice a day for 3 days to prevent infection. Rats were allowed to recover for a week before experimental studies were initiated.
Electrocorticography Recordings
After the recovery period, the animals were placed individually in plexiglass cages (40 × 40 × 40 cm) for 30 min for habituation. The electrocorticography (EcoG) signals of the electrodes were amplified at the BioAmp interface (AD Instruments, Castle Hill, Australia) and connected with a cable to the data acquisition unit PowerLab 4/SP (AD Instruments, Castle Hill, Australia). ECoG signals were recorded for 30 min (Fig. 2). EcoG recordings are filtered between 1 and 34 Hz to remove noise signals from the environment.
ECoG and video recordings were analyzed using PowerLab Chart (v7.0.3) program and calculated the seizure stage, onset time of first myoclonic jerk (FMJ), number of spikes per minute and percentage of seizure duration. The epileptic spike activity in EcoG simultaneously with the first limb contraction of the animal in the video recordings was interpreted as the first myoclonic jerk and its duration was recorded in seconds. Basal ECoG activity threshold of each rat was found by using PowerLab Chart software and its macro features. By introducing this threshold value to the program, the number of epileptic spikes per minute was calculated automatically. Then, seizure durations corresponding to epileptic spikes for 30 min were calculated with the help of the program and converted into percentages [(Seizure duration × 100)/1800; 1800 = seconds corresponding to 30 min].
Hippocampal GABA Assay
After the drug injections, animals were sacrificed by decapitation and their right brain tissue was removed. Extracted brain tissue was stored in a freezer (− 80 °C). Hippocampus was isolated from brain tissue. For the hippokampal tissue homogenization, 50 mM Tris–HCL (pH: 7.5) buffer was used and transferred to glass tubes under cold chain conditions. 2 ml Tris–HCL buffer was added to the tissue sample. Tissue samples were placed in eppendorf tubes and homogenized for 3 min at 28,672× g. Buffer was added at 5 times the weight of the compound. The centrifuged supernatants of each sample were used to measure the hippokampal GABA levels using GABA ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Immunohistochemical Analysis
Tissue samples of left cerebral hemispheres were fixed in 10% neutral buffered formalin for 30–36 h. Tissue samples were stored to + 4 °C after dehydration, transparency and paraffin embedding. After the blocks were kept at − 20 °C for a few hours, 4 μm thick sections were cut with a microtome (Leica RM2235, Nussloch, Germany). Each 12th section of serial sections was taken on a poly-L-lysine coated slide for evaluation, and 5 sections for each rat were evaluated for c-Fos immunoreactivity. c-Fos expression was determined by using primary antibodies and immunohistochemical staining of the sections. The sections taken on the slide were kept at 60 °C for 1.5 h and stained with hematoxylin–eosin (H&E). Dentate gyrus (DG), hippocampal CA1 and CA3 areas isolated from left brain tissue were examined at 400× magnification by light microscopy (Olympus BX5, Olympus corp. Tokyo, Japan). c-Fos protein in neurons is stained red with 3-Amino-9-ethylcarbazole (AEC), a chromogenic substrate that visualizes sections stained with secondary antibodies. c-Fos positive cells of the DG and hippocampal areas were quantitatively analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Statistical analysis of the numerical data obtained from the electrophysiological records was performed using the SPSS program (SPSS 22.0 for windows, Chicago, IL, USA). Data are expressed as mean ± SEM. Normal distribution was assessed in accordance with the Shapiro Wilk’s test. One-way analysis of variance (ANOVA), followed by Tukey's HSD multiplex comparisons, was used for the analysis of seizure parameters and immunohistochemical data that were normally distributed and satisfied the parametric test assumptions. GABA results were compared using Kruskal–Wallis nonparametric analysis of variance followed by Dunn's comparisons test. Statistical significance level was accepted as p < 0.05.
Results
After a one-week recovery period of all rats, drug injections were made and 30-min ECoG measurements were performed, respectively. The mean of the measurement data of 8 rats in the groups was used for statistical analysis.
