Abstract
Rare earth elements (REEs) are used in many fields for their diverse physical and chemical properties. Surveys have shown that REEs can impair learning and memory in children and cause neurobehavioral defects in animals. However, the mechanism underlying these impairments has not yet been completely elucidated. Lanthanum (La) is often selected to study the effects of REEs. The aim of this study was to investigate the spatial memory impairments induced by lanthanum chloride (LaCl3) and the probable underlying mechanism. Wistar rats were exposed to LaCl3 in drinking water at 0 % (control, 0 mM), 0.25 % (18 mM), 0.50 % (36 mM), and 1.00 % (72 mM) from birth to 2 months after weaning. LaCl3 considerably impaired the spatial learning and memory of rats in the Morris water maze test, damaged the synaptic ultrastructure and downregulated the expression of p-MEK1/2, p-ERK1/2, p-MSK1, p-CREB, c-FOS and BDNF in the hippocampus. These results indicate that LaCl3 exposure impairs the spatial learning and memory of rats, which may be attributed to disruption of the synaptic ultrastructure and inhibition of the ERK/MSK1 signaling pathway in the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) include fifteen lanthanides, scandium and yttrium. As a result of their various properties, REEs have been widely used as industrial materials [1, 2]. Because REEs are capable of stimulating the growth of vegetables, crops, livestock, poultry and aquaculture, they are also used as important trace fertilizers and feedstuffs in agriculture [3, 4]. In addition, REEs can be used to treat chronic renal failure (CRF) due to their pharmacological properties [5–7], and long-term treatment with REEs can reduce mineral and bone abnormalities in rats with CRF [8]. With extensive application, more and more REEs enter the environment and eventually accumulate in the human body through the inhalation of atmospheric particles or ingestion via food. Therefore, it is important to clarify the environmental and biological effects of REEs.
REEs are non-essential elements for living organisms. Surveys of children in regions with high levels of REEs demonstrated that mental age and IQ were much lower than in children in regions with low levels of REEs [9, 10]. Experimental data also have shown that REEs damage neurobehavioral performance in animal [11, 12]. La, a widely used REE, is one of the most reactive REEs and is often selected to explore the neurological adverse effects of REEs. A study reported that daily injection with 2 mg/kg LaCl3 from E9 to E16 could significantly impair long-term memory in day-old chick [13]. He et al. [14] also confirmed that the behavioral performance of rats in the Morris water maze test could be significantly damaged by chronic exposure to LaCl3. These findings indicated that La could adversely affect learning and memory. This impairment might be attributable to the homeostatic disturbance among trace elements, enzyme activities or neurotransmitters and excessive neuronal apoptosis in the brain [15]. However, the mechanisms underlying these effects remain largely unclear.
Synaptic plasticity refers to the change in neurotransmitter transmission between two neurons or synapses, which is often accompanied by changes in synaptic morphology and function. Synaptic plasticity is believed to be the cellular mechanism underlying memory [16]. Synaptic plasticity or consolidation of memory requires gene transcription and subsequent de novo protein synthesis in neurons [17–19]. We have previously shown that LaCl3 exposure induced a significant impairment in spatial memory. This impairment of memory formation was paralleled by alterations in NF-κB signaling pathways and might be associated with decreased expression of hippocampal brain-derived neurotrophic factor (BDNF) and c-FOS in rats [20, 21]. The interacting signalling pathways that underlie the long-term spatial memory impairments caused by LaCl3 exposure remain unclear. Extracellular signal-regulated kinases (ERKs) play a key role in the long-lasting modification of synaptic efficacy and the formation of LTM [22–24]. ERKs can be activated by MEKs (MAPK kinases) and then phosphorylate MSK1 [25, 26]. Given that MSK1 is a potent CREB kinase [27] and that phosphorylated CREB can initiate the transcription of its target genes, including the expression of key proteins involved in synaptic plasticity and memory consolidation in the hippocampus, such as c-FOS and BDNF [28–30], it may be speculated that phosphorylation of the ERK/MSK1 signaling pathway and subsequent transcription of c-FOS and BDNF provide important clues to elucidate the mechanism underlying the memory impairments caused by LaCl3.
