Abstract
The mechanism of cognitive dysfunction in diabetes is still unclear. Recently, studies have shown that the cerebellum is involved in cognition. Furthermore, diabetes-induced cerebellar alterations is related to vascular changes. Therefore, we aimed to explore the roles of vascular function in diabetes-induced cerebellar damage and motor learning deficits. Type 1 diabetes was induced by a single injection of streptozotocin in Sprague–Dawley rats. Motor learning was assessed by beam walk test and beam balance test. The pathological changes of the cerebellum were assessed by Hematoxylin and eosin staining and Nissl staining. Apoptosis was evaluated by anti-caspase-3 immunostaining. Protein expression was evaluated by western blotting and double immunofluorescence. Our results have shown that motor learning was impaired in diabetic rats, coupled with damaged Purkinje cells and decreased capillary density in the cerebellum. In addition, the protein expression of neuronal NOS, inducible NOS, endothelial NOS, total nitric oxide, vascular endothelial growth factor and its cognate receptor Flk-1 was decreased in the cerebellum. Gastrodin treatment ameliorated neuronal damage and restored protein expression of relevant factors. Arising from the above, it is suggested that vascular dysfunction and NO signaling deficits in the cerebellum may be the underlying mechanism of early manifestations of cognitive impairment in diabetes, which could be ameliorated by gastrodin intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM), a systemic disease characterized by hyperglycemia, is often complicated by dysfunction of multiple organs in the body [1]. It has been reported as a risk factor for cognitive dysfunctions, such as vascular dementia and Alzheimer's disease (AD) [2], but the underlying mechanism of cognitive dysfunction in diabetes is still unclear.
The cerebellum is an important part of the central nervous system (CNS). Studies have shown that the cerebellum is involved in motor learning through adjusting the cognitive processes [2]. Inside the cerebellum, cerebellar cortex was regarded as an important region closely related to motor learning [3, 4]. This study aims to investigate potential mechanisms in DM-induced motor learning impairment.
VEGF (Vascular endothelial growth factor) is a key regulator of vasculogenesis and angiogenesis [5, 6]. In the CNS, VEGF could promote neuronal growth, survival and regeneration [7, 8]. It is also involved in neuroprotection, showing neurotrophic effects [9, 10]. Studies have indicated that diabetes could lead to abnormal VEGF signaling and pathogenic vessel remodeling [11, 12]. Flk-1(VEGF-R2) is one of the VEGF receptors, which acts as a major mediator of angiogenesis and vascular permeability [12].
Nitric oxide (NO) is an important signaling molecule widely distributed in the nervous system. Under physiological conditions, NO contributes to the proliferation, survival, and differentiation of neurons. Moreover, NO is involved in the synaptic activity, angiogenesis, neural plasticity, and memory function [13,14,15]. Cerebellar NO in particular participates in the induction of the long-term depression of parallel fiber inputs [16]. Nitric oxide synthase (NOS) is a family of enzymes catalyzing the production of NO from l-arginine [17]. The family contains three distinct genes encoding NOS isozymes: neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). nNOS is expressed in the central and peripheral nervous systems; its role in the CNS includes regulating synaptic plasticity, blood pressure [18], endocrine system, neural signal transduction and the differentiation of neural progenitor cells [19]. iNOS is expressed in many cell types in response to lipopolysaccharide and generate a large amount of NO indispensable for immune system [17]. It can react with superoxide, leading to peroxynitrite formation and cell toxicity [20]. eNOS, mainly expressed in endothelial cells, is vasoprotective and critical for maintaining of blood pressure and dilated blood vessels [18]. In the cerebellum, NOS is expressed by local stellate, basket, and granule cells.
Gastrodin, a phenolic glycoside, is a widely used Chinese herb that has been traditionally used for the treatment of headache, dizziness, spasm, epilepsy, stroke, amnesia and other disorders for centuries. The chemical structural formula of gastrodin is shown as Supplementary Fig. 1. There is ample evidenc showing that gastrodin can exert various therapeutic effects, including sedation, improvement of blood supply to the inner ear, protection of neurons, enhancement of immunity, anti-inflammation and anti-oxidation [21, 22]. In the CNS, many studies have shown that gastrodia elata has the effect of improving CNS disorders including epilepsy, Alzheimer's disease, cerebral ischemia/reperfusion, Parkinson's disease and cognitive impairment [22]. However, it remains uncertain whether gastrodin would protect motor learning through NO signaling.