The Effects of DEX and ATI on Seizure Stage
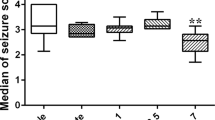
According to the Racine scale, one-way ANOVA revealed a significant effect (F2,21 = 5.25, p < 0.01) and further post hoc analysis showed that the mean (± SEM) of the DEX group (3.16 ± 0.42) significantly decreased in compared to the epileptic control PTZ group (4.83 ± 0.40) (p < 0.01; Fig. 3A). In contrast, ATI (4.82 ± 0.32) had no significant effect on seizure stage in PTZ-induced epileptic rats (p > 0.05). However, the difference between the DEX and ATI groups was statistically significant (p < 0.05).
The Effects of DEX and ATI on Onset Time of FMJ
FMJ onset time was significantly higher in the DEX group (131.3 ± 25.6) than the PTZ (53.1 ± 10.7) (F2,21 = 9.45; p < 0.01; Fig. 3B). There was no statistically significant difference between ATI (51.8 ± 9.0) and PTZ group. In addition, the onset time of FMJ was significantly reduced in the ATI group compared to DEX, and the difference between the means was statistically significant (p < 0.05).
The Effects of DEX and ATI on Spike Number
The number of epileptic spikes was significantly lower in the DEX (31.9 ± 3.9) than the PTZ group (83.7 ± 5.9) (F2,21 = 6.78; p < 0.01; Fig. 3C). On the contrary, the number of epileptic spikes increased significantly in the ATI group (120.9 ± 10.7) compared to the PTZ (p < 0.05). The difference between the DEX and ATI groups was statistically significant (p < 0.05).
The Effects of DEX and ATI on Percentage of Seizure Duration
The percentage seizure duration in the DEX (27.3 ± 2.7) group showed a significant decrease compared to the PTZ (50.0 ± 2.4) group (F2,21 = 5.83; p < 0.01; Fig. 3D). On the other hand, it was found that the percentage seizure duration significantly increased in the ATI (76.8 ± 4.4) group compared to the PTZ (p < 0.05). In addition, the difference between the mean seizure duration percentages in the DEX and ATI groups was significant (p < 0.05).
GABA Levels
The GABA level was significantly lower in PTZ group (83.3 ± 5.1) than the CTRL (122.1 ± 6.9) (p < 0.05; Fig. 4). On the other hand, the decrease in the mean GABA levels of the DEX (105.6 ± 5.9) and ATI (109.1 ± 6.1) groups were not significant compared to the CTRL (p > 0.05).
c-Fos Immunoreactivity
Light microscopic (a, b) and c-Fos immunohistochemistry (c, d, e) images of the CTRL group are displayed in section A of the figures (Figs. 5, 6, 7). In the B part of the figures, c-Fos positive cells of the drug groups are distinguished by an arrow.
Effect of drugs on c-Fos expression in the DG region. A CTRL group light microscopic image (a, X40 and b, X100) and c-fos immunohistochemistry (c, X40; d, X100 and e, X100 negatif control) image. B c-Fos immunoreactivity (X400). a, CTRL; b, PTZ; c, DEX and d, ATI. C Number of c-fos positive neurons in DG. *p < 0.01, compared to the CTRL group; **p < 0.05, compared to the PTZ group and #p < 0.05 compared to the DEX group. DG dentate gyrus. ↑, c-Fos positive neurons
Effect of drugs on c-Fos expression in the hippocampal CA1 region. A CTRL group light microscopic image (a, X40 and b, X100) and c-Fos immunohistochemistry (c, X40; d, X100 and e, X100 negatif control) image. B c-Fos immunoreactivity (X400). a, CTRL; b, PTZ; c, DEX; d, ATI. C Number of c-Fos positive neurons in hippocampal CA1 region. *p < 0.05, compared to the CTRL group; **p < 0.05, compared to the PTZ group and #p < 0.05 compared to the DEX group
Effect of drugs on c-fos expression in the hippocampal CA3 region. A CTRL group light microscopic image (a, X40 and b, X100) and c-fos immunohistochemistry (c, X40; d, X100 and e, X100 negatif control) image. B c-fos immunoreactivity (X400). a, CTRL; b, PTZ; c, DEX; d, ATI. C Number of c-fos positive neurons in hippocampal CA3 region. *p < 0.05, compared to the CTRL group; **p < 0.05, compared to the PTZ group and #p < 0.05 compared to the DEX group
The mean percentage of c-Fos positive neurons in the PTZ and ATI groups was found to be significantly increased compared to CTRL in the dentate gyrus (DG) sections (p < 0.01; Fig. 5). The number of c-Fos positive cells was significantly reduced in rats injected with DEX group compared to the PTZ (p < 0.01). This decrease in the DEX group was also statistically significant compared to the ATI group (p < 0.05).