In this study, we investigated the effects of LaCl3 exposure on spatial learning and memory by observing the behavioral performance of rats in the Morris water maze test and ultrastructural alterations of synapses in the hippocampus. Furthermore, we measured the expression levels of p-ERK1/2, p-MEK1/2, p-MSK1 p-CREB, c-FOS and BDNF in the hippocampus. Our study provides further support for the impact of LaCl3 exposure on spatial memory and indicates for the first time that such impairments may be associated with inhibition of the ERK/MSK1 signaling pathway.
Materials and Methods
Animals
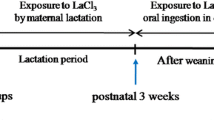
Wistar rats weighing 240–260 g were obtained from the Center for Experimental Animals of China Medical University (license number: SYXK-2003-0013). Rats were housed under standard laboratory conditions at a environmental temperature of 23 ± 1 °C at a 12/12 h light/dark cycle and humidity of 55 ± 5 %. Animals were observed for 1 week before mating (female:male = 1:1). After the pups were born, maternal rats were exposed to LaCl3 (Sinopharm Chemical Reagent Co., Ltd, China) in distilled drinking water at four different doses: 0 % (control, 0 mM), 0.25 % (18 mM), 0.50 % (36 mM), and 1.00 % (72 mM). The pups were exposed to LaCl3 by parental lactation for postnatal 3 weeks and then by oral ingestion in drinking water at 0, 0.25, 0.50 and 1.00 % for 2 months. The pups were then used in the following experiments. All experiments and surgical procedures were approved by Animal Care and Use Committee of China Medical University.
Determination of La Content
The pups were weighed and deeply anesthetized with an intraperitoneal injection of urethane (10 %). Perfusion with physiological saline was performed before decapitation. The hippocampus samples were rapidly removed and placed into Teflon tubes on ice. Then, 4 ml of 65 % nitric acid and 2 ml of H2O2 were added into the tube, which was sealed with a Teflon lid and placed into a steel bomb. The samples were sealed and heated to 180 °C for 5 h to decompose. Finally, the decomposed tissue samples were diluted with 2 % nitric acid, and the La contents were measured using inductively coupled plasma mass spectrometry.
Morris Water Maze Test
The water maze test was carried out according to the method described by previous investigation [31]. Behavioral training and testing were performed in a black circular pool (1.5 m in diameter, 60 cm high) filled water (22 ± 2 °C) to a depth of 30 cm. A hidden platform (15 cm in diameter) was submerged 1.5 cm below the water surface and located in the center of the second quadrants of the pool. Starch powder was used as opaque. The pups were initially habituated to the experimental room for 2 h prior to the experiment. Each pup was trained at the same time for 5 successive days. The animals were placed in the pool facing the interior wall from four quadrants in each trial. If the pup reached the submerged platform within 60 s, it was kept there for approximately 20 s. If the pup failed, it was guided and placed on the platform for 20 s. This procedure was adopted because spaced training is a better paradigm for facilating memory consolidation. After 5 days of training, all the pups rested for 1 week. Next, the spatial navigation and probe test were performed. Behavioral parameters such as escape latency, pathlength, and navigation path of the animals were measured. After 1 h, the platform was removed, and then the pups were placed in three randomly assigned locations of the pool, and allowed to swim for 60 s. The number of target quadrant crossings, time spent and distance travelled in the target quadrant, time from first entry to the target quadrant and track plots were measured for each trial as indicators of memory ability. The performance of the pup in each trial was monitored by a video camera, mounted on the ceiling above the center of the pool and analyzed using ANY-maze video tracking software (Stoelting Co., IL, USA).