In this study, we attempted to elucidate the possible mechanism of gastrodin on motor learning in diabetic rats. To investigate the role of NO signaling, the expression of nNOS, iNOS, eNOS, VEGF and its cognate receptor Flk-1 was examined. We report here the protective effect of gastrodin on motor learning through NO signaling.
Materials and Methods
Animal Use and Care
The Sprague–Dawley rats were purchased from Liaoning Changsheng Biotechonology Co., Ltd. A total of 100 male animals were used in this study (10 weeks old, weighing 250–300 g). Animal procedures were reviewed and approved by the Medical Ethics Committee of Kunming Medical University, Kunming, China. Rats were housed in our animal facilities in a temperature controlled, pathogen-free room, on a 12:12 light: dark cycle and allowed food and water intake ad libitum.
Diabetes Induction and Drug Administration
The rats were housed in plastic cages with standard bedding and the temperature was maintained at 22 °C.
The procedure of diabetes induction has been previously described [23]. Briefly, type 1 diabetes was induced by a single intraperitoneal injection of streptozotocin (65 mg/kg) after 2 weeks of adaptation. Blood glucose levels were assessed by a glucometer and animals were considered as diabetic if the blood glucose levels were higher than 16.7 mmol/l for three consecutive tests.
The rats were randomized into three groups: (i) NC6W group: normal control rats gavaged with normal saline daily (0.4 ml/100 g); (ii) DM6W + S group: diabetic rats gavaged with normal saline for 6 weeks at 3 weeks after diabetes induction; (iii) DM6W + G group, diabetic rats gavaged with gastrodin for 6 weeks (60 mg/k/day; dissolved in 0.9% saline) [23].
Beam Walk Test
Beam walk test was used for detecting motor learning deficit [24]. Rats were placed on a balance beam, which was 2 m long, 1.5 cm wide and elevated 50 cm above the ground. In the training phase, the rats were kept in a dark environment for 60 min and then put at the start of beam. Animals were trained 4 times daily for 3 days. In the test phase, the latency of crossing the beam was recorded for 3 times (up to 60 s) and the values obtained were averaged.
Beam Balance Test
Motor coordination was evaluated by beam balance test [25]. The rats were placed at the center of the same balance beam. Animals were trained 4 times a day for 3 days. In the test phase, the time staying on the balance beam without slipping (up to 60 s) was recorded for 3 times and the values obtained were averaged.
Western Blotting Analysis
Rats were anesthetized with 10% chloral hydrate administered intraperitoneally. The cerebellar tissues were rapidly dissected. The cerebellar cortex pivotal to motor learning, was rapidly isolated, frozen with liquid nitrogen and stored in − 80 °C. Proteins were extracted with RIPA buffer (9806; Cell Signaling Technology) containing phosphatase inhibitor cocktail (1:100; 5870; Cell Signaling Technology) and protease inhibitor cocktails (1:100; 5871; Cell Signaling Technology). All procedures were carried out at 4 °C. After complete lysis of the tissue, homogenates were centrifuged at 12,000×g for 10 min and the supernatant were collected. The protein concentration was determined by BCA protein assay kit. 30 μg of protein was loaded unto 8% SDS-PAGE gels and transferred to PVDF membranes. After that, the membranes were blocked for 2 h at room temperature using 5% skim milk and incubated at 4 °C overnight with primary antibodies. The following primary antibodies were used: mouse anti-nNOS antibody (1:1500; cat. no. sc-55521; Santa Cruz Biotechnology); mouse anti-iNOS antibody (1:1000; cat. no. sc-7271; Santa Cruz Biotechnology); mouse anti-eNOS antibody (1:1500; cat. no. sc-136977; Santa Cruz Biotechnology); mouse anti-VEGF antibody (1:1000; cat. no. sc-7269; Santa Cruz Biotechnology); mouse anti-Flk-1 antibody (1:1000; cat. no. sc-6251; Santa Cruz Biotechnology) and β-tubulin ((1:1000; cat. no. 15115; Cell Signaling Technology). After washing with TBS-0.1% Tween, the membranes were incubated for 2 h at room temperature with horseradish peroxidase-conjugated secondary antibody: goat-anti-rabbit (1:1,000; cat. no. 31460; Thermo Fisher Scientific) and goat-anti-mouse (1:1,000; cat. no. 31430). The blots were developed by enhanced chemiluminescence and developed on film with Image J software (version 1.4.3.67; National Institutes of Health, Bethesda, MD, USA).
