Abstract
Apelin-13, as an endogenous neuropeptide, is the ligand for the G-protein-coupled receptor, APJ, which has recently been demonstrated to be involved in the process that contributes to learning and memory. Previous studies showed that apelin may be required for certain forms of learning and memory. Up to date, the role of apelin in fear memory has not been explored. In the present study, we tested the effects of apelin-13 (1.0, 2.0 and 4.0 µg/rat) on contextual fear conditioning (experiment 1), consolidation (experiment 2) and expression (experiment 3) in rats. A well established fear conditioning protocol was used, which contained three training phases: habituation, fear conditioning and test. Apelin-13 was i.c.v injected 10 min before conditioning (experiment 1), immediately after conditioning (experiment 2) or 10 min before testing (experiment 3). The values of percent freezing were used to measure fear. We found that only 2.0 µg apelin-13 administrations produced a decrease freezing in experiment 1. The most effective dose of apelin-13 (2.0 µg) was selected, but it had no effect on freezing in experiment 2 and 3. Furthermore, the decreased freezing in experiment 1 was not attributed to the deficits of locomotor activity and foot-shock sensitivity. These results, for the first time, indicated that apelin-13 impaired fear acquisition but not fear consolidation or expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropeptide apelin originally isolated from bovine stomach extracts through the methods of reverse pharmacology [1], is the endogenous ligand of a formerly orphan G protein coupled receptor (GPCR), APJ. The preproapelin of apelin contains 77 amino acids, and is cleaved into several biologically active forms, such as apelin-13, apelin-36, apelin-19 and apelin-17 in different tissues [2, 3]. Apelin-13, studied in our present study, is completely conserved across all species investigated [4], and has high activity at the receptor [5, 6]. It has been shown that apelin-13 was involved in the regulation of cardiovascular function [7, 8], fluid homeostasis [9], pulmonary function [10], and the pituitary–adrenal axis [9, 11].
Apelin receptors and apelin are widely distributed in the central nervous system (CNS) [12, 13], such as the hippocampus, amygdala and cerebral cortex. These suggest that apelin/APJ may be of importance in the regulation of learning and memory. But, up to date, there remain comparatively few studies that have explored the relationship between apelin and learning and memory. A behavioral study showed that apelin-13 facilitated the consolidation of passive avoidance learning in mice [14]. But apelin-13 blocked short-term memory (STM) formation and long-term memory (LTM) consolidation in novel object recognition task [15]. This discrepancy may be attributed to the task-dependent effects on different forms of learning and memory.
So the aim of the present study was to explore the effects of apelin on learning and memory using a fear conditioning paradigm, a procedure that involves pairing a cued or contextual conditioned stimulus (CS) with a footshock unconditioned stimulus (US). This results in the CS alone eliciting fear responding (e.g., freezing) after conditioning. It is well established that context, but not tone, learning requires the hippocampus [16, 17]. In vivo data obtained in rodents suggest that mGlu5, another type of GPCR,exhibited a role in fear conditioning to context [18, 19]. mGlu5 signaling plays a crucial role in acquisition but not consolidation of contextual conditioned fear [19]. Hence, we examined whether apelin-13 affected contextual fear conditioning, consolidation and expression in rats.
Materials and Methods
Subjects
The subjects were adult male Sprague–Dawley rats (270–300 g) obtained from the Laboratory Animal Center of Central South University, Changsha, Hunan, China. After arrival, the rats were housed one per cage(290 × 178 × 160 mm) under laboratory conditions (12:12 h light/dark cycle with lights on at 07:00 h, 25 °C, 45–55 % humidity, pelleted food and water ad libitum). All procedures occurred between 09:00 and 15:00 h. Before surgery, the rats were handled daily during a 7-day adaption period. All experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the Committee for Animal Care and Use, Central South University.