Transmission Electron Microscope
The rats were anesthetized deeply with urethane (10 %), and perfused with physiological saline followed by 4 % paraformal-dehyde before decapitation. Next, 1 mm3 tissue sample from the hippocampus CA1 pyramidal cell layer were removed, placed quickly on ice within and fixed in 2.5 % glutaraldehyde at 4 °C for 24 h. The samples were rinsed 3 times with PBS (PH = 7.2–7.4), post-fixed with 1 % osmium tetroxide for 2 h at room temperature, and dehydrated in graduated ethanol solutions from 50–70 % (each for 30 min) and 100 % acetone (three times, each for 10 min). The samples were placed on a Teflon support, embedded in pure epoxy resin at 60 °C for 1 h and polymerized at 80 °C overnight. The samples were trimmed and sectioned into slices (70 nm thick), and stained with uranyl acetate and lead citrate. The ultrastructure of the hippocampal synapses was observed under a H-7650 type transmission electron microscope (Hitachi, Japan). ImagePro Plus 6.0 image (Media Cybernetics., Silver Spring, MD, USA) analysis software was used to quantify the thickness of the postsynaptic density (PSD), the length of the active zone, and the synaptic curvature. Fifty synapses from each group were examined. The thickness of the PSD and the length of the active zone were measured according to the method of Guldner [32], and the synaptic curvature (expressed by the ratio of the synaptic arch length and chord length) was determined according to Jones and Devon [33].
Western Blotting
Rats were killed after the Morris water maze test, and the hippocampus was immediately dissected and homogenized in ice-cold buffer (NaCl, 50 mM; pepstatin, 0.03 mM; aprotinin, 0.6 μM; PMSF, 1 mM; EDTA, 5 mM; EGTA, 10 mM; Tris–HCl, 50 mM, pH7.5; NaF, 2 mM; NaVO3, 1 mM) on ice and incubated at 4 °C overnight. The sample was then centrifuged at 25,000g for 1 h. The supernatant was used for analysis and protein concentrations were measured using the test kit (Baotaike, China). Forty micrograms of protein from each sample was separated by 10 % SDS-PAGE and then transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5 % bovine serum albumin in PBST (0.1 % Tween 20, 100 mM Tris–HCl and 0.9 % NaCl) at 4 °C for 2 h, then washed three times with PBST and incubated with primary antibody overnight at 4 °C. The primary antibodies used in this study were MEK1/2, phospho-MEK1/2, ERK1/2, phospho-ERK1/2, MSK1, phospho-MSK1 CREB, p-CREB, c-FOS (Cell Signaling Technology, Inc., Danvers, MA, USA) and BDNF (Stanta Cruze, USA). All the antibodies were diluted 1:1,000. The membranes were washed four times in PBST and incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase (1:1,000; Zhong shan Biotechnology, Inc., China) at room temperature for 1.5 h. The reaction products were visualized using ECL reagents (Zhongshan Biotechnology, Inc., China) with autoradiography and the protein bands were recorded with BioMax MR films (Kodak Scientific Imaging Systems, USA). The relative protein expression levels were normalized to GAPDH. The integrated density value (IDV) was used to represent the level of protein expression.