c-Fos protein expressions of the PTZ and ATI groups were significantly increased compared to the CTRL group in the hippocampal CA1 region (p < 0.01; Fig. 6). In addition, the number of c-Fos positive cells of DEX group was significantly lower than PTZ group (p < 0.05). The difference between the means of ATI and DEX group was statistically significant (p < 0.05).
The expression of c-Fos in the PTZ group in the hippocampal CA3 region was significantly increased compared to the CTRL group (p < 0.05; Fig. 7). Additionally, the number of c-Fos positive cells of DEX group was significantly lower than the PTZ group (p < 0.05). Furthermore, the difference between the means of ATI and DEX group was statistically significant (p < 0.05).
Discussion
In this study, the effects of alpha-2 adrenoceptor (α2-AR) receptor agonist have been demonstrated on epileptic seizures, hippocampal GABA levels and c-Fos expression in PTZ kindling model of epilepsy. The findings showed that the α2-AR agonist DEX decreased the seizure stages according to the Racine scale and prolonged the onset time of FMJ. Similarly, there was a significant decrease in the number of spikes and the percentage of seizure duration. In contrast, the α2-AR antagonist ATI increased the seizure stages, the number of spikes, and percentage of seizure duration. The GABA level did not show a significant decrease in other groups except the epileptic PTZ group. In addition, DEX significantly reduced the number of c-Fos positive cells in rat brain. All these data indicate that DEX produces anti-seizure effects. In addition, these study results show that DEX exerts its anti-seizure effects via α2-adrenergic receptors. Likewise, the α2-adrenergic receptor antagonist ATI increases seizure susceptibility by lowering the seizure threshold.
Noradrenergic receptors are widely distributed in brain tissue. It is especially found in areas associated with the formation of seizures, such as the hippocampus, cortex, and amygdala [15]. Noradrenaline (NA) has a strong anti-epileptogenic effect and it exerts this effect especially through α2-ARs [9]. Therefore, decreasing the endogenous NA level increases susceptibility to seizures [28]. Stimulation of the locus soeruleus, where noradrenergic neurons are in high density, inhibits seizure activity [29]. In contrast, blocking of NA activity in the locus ceruleus significantly facilitates seizure formation [30]. These study results appear to be consistent with the anti-seizure activity of DEX.