Surgery
The rats were anesthetized with sodium pentobarbital (45 mg/kg; Sigma-Aldrich, USA) and placed in a stereotaxic frame (RWD Life Science Co., Ltd., Shenzhen, Guangdong, China). A stainless-steel guide cannula was introduced into the right lateral ventricle (1 mm posterior to bregma, 1.6 mm lateral to midline, 3.8 mm ventral to skull surface) according to the atlas of Pellegrino et al. [20] and fixed to the skull with dental cement and acrylic resin. The rats were allowed to recover for 1 week. After completion of experiment, the proper injection site was verified by administration and localization of methylene blue dye. Only rats with the correct location of the cannula were used to evaluate the experiments.
Drugs and Treatments
Apelin-13 (Abbiotec Co., San Diego, USA) was freshly dissolved in sterile pyrogen-free 0.9 % saline. It was microinjected into the cella lateralis through an infusion cannula (RWD Life Science Co., Ltd., Shenzhen, Guangdong, China) which extended 0.5 mm below the ventral tip of the implanted guide cannula. Apelin-13 (1.0, 2.0, 4.0 µg/rat) or vehicle (4 µl) was infused over a period of 3 min via a 25 µl Hamilton microsyringe (Hamilton) mounted on a microdrive pump (KD Scientific). The infusion cannula was left in place for 1 min after the end of the infusion period. For the fear consolidation, fear expression,locomotor activity test and foot-shock sensitivity test, the most effective dose of apelin-13 (2.0 µg) was selected.
Apelin-13 or vehicle was injected 10 min before fear conditioning in experiment 1 (Fig. 1a), immediately after fear conditioning in experiment 2 (Fig. 2a) or 10 min before testing in experiment 3 (Fig. 3a). And in experiment 4, rats received apelin-13 or vehicle 10 min before the locomotor activity test and foot-shock sensitivity test.
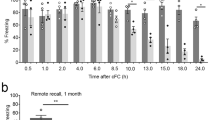
Apelin-13 following a single i.c.v. administration impaired the acquisition of contextual fear response in rats. a Schematic of the behavioral procedure used. Behavior procedure involved three training phases: habituation, fear conditioning and test. Rats were administrated apelin-13 10 min before conditioning phase. b Percent freezing to the context in the 60 s periods before and after each footshock presentation during fear conditioning in the vehicle group (n = 8; MN = 1), 1.0 µg apelin-13 group (n = 8; MN = 2), 2.0 µg apelin-13 group (n = 8; MN = 2) and 4.0 µg apelin-13 group (n = 8; MN = 3). c Percent freezing to the context during test phase. MN: missed number. *p < 0.05, **p < 0.01 for comparisons between every apelin-13 group and the vehicle group. All data are represented as mean ± SEM
Apelin-13 following a single i.c.v. administration does not affect fear consolidation. a Schematic of the behavioral procedure used. Behavior procedure involved three training phases: habituation, fear conditioning and test. Rats were administrated apelin-13 immediately after fear conditioning. b Percent freezing to the context in the 60 s periods before and after each footshock presentation during fear conditioning in the vehicle group (n = 9; MN = 1) and 2.0 µg apelin-13 group (n = 8; MN = 2). c Percent freezing to the context during test phase. MN: missed number. All data are represented as mean ± SEM
Apelin-13 following a single i.c.v. administration does not affect fear expression. a Schematic of the behavioral procedure used. Behavior procedure involved three training phases: habituation, fear conditioning and test. Rats were administrated apelin-13 10 min before test phase. b Percent freezing to the context in the 60 s periods before and after each footshock presentation during fear conditioning in the vehicle group (n = 8; MN = 1) and 2.0 µg apelin-13 group (n = 7; MN = 3). c Percent freezing to the context during test phase. MN: missed number. All data are represented as mean ± SEM
Behavioral Apparatus
Habituation, conditioning and testing sessions were conducted in a 46 cm × 46 cm × 46 cm cm chamber (context) which was located in a sound-attenuating cabinet (Huaibei Zhenghua Biological Equipment Co. Ltd., Anhui, China). On the right wall of the cabinet, there was a ventilation fan to supply a 60 dB background noise. Illumination was provided by a 8 W white house light installed on the ceiling of cabinet. The grid floor of the chamber consisted of 23 stainless steel rods, each measuring 6 mm in diameter and distant 20 mm (center to center), that were linked to a scrambled shocker for delivering foot-shock USs. A computer program was used to control stimuli appearance as scheduled. The chamber was washed with a 100 % ethanol solution before each session.