Double Immunofluorescence
The cerebellum was removed, fixed in 4% formaldehyde, dehydrated, cleared with xylene and embedded in paraffin blocks. Paraffin sections of 4 μm thickness were incubated in citrate buffer for antigen retrieval and were incubated with 5% goat serum (Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) for 2 h at room temperature. Subsequently, sections were incubated in PBS containing 0.2% TX-100 at 4 °C overnight with primary antibodies: mouse anti-nNOS antibody (1:100; cat. no. sc-55521; Santa Cruz Biotechnology); mouse anti-iNOS antibody (1:100; cat. no. sc-7271; Santa Cruz Biotechnology); mouse anti-eNOS antibody (1:100; cat. no. sc-136977; Santa Cruz Biotechnology); mouse anti-VEGF antibody (1:100; cat. no. sc-7269; Santa Cruz Biotechnology); rabbit anti-Calbindin D-28k antibody (1:400; Swant); Caspase-3 (1:100, Wanleibio, Shenyang, China). After this, goat anti-rabbit Alexa Fluor 488 (1:200; Thermo Fisher Scientific) and goat anti-mouse Alexa Fluor 546 (1:200; Thermo Fisher Scientific) secondary antibodies were added and incubated for 2 h. The images were captured under a confocal microscope.
Hematoxylin and Eosin (H&E) Staining and Nissl Staining
The preparation of paraffin sections has been described above in the procedure under double immunofluorescence. After dewaxing and hydration, the aforementioned tissue sections were processed for routine H&E staining and Nissl staining (Beyotime Institute of Biotechnology, Shanghai, China). The photoimages were captured under a light microscope (magnification, × 400) in a blinded manner.
Measurement of Microvessel Number and VASCULAR DEnsity
The microvessel number was measured by H&E staining. Three samples from each group were selected for counting of microvessels. In each sample, five areas of the cerebellar cortex ar the corresponding areas of each section were selected at × 200 magnification. ImageJ statistical software was then used to count the number of vessels.
The vascular density was measured by lectin immunostaining. Three samples from each group were selected for counting. One section was chosen from each sample and two areas were selected in the corresponding site of each section (Magnification: × 400). ImageJ statistical software was used to count the vascular density.
Measurement of Nitric Oxide Production
The supernatant derived from the fresh cerebellar tissues was used for nitric oxide (NO) content measurement. Total NO production was assessed by spectrophotometric measurement of nitrite concentrations using a Total Nitric Oxide Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China). Optical density at 540 nm was measured using a microplate reader. Concentrations were calculated by comparing absorption with a standard curve.
Statistical Analysis
Data were expressed as mean ± SD and analyzed with one-way analysis of variance and pairwise comparison (SPSS 17.0 statistical software, USA) to determine the statistical significance of differences. p < 0.05 was considered statistically significant.
Results
Early Intervention of Gastrodin Improved Cerebellar Motor Learning in Diabetic Rats
The beam walk test showed that the latency to traverse the beam was significantly increased in diabetic rats (NC6W: 6.81 ± 4.13 s; DM6W + S: 20.42 ± 7.74 s). Gastrodin treatment significantly reduced the duration of time in diabetic rats (DM6W + G: 15.57 ± 4.83 s) (Fig. 1A). The beam balance test showed that the time of remaining on the beam without slipping was significantly decreased in diabetic rats compared with that of the NC6W group (NC6W: 56.37 ± 6.75 s; DM6W + S: 18.57 ± 11.94 s), which was significantly increased in DM6W + G group (DM6W + G: 36.97 ± 21.94 s) (Fig. 1B).