Quantitative Real-Time PCR (qRT-PCR)
The hippocampus was dissected and stored at −80 °C before detection. Frozen tissues were homogenized in TRIzol Reagent (Invitrogen Inc., Burlington, Canada) and total RNA was resuspended in 20 μl RNA deionized water. The RNA concentrations were evaluated by nucleic acid determination. The first strand complementary DNA (cDNA) was synthesized with the PrimeScript® RT reagent Kit (Takara, Dalian, China) and SYBR green fluorescence dye attached to double stranded DNA was used to assess the PCR product levels. Primer sequences (Takara, Dalian, China) were designed using Primer Express software (Applied Biosystems) and the sequences were listed as bellow: BDNF (forward: 5′-ATCCACTGAGCAAAGCCGAAC-3′, reverse: 5′-CAGCCT TCATGC AACCGAAGTA-3′). c-FOS (forward: 5′-AGCCGACTCCTTCTCCAGCA-3′, reverse: 5′-AAGTTGGCACTAGAGACGGACAGAT-3′). GAPDH (forward: 5′-GGAGATTACTGCCCTGGCTCCTA-3′, reverse: 5′-GACTCATCGTACTCCTGCTTGCTG-3′). Real-time PCR amplification was performed at least in triplicate using a 7500HT real-time PCR system (Applied Biosystems). The reaction mixture contained SYBR Green PCR Master Mix 1× (Applied Biosystems), 100 ng of cDNA template and 0.5 pmol/μl of each forward and reverse primers in a final volume of 25 μl. The PCR cycle parameters were 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The results were analyzed by the 2−ΔΔCt method as described previously [34] and GAPDH was used to normalize the relative amounts of mRNA expression.
Data Analysis
Statistical analysis was carried out by SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The results were expressed as the mean ± SD. Differences among groups were evaluated with a one-way ANOVA followed by post hoc Newman-Keuls tests. P < 0.05 were considered to be the statistically significant level.
Results
La Contents in the Hippocampus
The La contents in the hippocampus of rats were shown in Fig. 1. The La contents in the hippocampus of the LaCl3-treated groups were significantly higher than those of the control group (F = 85.36, P < 0.05). With the dose increasing, the accumulation of La in the hippocampus increased. La accumulation in the hippocampus was positively related with administrated-dose of La (r = 0.945, P < 0.05).
The Effect of La on Spatial Learning and Memory in Rats
The effects of La on spatial learning and memory in rats were shown in Fig. 2. As shown in Fig. 2a, there were no differences in the escape latency between the three LaCl3-treated groups and the control on the first or second days of training (Day 1, F = 1.838, P = 0.163; Day 2, F = 0.512, P = 0.678). On the third training day, the escape latencies of the LaCl3-treated groups were significantly longer than that of the control group (Day 3: F = 11.066, P < 0.05). On the fourth training day, the escape latencies of the 0.5 and 1.0 % LaCl3-treated groups were significantly longer than that of the control group (Day 4: F = 12.651, P < 0.05). However, on the fifth day, there were no differences in the escape latencies between the three LaCl3-treated groups and the control (F = 1.044, P = 0.382). The mean distance traveled during the trial was also measured for each group. As shown in Fig. 2b, on the first and second days of training, there were no differences in the total traveled distance between the three LaCl3-treated groups and the control (Day 1, F = 0.442, P = 0.725; Day 2, F = 1.736, P = 0.182). On the third training day, the traveled distance of the 1.00 % LaCl3-treated group was significantly longer than that of the control group (Day 3, F = 2.546, P = 0.076). On the fourth training day, the traveled distance of the 0.50 and 1.00 % LaCl3-treated group was significantly longer than that of the control group (Day 4, F = 4.152, P = 0.015). On the fifth days, there was no differences in the traveled distance between the three LaCl3-treated groups and the control (Day 5, F = 2.108, P = 0.122), which suggested that there were similar learning performances in the LaCl3-treated groups and the control at this time.