Experimental Procedure and Treatment
Contextual Fear Conditioning and Test
The behavioral procedure (Fig. 1a) was implemented on 3 consecutive days. On Day 1 (habituation phase), rats were placed in the conditioning chamber to habituate context for 20 min with no stimuli presented. On Day 2 (fear conditioning phase), rats were put in the chamber and after 3 min were subjected to five 0.8 mA unsignaled footshocks (1.0 s duration) with 60 s inter-trial intervals. The rats remained in the chamber for 60 s after the last footshock, then were put back to their home cages. Fear response was measured by freezing which was defined as no visible movement except that associated with respiration. Freezing activity on each time block during which no footshock was presented was scored with a digital stopwatch from videotapes and expressed as the percentage of time (time spent freezing/total time × 100). Observers scoring freezing were blind to the treatment of each rat. On Day 3 (testing phase), rats were reintroduced in the chamber for 5 min without any shock, and freezing behavior was scored during 5 min context exposure.
Locomotor Activity Test
Two additional groups of rats received i.c.v. injections of apelin-13 (2.0 µg) or saline were used to evaluate the effect of apelin-13 on locomotor activity. The locomotor activity test was performed 10 min after the injection. Each rat was individually introduced to the locomotor test chamber (46 cm × 46 cm × 46 cm) and allowed to explore the arena freely for 10 min. A video camera installed on the chamber ceiling was used to record the rat behaviours during the session, and connected to a computer with the commercial software (Huaibei Zhenghua Biological Equipment Co. Ltd., Anhui, China). The traveling distance of each rat was analyzed by the commercial software to assess locomotor activity. The chamber was thoroughly cleaned with 100 % ethanol solution before and after use.
Foot-Shock Sensitivity Test
Shock sensitivity was tested 10 min after apelin-13 (2.0 µg) or vehicle injection, a time point in accordance with fear conditioning. According to a previous study [21], rats were transported from their home cages to the fear conditioning chambers. After 3 min, the rats were subjected to unsignaled foot shocks (1.0 s, 60 s inter-trial interval) increasing from 0.05 to 0.8 mA. The incremental amplitude was 0.05 mA. Foot shock was stopped until the thresholds of three kinds of response were reached: noticing (an orienting head movement), flinching (hind paws briefly raised off the bars) and vocalizing.
Data Analysis
Differences of experimental groups during fear conditioning phases were analyzed using two-way repeated-measures ANOVA. Differences during test phases in experiment 1 were analyzed using one-way ANOVA. Post hoc comparisons were performed with the Tukey HSD method when ANOVA was significant. Differences between two groups during test phases in experiment 2 and 3 as well as in experiment 4 were analyzed using Student’s t test. All data were expressed as mean ± SEM. p < 0.05 were regarded as statistically significant. Statistical analysis were performed using SPSS (Version 19; SPSS, Chicago, IL).
Results
Experiment 1: Effects of Apelin-13 on Contextual Fear Conditioning
During the fear conditioning phase (Fig. 1b), two-way repeated-measures ANOVA of percent freezing showed significant effects of time block [F(5 140) = 111.72, p < 0.001] and group [group, F(3,28) = 3.507, p < 0.05; group × time block, F(5, 140) = 3.182, p < 0.05]. Post hoc analysis showed that compared with vehicle group, 2.0 µg apelin-13 group presented a significant decrease of freezing response (time block 4, p < 0.05; time blocks 5 and 6, p < 0.01). During the test phase (Fig. 1c), apelin-13 (2.0 µg) group showed significantly lower freezing in comparison to the vehicle group [F(3, 28) = 2.756]; p < 0.01. These data suggested that apelin-13 impaired the acquisition of contextual fear response.
Experiment 2: Effects of Apelin-13 on Fear Consolidation
During the conditioning phase (Fig. 2b), there was a significant time block effect in freezing [F(5, 75) = 151.864, p < 0.001] but no significant differences between groups [group, F(1,15) = 0.020, p > 0.05 and group × time block, F(5, 75) = 0.827, p > 0.05, respectively], suggesting that the two groups showed equivalent fear learning. During the test phase (Fig. 2c), there was no significant difference in the level of freezing between the two groups [t(15) = 1.050; p > 0.05]. These data suggested that apelin-13 did not affect fear consolidation.