Clonidine, a α2-AR agonist, prevents tonic convulsions induced by strychnine and maximal electroshock in rats [31]. While clonidine does not alter seizure activity at low doses, it exerts a proconvulsant effect at high doses [14]. In mice with PTZ induced epilepsy, high dose (0.5 mg/kg) of clonidine exerts an anticonvulsant and yohimbine (α2-AR antagonist) acts as a proconvulsant [32]. These results are supported by the results of Lazarova et al. [33] study on rats with seizures. In this study, it was observed that clonidine prolonged the onset time of FMJ in epileptic animals. DEX (α2-AR agonist) prevents contractions in the kainic acid model of epilepsy, whereas atipemazole (α2-AR antagonist, ATI) causes more severe contractions in rats and increases seizure stages to 4 or 5 according to the Racine scale [34]. Consistent with this study, it was found that the seizure stages in rats injected with DEX and ATI were stage 3 and stage 5, respectively. DEX significantly prolonged the onset time of FMJ and decreased the number of epileptic spikes and the percentage of seizure duration. There is a great deal of evidence showing the neuroprotective effects of α2-AR agonists in the brain under ischemic conditions. DEX improves pathologies associated with ischemia-induced neurons in rats [35] and protects against ischemia-induced neuronal damage in rabbits [36]. There is a close relationship between neuronal ischemia and epilepsy. Hence, prevention of ischemia-induced neuronal death by correcting ischemia can prevent epileptic seizures [37].
Experimental studies show that the reduction in GABAA signal triggers epileptiform activity [13]. At the same time, the increase in the number of GABAergic synapses in the brains of people with epilepsy and animals with experimental epilepsy is interpreted as a compensatory mechanism [38]. It has been shown that DEX (5 µg/kg) and ATI (0.1 mg/kg) do not have a significant effect on extracellular GABA levels [39]. Consistent with this, in our study, the hippocampal GABA level was found to be significantly lower in epileptic rats given only PTZ. However, the GABA level did not demonstrate a significant decrease when other drugs administration to rats.
Increased c-Fos expression detected during seizures is accepted as an indicator of neuronal activity. In addition, there is a proportional relationship between the severity of PTZ-induced seizures and c-Fos expression in the brain [40]. Seizures up to stage 3 according to the Racine scale do not cause detectable c-Fos expression in the brain. Increased c-Fos expression in the cortex is more common after stage 4 and 5 seizures. However, a stage 5 seizure is required to show c-Fos proteins in the hippocampus [41, 42]. It has been observed that c-Fos expression increased significantly in the striatum, accumulate nucleus and prefrontal cortex of rats induced seizures with kainic acid [41]. α2-AR receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region [43]. Increased mRNA expression of c-Fos is considered an early marker of neuronal cell death and neurodegeneration. Highly increased c-Fos expression is detected in the cerebral cortex and dentate gyrus of rats (10–21 days old) with PTZ-induced status epilepticus [44]. In contrast, c-Fos expression cannot be detected in the immature rat brain. Thus, c-Fos immunoreactivity can be considered as a good sign of neuronal activation. In our study, PTZ significantly increased c-Fos expression in rats. In addition, the number of c-Fos positive cells in the dentate gyrus (DG) and hippocampal CA1 and CA3 areas was significantly reduced in DEX-administered rats.
Conclusions
In conclusion, α2-AR selective agonist DEX showed anticonvulsant effects on PTZ-induced seizures in rats. On the contrary, α2-AR antagonist ATI facilitated epileptic seizures. However, administration of DEX to rats produced neuroprotective effects with a significant reduction in the number of c-Fos positive cells. Further studies are needed for this drug to be used for therapeutic purposes in epileptic seizures.