Early Intervention of Gastrodin Ameliorated Neuronal Injury Through Reducing Apoptosis in the Cerebellar Cortex
H&E staining showed that the Purkinje cells in the NC6W group were arranged in regular order and well-defined. In the DM6W + S group, Purkinje cells were loosely arranged with irregular morphology. Neuronal swelling and glial reaction were evident. Furthermore, the number of Purkinje cells and granular cells was decreased in the DM9W + S group. Neuronal damage was less evident in the DM6W + G group (Fig. 2A–C). Nissl staining showed that the number of Nissl bodies was decreased in the DM6W + S group, along with reduced Nissl substance. The DM6W + G group exhibited more Nissl bodies than the DM6W + S group (Fig. 2D–F).
Effects of gastrodin on histopathological alterations in the cerebellar cortex of diabetic rats. H&E staining(A–C) and Nissl staining (D–F) of the NC6W, DM6W + S and DM6W + G groups. Neuronal swelling (a), neuroglial aggregation (b) and neuron loss (c) can be observed in H&E staining. n = 6. Magnification: × 400. Bar = 50 µm
Diabetes-induced apoptosis was observed in the Purkinje cell layer and granule cell layer with caspase-3 immunofluorescence labeling. The number of cells undergoing apoptosis was markedly increased in the DM6W + S group compared with that of the NC6W group. In the DM6W + G group, the number of caspase-3-positive cells was decreased compared with that of the DM6W + S group (Fig. 3).
Effects of gastrodin on the apoptosis of Purkinje cells in diabetic rats. Representative immunofluroscent images of caspase-3 (red) and DAPI (blue) double staining of the cerebellar cortex in the NC6W group, DM6W + S group, and DM6W + G group. Note the increase in incidence of caspase-3 positive cells in DM6W + S (single arrow) compared with the NC6W and DM6W + G group. Magnification: × 400. Bar = 50 µm (Color figure online)
Early Intervention of Gastrodin Increased the Vascular Area Through Upregulating the Expression of VEGF and Flk-1
Immunofluorescence staining of lectin showed that the capillary density in the DM6W + S group was significantly decreased in comparison with the NC6W group and DM6W + G group. However, the number of arterioles showed no obvious change among three groups (Fig. 4).
Effects of gastrodin on capillary density in diabetic rats. (A) Representative photographs showing lectin expression in the cerebellar cortex of the NC6W group, DM6W + S group and DM6W + G group. Bar graphs represent capillary density (B) and the number of arterioles counted in sections of H&E staining (C). Magnification: × 400. Bar = 50 µm. *p < 0.05, **p < 0.01
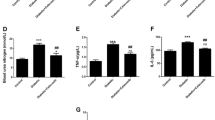
Western blot analysis showed that the expression of VEGF protein was significantly decreased in the diabetic rats (p < 0.01), which was significantly enhanced after gastrodin intervention (p < 0.01). Furthermore, DM6W + S group exhibited lower protein level of Flk-1 than NC6W (p < 0.01) and DM6W + G group (p < 0.05) (Fig. 5A, B).
Gastrodin upregulated the expression of VEGF and Flk-1. Western blot analysis of VEGF (A) and Flk-1 (B) expression levels in the cerebellar cortex, including the immunoreactive bands of VEGF (21 kDa), Flk-1 (230 kDa) and β-Tubulin (55 kDa). Bar graphs representing optical density (mean ± SD) of VEGF and Flk-1 normalized with β-Tubulin. (C) Immunoexpression of VEGF in the cerebellar cortex of NC6W group, DM6W + S group, and DM6W + G group. Calbindin D-28k was shown in green (Alexa 488) and nNOS in red (Alexa Fluor 546). Note the diminution of VEGF immunofluorescence in Purkinje cells in the DM6W + S group (single arrow) as compared with the normal control (NC6W). Magnification: × 400. Bar = 50 µm. *p < 0.05, ** p < 0.01 (Color figure online)
Double immunofluorescence of Calbindin D-28k and VEGF in cerebellar Purkinje cell layer has shown that VEGF was localized primarily in the cytoplasm of Purkinje cells. The immunoexpression of VEGF was decreased in the DM6W + S group compared with that of the NC6W and DM6W + G group (Fig. 5C).