Experiment 3: Effects of Apelin-13 on Fear Expression
During the conditioning phase (Fig. 3b), there were significant differences across time blocks [F(5, 65) = 168.093, p < 0.001]. But there was no effect of group [F(1,13) = 0.003, p > 0.05] or interaction of group and time block [F(5, 65) = 4.193, p > 0.05]. Thus, the two groups showed equivalent fear learning. During the test phase (Fig. 3c), no significant difference in the level of freezing was observed between the two groups [t(13) = 1.176; p > 0.05]. These data suggested that apelin-13 did not affect fear expression.
Experiment 4: Nonspecific Response Measurement
Locomotor Activity Test
No significant difference in the total traveling distance was observed between the apelin-13 group and vehicle group during the 10 min test session [t(10) = 1.279; p > 0.05, Fig. 4a], indicating that apelin-13 did not significantly affect locomotor activity.
Nonspecific responses measurement in the apelin-13 group and vehicle group. a Locomotor activity test. Apelin-13 following a single i.c.v. administration does not affect locomotor activity. Rats were administrated apelin-13 10 min before the locomotor activity test. The total traveling distance was showed for the vehicle group (n = 6; MN = 2) and 2.0 µg apelin-13 group (n = 6; MN = 2) during the 10 min test session. b Foot-shock sensitivity test. Apelin-13 following a single i.c.v. administration does not affect shock sensitivity. The three response thresholds, including noticing, flinching and vocalizing, were showed for the vehicle group (n = 7; MN = 1) and 2.0 µg apelin-13 group (n = 8; MN = 0). MN: missed number. All data are represented as mean ± SEM
Foot-Shock Sensitivity Test
There was no significant difference between the apelin-13 group and vehicle group as concerned with three response thresholds to foot shock (all, p > 0.05) (Fig. 4b). Thus, apelin-13 did not significantly change sensitivity to foot shock during fear conditioning.
Discussion
The present results, for the first time, demonstrate that apelin-13 (2.0 µg) following a single i.c.v. administration did impair the acquisition of contextual fear response in rats, but had no effect on fear consolidation or expression. Our further results showed that the disruptive effect of apelin-13 on fear acquisition was not attributed to the alteration of foot-shock sensitivity.
Previous studies showed that i.c.v. administration of apelin-13 to rats did not influence the spontaneous motor activity [11, 22]. We found a similar result when locomotor activity test was conducted 10 min after apelin-13 injection. Thus, it was unlikely that the deficit in the acquisition of fear was ascribed to nonspecific changes of locomotor activity that followed apelin-13 injection.
Apelin and APJ receptor are widely expressed in neurons and gliocytes in central nervous system [23], which suggests that apelin plays an important role in the neuronal signaling pathway [13]. Previous observations [13, 24] found that hippocampus has high levels of apelin and its receptor APJ. Apelin-13 has been found to impair LTM of novel object recognition memory which is hippocampus-dependent learning [15]. Consistent with previous studies, our current study showed that apelin-13 impaired contextual fear acquisition, also a hippocampus-dependent task. We also observed that apelin-13 (1.0–4.0 µg/rat) produced a dose-related damage effect on fear acquisition, induced effectively by a dose of 2 µg/rat. The “U-shaped” dose response might indicate that apelin-13 is an agonist at low doses but reveals partial reversal of agonistic activity at higher concentrations [25, 26]. Additionally, apelin-13 did not block the expression of freezing in rats. This is in accordance with a previous observation that no effect of apelin-13 was observed when apelin-13 was given prior to testing 24 h after novel object recognition memory training in mice [15]. In summary, apelin-13 selectively produced an impairment effect on the acquisition but not on the expression of conditioned fear. These results indicate that apelin-13 regulates the plasticity of fear memory, but not prevents the synaptic transmission of information [27].