Effect of La on the spatial memory of rats in Morris water maze. a, b Bar graphs for latency and traveled distance in the five training days of rats. c, d Bar graphs for latency and traveled distance in place navigation test. e, f Bar graphs for number of target quadrant crossing and time spent in target quadrant in spatial probe test. d, h Bar graphs for traveled distance in target quadrant and latency of first entry to the target quadrant. i, j Representative images for search tracks in spatial probe test. Each column represented as the mean ± SD. n = 8 for each group. Compared with the control group, *P < 0.05; compared with 0.25 % LaCl3 group, #P < 0.05; compared with 0.50 % LaCl3 group, ∆P < 0.05
To test the spatial memory of the rats, they were subjected to two tests after 1 week of rest. The effect of La on spatial memory was shown in Fig. 2c–j. In the place navigation test, the rats were placed in the water maze, and the escape latency and traveled distance were recorded again. As shown in Fig. 2c, d, the escape latencies of the three LaCl3-treated groups were significantly higher than that of the control group. The traveled distance of the 0.50 and 1.00 % LaCl3-treated groups were significantly longer than that of the control group. Both the escape latency and traveled distance were positively correlated with the doses of LaCl3 (r = 0.969, P < 0.05 and r = 0.956, P < 0.05, respectively). As shown in Fig. 2i, diverse search strategies were presented among four groups, the rats in control group could find the platform directly, but the rats in LaCl3-treated groups didn’t show this tendency. Even though the 0.25 and 0.50 % LaCl3-treated rats could finally reach onto the platform, they spent longer time and swam in a much more indirect road than control rats. In the spatial probe test, the number of crossing target quadrant for all groups was shown in Fig. 2e. It was significantly lower in all three LaCl3-treated groups compared to the control group (F = 43.70, P < 0.05). As shown in Fig. 2f, the LaCl3-treated rats spent significantly less time in the target quadrant compared to the control rats (F = 16.99, P < 0.05). The traveled distance in target quadrant was shown in Fig. 2g. The LaCl3-treated rats swam significantly shorter compared to the control rats (F = 43.70, P < 0.05). As shown in Fig. 2h, the LaCl3-treated rats spent significantly more time for the first entry to the target quadrant. In Fig. 2j, the rats in different groups seemed to perform distinct search strategies. The control rats used a focus searching technique, passing through the target quadrant repeatedly. In contrast, the LaCl3-treated rats, especially 1.0 % LaCl3-treated rats, swam around aimlessly and even seldom swam into the target quadrant. All above results indicated that LaCl3-treated rats have weaker spatial memory than the control rats. LaCl3 might impair the spatial memory of rats.
The Effect of La on Synaptic Ultrastructure in the Hippocampus of Rats
The ultrastructural parameters were shown in Table 1. There were significant differences in the average of the thickness of PSD (F = 511.421, P < 0.05), the length of active zone (F = 813.654, P < 0.05), and the synaptic curvature (F = 14.055, P < 0.05) between groups. The PSD in the LaCl3-treated groups were thicker, the active zone became shorter and the synaptic curvature turned smaller (Fig. 3). These indicated that LaCl3 exposure could injure the synaptic ultrastructure in hippocampus.
Effect of La on the ultrastructure of synapses in hippocampus. Representative photomicrographs of synapses in the hippocampus CA1 area from control (a), 0.25 % LaCl3, (b) 0.50 % LaCl3 (c), and1.00 % LaCl3 (d). The position of the thickness of PSD is indicated by a black arrow; the length of active zone are indicated by two white triangles; the synaptic curvature is indicated by a white arrow. Scale bar = 0.2 μm
The Effect of La on the Expression of MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1 in the Hippocampus of Rats
The effects of La on the expression of MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1 in the hippocampus of rats were shown in Fig. 4. p-MEK1/2 expression in the three LaCl3-treated groups was significantly lower than in the control group (F = 933.265, P < 0.05), and equal to 94.60, 72.24 and 53.15 % of control value, respectively. The p-ERK1/2 expression in the three LaCl3-treated groups was significantly lower than in the control group (F = 1.098, P < 0.05), and equivalent to 83.57, 69.64 and 58.78 % of control value, respectively. Meanwhile the p-MSK1 expression in the three LaCl3-treated groups was also significantly lower than that of control group (F = 364.917, P < 0.05), and equivalent to 87.13, 61.20 and 32.52 % of control value, respectively. Expressions of p-MEK1/2, p-ERK1/2, p-MSK1 in the hippocampus were negatively correlated with the dose of La (r p-MEK1/2 = −0.954, P < 0.05, r p-ERK1/2 = −0.995, P < 0.05 and r p-MSK1 = −0.974, P < 0.05, respectively). However, there were no significant differences in the expression of MEK1/2, ERK1/2 and MSK1 between the three LaCl3-administrated groups and the control group (F p-MEK1/2 = 0.365, P = 0.779, F p-ERK1/2 = 0.631, P = 0.603 and F p-MSK1 = 1.824, P = 0.175, respectively). These data indicated that LaCl3 exposure significantly down-regulated the p-MEK1/2, p-ERK1/2 and p-MSK1 expression in the hippocampus of rats.