Early Intervention of Gastrodin Restored the Protein Expression of NOS in the Cerebellar Cortex of Diabetic Rats
Western blot analysis showed that the expression of nNOS was significantly decreased in the DM6W + S group, in comparison with that of the NC6W group (p < 0.01). Gastrodin intervention could restore the expression of nNOS (p < 0.01). Similarly, the expression of eNOS and iNOS was lower in the cerebellar cortex of diabetic rats (p < 0.01), which was significantly increased in the DM6W + G group (Fig. 6A–C). Compared with the NC6W group, NO level was significantly decreased in the cerebellar cortex of diabetic rats (p < 0.05). In diabetic rats given gastrodin treatment, the level of NO exhibited the trend of elevation.
Gastrodin upregulated the expression of nitric oxide in diabetic rats. Western blot analysis of nNOS (A), eNOS (B) and iNOS (C) expression levels and NO content assay (D) in the cerebellar cortex, including the immunoreactive bands of nNOS (155 kDa), iNOS (130 kDa), eNOS (140 kDa), and β-Tubulin (55 kDa). Bar graphs represented optical density (mean ± SD) of these factors normalized with β-Tubulin. *p < 0.05; **p < 0.01
The NO levels as determined by nitrite level, were significantly decreased (P < 0.05) in the DM6W + S group, as compared with the NC6W group. In the DM6W + G group, NO levels were increased compared with DM6W + S group, but the decrease was not statistically significant (Fig. 6D).
Double immunofluorescence of Calbindin D-28k and nNOS showed that nNOS was localized primarily in the cytoplasm and neurites of Purkinje cells. The immunoexpression of nNOS was notably reduced in the DM6W + S group compared with that of the NC6W group, which was significantly increased compared with the DM6W + G group (Fig. 7).
Immunostaining of nNOS in the cerebellar cortex of the NC6W group, DM6W + S group, and DM6W + G group. Calbindin D-28k was shown in green (Alexa 488) and nNOS in red (Alexa Fluor 546). Note the drastic reduction of nNOS/CD28k + Purkinje cells in the DM6W + S group compared with that of the NC6W group (single arrow). Magnification: × 600. Bar = 25 µm (Color figure online)
Discussion
The present results have shown that diabetes could result in impairments in motor learning and cerebellar lesion. Of note, gastrodin could ameliorate the pathological changes of cerebellar cortex through decreasing the apoptosis of Purkinje cells in diabetic rats. To further clarify the underlying mechanism of gastrodin, we investigated the relation of neuronal and vascular changes. It was found that capillary density assessed by lectin staining was decreased in the cerebellar cortex, coupled with decreased expression of VEGF and nitric oxide synthase in diabetes. It is therefore suggested that decreased VEGF expression, which was affected by nitric oxide, may result in reduced cerebellar blood flow and pathological changes.
It is widely acknowledged that diabetes could lead to pathogenic vascular remodeling, coupled with abnormal VEGF signaling. Restoring VEGF expression in an animal model of diabetes ameliorated blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment [26]. In animals with brain injury, cognitive function could be improved by enhancing VEGF signaling [27,28,29]. VEGF deficiency may affect motor learning through the following mechanisms. On the one hand, VEGF deficiency may change the synaptic plasticity of neurons [30], which was supported by expression of VEGF in the cytoplasm of cerebellar Purkinje cells and granule cells. On the other hand, it may cause progressive and insidious damage to vessels, which could be an important cause of diabetes-related cognitive impairments [31, 32]. Therefore, VEGF deficiency is critical for the pathogenesis of cerebellar alterations.