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Weltha L, Reemmer J, Boison D (2019) The role of adenosine in epilepsy. Brain Res Bull 151:46–54
Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F (2004) The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev 28:507–524
Moezi L, Mansoori E, Niknahad H, Shafaroodi H (2014) The role of alpha-2 adrenoceptors in the anticonvulsant effects of adenosine on pentylenetetrazole-induced seizure threshold in mice. Pharmacol Biochem Behav 126:36–42
Meldrum BS, Akbar MT, Chapman AG (1999) Glutamate receptors and transporters in genetic and acquired models of epilepsy. Epilepsy Res 36:189–204
Gunes H, Ozdemir E, Arslan G (2019) Coenzyme Q10 increases absence seizures in WAG/Rij rats: The role of the nitric oxide pathway. Epilepsy Res 154:69–73
Taskıran AS, Ozdemir E, Gumus E, Ergul M (2020) The effects of salmon calcitonin on epileptic seizures, epileptogenesis, and postseizure hippocampal neuronal damage in pentylenetetrazole-induced epilepsy model in rats. Epilepsy Behav 113:107501
Loscher W, Kohling R (2010) Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav 19:105–113
Strac DS, Pivac N, Smolders IJ, Fogel WA, Deurwaerdere PD, Giovanni GD (2016) Monoaminergic mechanisms in epilepsy may offer innovative therapeutic opportunity for monoaminergic multi-target drugs. Front Neurosci 10:492
Szot P, Lester M, Laughlin ML, Palmiter RD, Liles LC, Weinshenker D (2004) The anticonvulsant and proconvulsant effects of α2-adrenoreceptor agonists are mediated by distinct populations of α2a-adrenoreceptors. Neuroscience 126:795–803
Van Gaalen M, Kawahara H, Kawahara Y, Westerink BHC (1997) The locus coeruleus noradrenergic system in the rat brain studied by dual-probe microdialysis. Brain Res 763:56–62
Kawahara Y, Kawahara H, Westernik BHC (1999) Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studies by dual-probe microdialysis. Brain Res 823:42–48
Bucheler MM, Hadamek K, Hein L (2002) Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience 109:819–826
Amabeoku G, Chikuni O, Bwakura E (1994) Gamma aminobutyric acid mediation of the anticonvulsant effect of clonidine on pentylenetetrazol-induced seizures in mice. Pharmacol Res 29:273–280
Fletcher A, Forster EA (1988) A proconvulsant action of selective alpha 2-adrenoceptor antagonists. Eur J Pharmacol 151:27–34
Loscher W, Czuczwar SJ (1987) Comparison of drugs with different selectivity for central alpha 1-and alpha 2-adrenoceptors in animal models of epilepsy. Epilepsy Res 1:165–172
Chermat R, Doare L, Lachapelle F, Simon P (1981) Effects of drugs affecting the noradrenergic system on convulsions in the quaking mouse. Naunyn Schmiedebergs Arch Pharmacol 318:94–99
Tacke U, Kolonen S (1984) The effect of clonidine and yohimbine on audiogenic seizures (AGS) in rats. Pharmacol Res Commun 16:1019–1030
Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF (1991) Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology 75:328–332
Whittington RA, Virag L, Vulliemoz Y, Cooper TB, Morishima HO (2002) Dexmedetomidine increases the cocaine seizure threshold in rats. Anesthesiology 97:693–700
Zhai M-Z, Wu H-H, Yin J-B, Cui Y-Y, Mei X-P, Zhang H et al (2016) Dexmedetomidine dose-dependently attenuates ropivacaine-induced seizures and negative emotions via inhibiting phosphorylation of amygdala extracellular signal-regulated kinase in mice. Mol Neurobiol 53:2636–2646
Wang L, Tang S, Wang Z, Chen H, Rajcha SS, Qian J (2019) The administration of dexmedetomidine changes microRNA expression profiling of rat hearts. Biomed Pharmacother 120:109463
Nissinen J, Andrade P, Natunen T, Hiltunen M, Malm T, Kanninen K et al (2017) Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res 136:18–34
Mirsky MAZ, Rossell LA, McPherson RW, Traystman RY (1994) Dexmedetomidine decreases seizure threshold in a rat model of experimental generalized epilepsy. Anesthesiology 81:1422–1428