The effects of La on the expression of MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1 in the hippocampus after Morris water maze. a–c Representative western blotting photograms for MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1 in the hippocampus of control and different LaCl3-treated rats. d-i IDV was used as the parameter representing the expression levels of MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1. The expression levels of MEK1/2, p-MEK1/2, ERK1/2, p-ERK1/2, MSK1 and p-MSK1 were all normalized to GAPDH. Each column represented the mean ± SD, n = 6 for each group; Each determination was assayed in triplicate. Compared with control group, *P < 0.05; compared with 0.25 % LaCl3 group, #P < 0.05; compared with 0.50 % LaCl3 group, ∆P < 0.05
The Effect of La on the Expression of CREB and p-CREB in the Hippocampus of Rats
As shown in Fig. 5, the p-CREB expression in the three LaCl3-treated groups was significantly lower than in control group (F = 607.627, P < 0.05), and equal to 90.45, 71.44 and 56.80 % of the control value, respectively. Expression of p-CREB was negatively related with the dose of LaCl3 (r = −0.999, P < 0.05). However, there were no significant differences of the CREB levels in the hippocampus between the LaCl3-treated groups and control group. This data indicated that expression of p-CREB in the hippocampus of rats was considerably inhibited by LaCl3 exposure.
The effects of La on the expression of CREB, p-CREB in the hippocampus after Morris water maze. a Representative western blotting photograms for CREB, p-CREB in the hippocampus of the control and LaCl3-treated rats. b, c IDV was used as the parameter representing the expression levels of CREB, p-CREB. The expression levels of CREB, p-CREB were normalized to GAPDH. Each column represented the mean ± SD, n = 6 for each group; each determination was repeated three times. Compared with control group, *P < 0.05; compared with 0.25 % LaCl3 group, #P < 0.05; compared with 0.50 % LaCl3 group, ∆P < 0.05
The Effect of La on the Expression of c-FOS and BDNF in the Hippocampus of Rats
To investigate the effect of La, the c-FOS and BDNF mRNA and protein expression levels in the hippocampus of rats were measured, and the results are shown in Fig. 6. The c-FOS mRNA expression (Fig. 6a) in the three LaCl3-treated groups was significantly lower than in the control group (F = 385.245, P < 0.05), and equal to 0.760, 0.513 and 0.284 of control value, respectively. The BDNF mRNA expression (Fig. 6d) in the three LaCl3-treated groups was significantly lower than in control group (F = 135.344, P < 0.05), and equal to 0.912, 0.669 and 0.430 of control value, respectively. The c-FOS protein expression (Fig. 6b, c) in the three LaCl3-treated groups decreased significantly compared with the control group (F = 1.848, P < 0.05), and equal to 83.27, 63.67 and 53.99 % of the control value, respectively. The BDNF protein expression (Fig. 6e, f) in the three LaCl3-treated groups was significantly lower than that in the control group (F = 1.015, P < 0.05), and equal to 86.00, 61.85 and 49.64 % of control value, respectively. The c-FOS mRNA and protein expression in the hippocampus was negatively correlated with the dose of LaCl3 (r c-FOS mRNA = −0.995, P < 0.05 and r c-FOS pro = −0.985, P < 0.05, respectively). The of BDNF mRNA and protein expression in the hippocampus were also negatively correlated with the dose of LaCl3 (r BDNF mRNA = −0.978 and P < 0.05; r BDNF pro = −0.985, P < 0.05, respectively). These results indicated that the expression levels of c-FOS and BDNF in the hippocampus of rats were significantly impaired by LaCl3 exposure.