The decreased expression of VEGF may be caused by reduced NO expression in the cerebellar cortex. The present results have shown that NO levels were significantly decreased at 6 weeks after diabetes induction, which is consistent with our previous study as well as studies by others [33,34,35]. NO can exacerbate nervous injury in multiple pathological processes, including ischemia, inflammatory disorders, axonal injury, glutamate neurotoxicity [36,37,38]. High glucose can trigger endothelial cell apoptosis by de-activation of eNOS [39]. Since vasogenic NO is the major mediator of the vasodilatation and vascular permeability [17, 18, 40], the deficiency of eNOS could be the cause of vasodilatory disorder, causing insufficiency of blood supply. In addition, poor control of blood glucose can cause vasculopathy and abnormal blood flow [41,42,43]. Therefore, it is reasonable to suggest that chronic hyperglycemia may impair normal blood flow through suppressing NOS function.
Other than affecting vascular function, NOS function could directly lead to neuronal damage. A separate research found that STZ-induced diabetic rats had significantly decreased nNOS activity and mRNA level in the cerebellum [34]. nNOS deficiency was reported to promote apoptotic cell death [44]. Furthermore, cerebellar defects could be detected in nNOS knock-out mice [45]. nNOS could protect cultured cerebellar granule neurons against alcohol-induced cell death by stimulating the cAMP pathway [46]. Very strikingly, the expression of nNOS was elevated following gastrodin intervention, suggesting that gastrodin may help restore the normal function of NO by increasing the expression of nNOS. This would be consistent with the finding that gastrodin can either pass through the BBB or be metabolized into p-Hydroxybenzyl alcohol, which has similar pharmacological effects on central nervous system disease [22]. It is noteworthy that gastrodin and p-Hydroxybenzyl alcohol can be detected in the cerebellar tissue and other areas including the frontal cortex, hippocampus and thalamus [47].
Notwithstanding of the above, it is clear that there are some limitations to our present study. Firstly, all our experiments were conducted in vivo, which makes it hard to demonstrate the pathways involved. It would be desirable to directly investigate the interaction between endothelial cells and Purkinje cells using an in vitro cell model. In addition, iNOS expression was also upregulated by gastrodin treatment, which is different from previous studies [48, 49]. Very interestingly, it has been reproted that caffeic acid para-nitro phenethyl ester ameliorated diabetic changes by increasing NO content via upregulating iNOS expression [50], which is consistent with our present finding. Nonetheless, the exact mechanism of iNOS is still unclear and requires further investigation.
Conclusion
In summary, we have found that the expression of nNOS, iNOS, eNOS, NO, VEGF and Flk-1 was decreased after diabetes induction; concomitant to this was neuronal damage and motor learning impairment [51]. The underlying mechanism of diabetes-induced motor learning deficits may be through downregulation of VEGF and NOS expression (Fig. 8). Of note, the results have revealed that the effects of gastrodin may be exerted through restoring VEGF and NO signaling pathway in the cerebellar cortex of diabetic rats.