Our findings of the role for apelin-13 in fear consolidation are inconsistent with the previous literatures [14, 15]. Previous reports showed that central administration of apelin-13 facililated the consolidation of passive avoidance memory in mice [14] or impaired object recognition memory consolidation [15]. While in our current study, there was comparable freezing level in fear consolidation test between rats with 2.0 µg (1.29 nmol) apelin-13 and with vehicle. These disagreements of apelin-13 on fear consolidation are difficult to explain. In our studies, the dose of apelin-13 (2 µg/1.29 nmol) were roughly equal to that (2 µg or 1 nmol) used in the previous studies, thus the different doses of apelin-13 seem not to be enough to account for the inconsistent results. We infer that this discrepancy may attributed to different task types. Studies on the mechanism suggest that there exist some differences in the biochemical processes involved in different task [28–30]. In addition, this inconsistency may be related to different animal species (rats vs mice). Taken overall, our present results and previous studies that produced contradictory findings suggested that different influences of apelin-13 on memory may be attributed to different task types, processing modes or animals used. Anyway, future studies are need to be illuminated the molecular mechanisms that underlie these discrepancies. Overall our observation suggests that apelin-13 negatively regulates the acquisition of fear conditioning. However, the underlying mechanisms are still unknown. Previous studies have found apelin/APJ system could negatively regulate the cAMP pathway [1], which is important for contextual fear conditioning [31]. And apelin has been showed to attenuate N-methyl-d-aspartate (NMDA) receptor-mediated Ca2+ accumulation and excitotoxicity in the hippocampal neurons [32, 33], indicating that apelin could block the NMDA receptor pathway. While acquisition of fear memory relies on NMDA receptor signaling [34, 35]. Base on the above researches, we speculated that apelin impair the acquisition of fear memory by blocking cAMP pathway or NMDA receptor signaling. However, it should be mentioned that mGlu5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) attenuated contextual fear learning, which suggests that mGlu5 facilitates the acquisition of contextual fear in mice [19]. While our results found that apelin-13, the agent of another GPCRs, impaired the acquisition of contextual fear in rats. This disagreement may be mainly caused by the following two factors. First, different animal species (mice vs rats) may be relative to this disagreement. Second, different kinds of GPCRs bound by ligand may produce different, even contrary effects. Anyhow, we fully agree with other authors [14, 15] that apelin/APJ system may be a potential target for the regulation of memory and well worth further exploration.
Conclusions
In summary, data from our present experiments strongly indicates, for the first time, that i.c.v. administration of apelin-13 (2.0 µg) impaired contextual fear acquisition but not consolidation or expression. These findings suggested that apelin were specific to certain aspects of the learning experience, which extended previous research on the effects of apelin on learning and memory. Additionally, it is widely accepted that the acquisition of fear is involved in some anxiety disorders, such as post-traumatic stress disorder (PTSD), which are marked by excessive fear [36]. Thus, the Apelin/APJ system may be considered as a novel target to treat anxiety disorders by decreasing fear. Further studies are needed to fully delineate it.