The effects of La on the expression of c-FOS and BDNF in the hippocampus after Morris water maze. a, b Representative c-FOS and BDNF mRNA expression in the hippocampus of the control and LaCl3-treated rats. The value of 2−∆∆Ct was used as the parameter representing the expression levels of c-FOS and BDNF mRNA. The expression levels of c-FOS and BDNF mRNA were normalized to GAPDH mRNA levels. Each column represented the mean ± SD, n = 6 for each group. c–f Representative western blotting photograms and expression levels for c-FOS and BDNF in the hippocampus of control and LaCl3-treated rats. IDV was used as the parameter indicating the expression levels of c-FOS and BDNF. The expression levels of c-FOS and BDNF were normalized to GAPDH. Each column represented the mean ± SD, n = 6 for each group; All experiment were assayed in duplicate. Compared with control group, *P < 0.05; compared with 0.25 % LaCl3 group, #P < 0.05; compared with 0.50 % LaCl3 group, ∆P < 0.05
Discussion
La can penetrate blood–brain barrier and cause long-term accumulation in the brain [35]. Oral exposure to 40 mg/kg/day LaCl3, from postnatal week 4–6 months [15] or from maternal gestation to 6 months of age [14] caused a significant accumulation of La in the hippocampus of rats. Our previous research showed a higher accumulation of La in the hippocampus of pups treated with LaCl3 [36, 37]. Without exception, dose-related La retention in the hippocampus of rats was also observed in the current study. As the hippocampus is the key region of the brain for spatial learning and memory, we hypothesized that La accumulation in the hippocampus might have negative impact on behavioral performance of animals.
Water maze test is regarded as one of typically behavioral tasks to assess hippocampus-dependent spatial learning and memory abilities of rodents. Poucet suggested that the rat hippocampus contained place cells, which could allow the rapid acquisition of novel information underlying the spatial learning and memory [38]. Several studies demonstrated that oral exposure to LaCl3 significantly reduced the behavioral performance of rats in the Morris water maze test [14, 15]. In agreement with these studies, we also found that LaCl3 exposure could impair the learning and memory of rats in Morris water maze test. In the middle of the training/learning period, LaCl3-treated pups found the platform more slowly than the control ones. By the end of training period, there were similar learning performances among all groups, which indicated that LaCl3-treated rats learned slowly than did control ones. To assess long-term spatial memory, we allowed rats to rest for 1 week after training. Then the animals were subjected to two tests. In the place navigation test, LaCl3-treated rats spent more time and swam in a much more indirect pattern to reach the platform, and the escape latency and traveled distance were both longer than the corresponding values for control animals. A dose-dependent impairment in LTM was observed. In the spatial probe test, LaCl3-treated rats showed fewer target quadrant crossing number, stayed less time and swam shorter distance in the target quadrant than control rats. Furthermore, the time that they took for first entry to the target quadrant was much longer. Although the rats in the LaCl3-treated groups displayed the same focused searching technique as the controls during the probe trial, they searched the inappropriate location. These results demonstrate that La accumulation in hippocampus could impair spatial memory of animals.
The pups in this study were exposed to LaCl3 from birth to 2 month after weaning, which is an important period of central nervous system development. A neuropathological study using Nissl staining showed that La caused pyramidal cell breakdown and loss in the CA3 subfield of the hippocampus [14]. A previous report from our laboratory also showed that La exposure could lead to the significant decrease of neuronal Nissl bodies in the CA1, CA3, and DG areas of the hippocampus [39]. In this study, we found that LaCl3 could significantly change the synaptic ultrastructure of the hippocampus of rats, including shorter active synaptic zone, uneven synaptic curvature and thinner postsynaptic density, indicating reduced synaptic activity. Because the synaptic ultrastructure is important for synapse plasticity and memory consolidation, the spatial memory impairment provoked by LaCl3 might be, at least partially, attributed to abnormal alteration of the synaptic ultrastructure in the hippocampus during brain development.