Diagram illustrating the effects of diabetes on Purkinje cells and vascular endothelial cells, which has been indicated by red arrows. Diabetes could reduce the neuronal secretion of VEGF, which affects the expression of eNOS and NO in endothelial cells. As a result, the capillary density was decreased, causing downregulation of nNOS in Purkinje cells and neuronal apoptosis (Color figure online)
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
References
Tao Z, Shi A, Zhao J (2015) Epidemiological perspectives of diabetes. Cell Biochem Biophys 73:181–185
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74
Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S (2006) Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience 139:767–777
Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P (2011) Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21:1761–1770
Shibuya M (2008) Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 41:278–286
Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A (2003) Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol 262:225–241
Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P (2009) Role and therapeutic potential of VEGF in the nervous system. Physiol Rev 89:607–648
Foehring D, Brand-Saberi B, Theiss C (2012) VEGF-induced growth cone enhancement is diminished by inhibiting tyrosine-residue 1214 of VEGFR-2. Cells Tissues Organs 196:195–205
Zachary I (2005) Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals 14:207–221
Beazley-Long N, Hua J, Jehle T, Hulse RP, Dersch R, Lehrling C, Bevan H, Qiu Y, Lagreze WA, Wynick D, Churchill AJ, Kehoe P, Harper SJ, Bates DO, Donaldson LF (2013) VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. Am J Pathol 183:918–929
Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A (2012) Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes 61:1533–1542
Reeson P, Tennant KA, Gerrow K, Wang J, Weiser Novak S, Thompson K, Lockhart KL, Holmes A, Nahirney PC, Brown CE (2015) Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci 35:5128–5143
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16:435–452
Moncada S, Bolanos JP (2006) Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 97:1676–1689
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27:2783–2802
Casado M, Isope P, Ascher P (2002) Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron 33:123–130
Menshikova EB, Zenkov NK, Reutov VP (2000) Nitric oxide and NO-synthases in mammals in different functional states. Biochemistry (Mosc) 65:409–426
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(829–837):837a–837d
Park SY, Kang MJ, Han JS (2017) Neuronal NOS induces neuronal differentiation through a PKCalpha-dependent GSK3beta inactivation pathway in hippocampal neural progenitor cells. Mol Neurobiol 54:5646–5656
Mungrue IN, Husain M, Stewart DJ (2002) The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev 7:407–422
Zhan HD, Zhou HY, Sui YP, Du XL, Wang WH, Dai L, Sui F, Huo HR, Jiang TL (2016) The rhizome of Gastrodia elata Blume: an ethnopharmacological review. J Ethnopharmacol 189:361–385
Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P, Xu Y, Zhao Q, Si G (2018) A review on central nervous system effects of gastrodin. Front Pharmacol 9:24
Qi YH, Zhu R, Wang Q, Li Q, Liu YD, Qian ZY, Yang ZH, Mu ZH, Liu XJ, Zhang MY, Wang X, Liao XY, Wan Q, Lu D, Zou YY (2019) Early intervention with gastrodin reduces striatal neurotoxicity in adult rats with experimentally induced diabetes mellitus. Mol Med Rep 19:3114–3122
Piot-Grosjean O, Wahl F, Gobbo O, Stutzmann JM (2001) Assessment of sensorimotor and cognitive deficits induced by a moderate traumatic injury in the right parietal cortex of the rat. Neurobiol Dis 8:1082–1093
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp 49:e2376
Taylor SL, Trudeau D, Arnold B, Wang J, Gerrow K, Summerfeldt K, Holmes A, Zamani A, Brocardo PS, Brown CE (2015) VEGF can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol Dis 78:1–11
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835
Ortuzar N, Rico-Barrio I, Bengoetxea H, Argandona EG, Lafuente JV (2013) VEGF reverts the cognitive impairment induced by a focal traumatic brain injury during the development of rats raised under environmental enrichment. Behav Brain Res 246:36–46
Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E (2011) Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA 108:5081–5086
Tillo M, Ruhrberg C, Mackenzie F (2012) Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell Adh Migr 6:541–546
Iadecola C (2010) The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120:287–296
Feinkohl I, Price JF, Strachan MW, Frier BM (2015) The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther 7:46
Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106:987–992
Gorgun FM, Gumustas MK, Altug T, Kokoglu E (2002) Vitamin E supplementation in streptozotocin-treated rats alters cerebellar and plasma nitric oxide metabolism. J Toxicol Environ Health A 65:631–637
Han Y, Li X, Zhou S, Meng G, Xiao Y, Zhang W, Wang Z, Xie L, Liu Z, Lu H, Ji Y (2012) 17ss-estradiol antagonizes the down-regulation of ERalpha/NOS-3 signaling in vascular endothelial dysfunction of female diabetic rats. PLoS ONE 7:e50402
Manucha W (2017) Mitochondrial dysfunction associated with nitric oxide pathways in glutamate neurotoxicity. Clin Investig Arterioscler 29:92–97
Madigan CA, Cambier CJ, Kelly-Scumpia KM, Scumpia PO, Cheng TY, Zailaa J, Bloom BR, Moody DB, Smale ST, Sagasti A, Modlin RL, Ramakrishnan L (2017) A macrophage response to mycobacterium leprae phenolic glycolipid initiates nerve damage in leprosy. Cell 170(973–985):e910
Keynes RG, Garthwaite J (2004) Nitric oxide and its role in ischaemic brain injury. Curr Mol Med 4:179–191
Lin LY, Lin CY, Ho FM, Liau CS (2005) Up-regulation of the association between heat shock protein 90 and endothelial nitric oxide synthase prevents high glucose-induced apoptosis in human endothelial cells. J Cell Biochem 94:194–201
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7:359–371
Kelly-Cobbs A, Elgebaly MM, Li W, Ergul A (2011) Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic Goto-Kakizaki rat in response to glycaemic control. Acta Physiol (Oxf) 203:245–251
Tennant KA, Brown CE (2013) Diabetes augments in vivo microvascular blood flow dynamics after stroke. J Neurosci 33:19194–19204
Rahman S, Rahman T, Ismail AA, Rashid AR (2007) Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab 9:767–780
Karacay B, Bonthius DJ (2015) The neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicity. Cell Mol Neurobiol 35:449–461
Kriegsfeld LJ, Eliasson MJ, Demas GE, Blackshaw S, Dawson TM, Nelson RJ, Snyder SH (1999) Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience 89:311–315
Karacay B, Li G, Pantazis NJ, Bonthius DJ (2007) Stimulation of the cAMP pathway protects cultured cerebellar granule neurons against alcohol-induced cell death by activating the neuronal nitric oxide synthase (nNOS) gene. Brain Res 1143:34–45
Wang Q, Chen G, Zeng S (2008) Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal 46:399–404
Zhou XY, Zhang F, Ying CJ, Chen J, Chen L, Dong J, Shi Y, Tang M, Hu XT, Pan ZH, Xu NN, Zheng KY, Tang RX, Song YJ (2017) Inhibition of iNOS alleviates cognitive deficits and depression in diabetic mice through downregulating the NO/sGC/cGMP/PKG signal pathway. Behav Brain Res 322:70–82
Ahlawat A, Sharma S (2018) A new promising simultaneous approach for attenuating type II diabetes mellitus induced neuropathic pain in rats: iNOS inhibition and neuroregeneration. Eur J Pharmacol 818:419–428
Wang X, Li D, Fan L, Xiao Q, Zuo H, Li Z (2017) CAPE-pNO2 ameliorated diabetic nephropathy through regulating the Akt/NF-kappaB/ iNOS pathway in STZ-induced diabetic mice. Oncotarget 8:114506–114525
Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, Han X, Zhu DY (2007) Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem 103:1872–1882
Funding
The present study was supported in part by the National Natural Science Foundation of China (Grant Nos. 81760149, 81360176 and 81200840), the Department of Science and Technology of Yunnan Province (Grant No. 2013HB078), the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University [Grant Nos. 2017FE468 (-171) and 2017FE468 (-012)], and the Yunnan Provincial Education Commission (Grant No. 2018JS156).
Author information
Authors and Affiliations
Contributions
YYZ, ZHM and JLS designed the project and finalized the manuscript. FZ, CKD and YJH performed the majority of the experiments, participated in discussion, analyzed the data, and prepared the first draft of the manuscript. YHM, YYW and YZ conducted the part of experiments, participated in discussion, and analyzed the data. ZYQ, WQZ and RDZ performed the paraffin embedding, sectioning, and H&E staining. BL, XS, XYW and GC conducted part of the experiments and helped with removal of tissue samples and took care of the experimental rats.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical Approval
Animal procedures were reviewed and approved by the Medical Ethics Committee of Kunming Medical University, Kunming, China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Deng, CK., Huang, YJ. et al. Early Intervention of Gastrodin Improved Motor Learning in Diabetic Rats Through Ameliorating Vascular Dysfunction. Neurochem Res 45, 1769–1780 (2020). https://doi.org/10.1007/s11064-020-03039-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03039-6