References
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251:471–476
Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O, Fujino M (2001) Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta 1538:162–171
Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortès C (2001) Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem 77:1085–1096
Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O’Dowd BF (2000) Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74:34–41
Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M (2000) Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 275:21061–21067
Masri B, Morin N, Pedebernade L, Knibiehler B, Audigier Y (2006) The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J Biol Chem 281:18317–18326
Azizi Y, Faghihi M, Imani A, Roghani M, Zekri A, Mobasheri MB, Rastgar T, Moghimian M (2015) Post-infarct treatment with [Pyr(1)]apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in rats. Eur J Pharmacol 761:101–108
Seyedabadi M, Goodchild AK, Pilowsky PM (2002) Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci 101:32–38
Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S (2002) The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun 291:1208–1212
Mishra A, Kohli S, Dua S, Thinlas T, Mohammad G, Pasha MA (2015) Genetic differences and aberrant methylation in the apelin system predict the risk of high-altitude pulmonary edema. Proc Natl Acad Sci USA 112:6134–6139
Jászberényi M, Bujdosó E, Telegdy G (2004) Behavioral, neuroendocrine and thermoregulatory action of apelin-13. Neuroscience 129:811–816
De Mota N, Lenkei Z, Llorens-Cortes C (2000) Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 72:400–407
O’Carroll AM, Selby TL, Palkovits M, Lolait SJ (2000) Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta 1492:72–80
Telegdy G, Adamik A, Jászberényi M (2013) Involvement of neurotransmitters in the action of apelin-13 on passive avoidance learning in mice. Peptides 39:171–174
Han RW, Xu HJ, Zhang RS, Wang R (2014) The role of apelin-13 in novel object recognition memory. Peptides 62:155–158
Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256:675–677
Wallenstein GV, Vago DR (2001) Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem 75:245–252
Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC (1997) Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 17:5196–5205
Handford Charlotte E, Tan Shawn, Lawrence Andrew J, Kim Jee Hyun (2014) The effect of the mGlu5 negative allosteric modulator MTEP and NMDA receptor partial agonist d-cycloserine on Pavlovian conditioned fear. Int J Neuropsychopharmacol 17:1521–1532
Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) A Stereotaxic Atlas of the Rat Brain. Plenum Press, New York
Quirk GJ, Russo GK, Barron JL, Lebron K (2000) The role of ventromedial prefrontal cortex inthe recovery of extinguished fear. J Neurosci 20:6225–6231
Lv SY, Qin YJ, Wang HT, Xu N, Yang YJ, Chen Q (2012) Centrally administered apelin-13 induces depression-like behavior in mice. Brain Res Bull 88:574–580
Choe W, Albright A, Sulcove J, Jaffer S, Hesselgesser J, Lavi E, Crino P, Kolson DL (2000) Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J Neurovirol 6:S61–S69
Kleinz MJ, Skepper JN, Davenport AP (2005) Immunoeytochemical localization of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept 126:233–240
Telegdy G, Jászberényi M (2014) Transmitter mediation of the anxiolytic action of apelin-13 in male mice. Neurobiology of learning and memory. Behav Brain Res 263:198–202
Xu N, Wang H, Fan L, Chen Q (2009) Supraspinal administration of apelin-13 induces antinociception via the opioid receptor in mice. Peptides 30:1153–1157
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Wang XQ, Li XL, Wang GW (2013) Effect of prefrontal infralimbic cortex GABAA receptor agitating on passive avoidance memory consolidation of rats. Dongwuxue Yanjiu 34:589–595
Walf AA, Koonce CJ, Frye CA (2015) Progestogens’ effects and mechanisms for object recognition memory across the lifespan. Behav Brain Res 294:50–61
Izquierdo I, Furini CR, Myskiw JC (2016) Fear memory. Physiol Rev 96:695–750
Preethi J, Singh HK, Venkataraman JS, Rajan KE (2014) Standardised extract of Bacopa monniera (CDRI-08) improves contextual fear memory by differentially regulating the activity of histone acetylation and protein phosphatases (PP1α, PP2A) in hippocampus. Cell Mol Neurobiol 34:577–589
Cook DR, Gleichman AJ, Cross SA, Doshi S, Ho W, Jordan-Sciutto KL, Lynch DR, Kolson DL (2011) NMDA receptor modulation by the neuropeptide apelin: implications for excitotoxic injury. J Neurochem 118:1113–1123
O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL (2007) Apelin an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem 102:1905–1917
DuPont CM, Coppola JJ, Kaercher RM, Lindquist DH (2014) Impaired trace fear conditioning and diminished ERK1/2 phosphorylation in the dorsal hippocampus of adult rats administered alcohol as neonates. Behav Neurosci 128:187–198
Gao C, Gill MB, Tronson NC, Guedea AL, Guzmán YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J (2010) Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus 20:1072–1082
Johnson LR, McGuire J, Lazarus R, Palmer AA (2012) Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology 62:638–646
Acknowledgments
This study was supported by A Project Supported by National Natural Science Foundation of China (81171281), A Project supported by Scientific Research Fund of Hunan Provincial Education Department (14C0128).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, L., Luo, H., Huang, F. et al. Apelin-13 Impaires Acquisition but Not Consolidation or Expression of Contextual Fear in Rats. Neurochem Res 41, 2345–2351 (2016). https://doi.org/10.1007/s11064-016-1948-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1948-3