Srivastava AK, Alex AB, Wilcox KS, White HS (2013) Rapid loss of efficacy to the antiseizure drugs lamotrigine and carbamazepine: a novel experimental model of pharmacoresistant epilepsy. Epilepsia 54:1186–1194
Treiman DM, Walton NY, Kendrick C (1990) A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res 5:49–60
Davoudi M, Shojaei A, Palizvan MR, Javan M, Mirnajafi-Zadeh J (2013) Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat. Epilepsy Res 106:54–63
Paxinos G, Watson C (1988) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Weiss R, Tomasula JJ, Sotolongo JR (1990) The effect of an alpha-2 agonist on bladder function and cord histology after spinal cord injury. J Urol 144:1527–1530
Fornai F, Ruffoli R, Giorgi FS, Paparelli A (2011) The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci 33:2169–2178
Corda MG, Orlandi M, Lecca D, Carboni G et al (1991) Pentylenetetrazol-induced kindling in rats: effect of GABA function inhibitors. Pharmacol Biochem Behav 40:329–333
Kulkarni SK (1981) Actions of clonidine on convulsions and behaviour. Arch Int Pharmacodyn Ther 252:124–132
Homayoun H, Khavandgar S, Dehpour AR (2002) The role of alpha2-adrenoceptors in the modulatory effects of morphine on seizure susceptibility in mice. Epilepsia 43:797–804
Lazarova M, Bendotti C, Samanin R (1984) Evidence that the dorsal raphe area is involved in the effect of clonidine against pentylenetetrazole-induced seizures in rats. Naunyn Schmiedebergs Arch Pharmacol 325:12–16
Halonen T, Kotti T, Tuunanen J, Toppinen A, Miettinen R, Riekkinen PJ (1995) α2-Adrenoceptor agonist, dexmedetomidine, protects against kainic acid-induced convulsions and neuronal damage. Brain Res 693:217–224
Savola JM, Virtanen R (1991) Central alpha2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol 195:193–199
Maier C, Steinberg GK, Sun GH, Zhi GT, Maze M (1993) Neuroprotection by the a2-adrenoreceptor agonist dexmedetomidine in a focal model of cerebral ischemia. Anesthesiology 79:306–312
Freund TF, Ylinen A, Miettinen R, Pitkinen A et al (1991) Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull 28:27–38
Rowley HL, Martin KF, Marsden CA (1995) Decreased GABA release following tonic-clonic seizures is associated withan increase in extracellular glutamate in rat hippocampus in vivo. Neuroscience 68:415–422
Valtonen P, Haapalinna A, Riekkinen P, Haionen T (1995) Effect of alfa-2-adrenergic drugs dexmedetomidine and atipamezole on extracellular amino acid levels in vivo. Eur J Pharmacol 285:239–246
Malhi SM, Jawed H, Hanif F, Ashraf M, Zubair F et al (2014) Modulation of c-Fos and BDNF protein expression in pentylenetetrazol kindled mice following the treatment with novel antiepileptic compound HHL-6. Biomed Res Int 2014:876712
Erdtmann-Vourliotis M, Riechter U, Mayer P, Grecksch G, Höllt V (1998) Pentylenetetrazol (PTZ)-induced c-fos expression in the hippocampus of kindled rats is suppressed by concomitant treatment with naloxone. Brain Res 792:299–308
Roe DL, Bardgett ME, Csernansky CA, Csernansky JG (1998) Induction of Fos protein by antipsychotic drugs in rat brain following kainic acid-induced limbic-cortical neuronal loss. Psychopharmacology 138(2):151–158
Jurgens CWD, Hammad HM, Lichter JA, Boese SJ, Nelson BW, Goldenstein BL et al (2007) α2A-adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region. Mol Pharmacol 71:1572–1581
Motte JE, da Silva Fernandes MJ, Marescaux C, Nehlig A (1997) Effects of pentylenetetrazol-induced status epilepticus on c-Fos and HSP72 immunoreactivity in the immature rat brain. Mol Brain Res 50:79–84
Funding
This study was supported by Cumhuriyet University Scientific Research Project, Sivas, Turkey (CUBAP, Grant Number: T-718).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cetindag Ciltas, A., Ozdemir, E., Gumus, E. et al. The Anticonvulsant Effects of Alpha-2 Adrenoceptor Agonist Dexmedetomidine on Pentylenetetrazole-Induced Seizures in Rats. Neurochem Res 47, 305–314 (2022). https://doi.org/10.1007/s11064-021-03445-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03445-4