Memory consolidation involves a series of important post-synaptic cellular events, including the activation of certain protein kinases, transcription and new protein synthesis and modulation of neuronal signaling pathways [40, 41]. Increasing evidences indicates that the activation of CREB is pivotal for synaptic plasticity facilitation and LTM formation [42–45]. It has been reported that up-regulation of CREB activity could improve long-term spatial memory in rats [46, 47]. However, down-regulation of CREB activity using antisense methodology or disruption of CREB-dependent transcription significantly impaired spatial memory in rats [48, 49]. The activation of CREB involves several kinase-mediated signaling pathways. Among them, the ERK/MSK1 signaling pathway is mainly related to CREB phosphorylation [50–52]. In the CNS, the key role of ERK has been shown to be the control of gene regulation required for neuronal plasticity and long-term memory [53, 54]. ERK, which includes ERK1 and ERK2, belongs to the members of MAPK family [55, 56]. Following its activation, ERK becomes uncoupled from MEK1/2 to enter the nucleus and activate MSK1. MSK1 has been shown to be a genuine CREB kinase, which can translocate to neuronal nuclei to phosphorylate CREB at Ser-133 and thereby activate nuclear factor [26, 57, 58]. It has been reported that activation of CREB by forskolin requires ERK activation, while MEK inhibitors prevent the activation of CREB [50]. Moreover, in MSK1-deficient mice, CREB activation is almost completely eliminated [59]. In this study, although the expression levels of MEK1/2, ERK1/2, MSK1 and CREB in the hippocampus of LaCl3-treated rats were normal, their phosphorylated forms were significantly reduced from the control levels. La clearly did not modulate the expression of these proteins, but inhibited their phosphorylation. Because phosphorylated-MEK1/2, ERK1/2, and MSK1 are the activated forms of the proteins and are essential for the activation of CREB, these results suggested that LaCl3 could inhibit the activation of CREB by suppressing the ERK/MSK1 signaling pathway.
Activated CREB can bind to the promoters of a variety of nuclear genes, such as c-FOS and BDNF, and regulate their expression. c-FOS is a member of immediate early genes (IEGs). IEG expression is one of the cellular events involved in the mechanism underlying long-term modification of synaptic responses [60], and therefore, it plays an important role in the modification of synaptic plasticity [61] and memory consolidation [30, 62]. It has been reported that c-FOS enables neurons to transcribe and translate the corresponding proteins which affects long-term cellular responses [63, 64] and LTM [30, 65, 66]. It has been confirmed that ERK can mediate the up-regulation of c-FOS expression through phosphorylating CREB [67–69]. BDNF is a critical neurotrophic factor for learning and memory [70–72]. BDNF was up-regulated in the hippocampus after spatial training [73], and inhibition of BDNF expression in the brain blocked spatial memory formation [74]. Studies have demonstrated that long-term potentiation (LTP) facilitation induced by BDNF requires activation of the ERK/MAPK-CREB cascade in hippocampus [75, 76]. In our study, the expression levels of c-FOS and BDNF mRNA and protein in the hippocampus of LaCl3-treated pups were significantly lower than the control values. Furthermore, the expression levels of two signal transduction proteins were negatively correlated with the dose of LaCl3. Therefore, synaptic plasticity and memory consolidation may be inevitably affected. Based on these findings, we suggest that repression of the ERK/MSK1 signaling pathway may be an important mechanism underlying LaCl3-impaired learning and memory.
Until now, the mechanisms by which La causes changes in synaptic plasticity and the ERK/MSK1 signaling pathway have remained unclear. Studies have shown that La3+ has similar properties to calcium (Ca2+) [77]. La3+ could initially recognize Ca2+ binding sites on the cell membrane [78], leading to an increase in intracellular free Ca2+ content. It is well known that Ca2+ mediates a variety of physiological responses in neurons. However, an overload of intracellular Ca2+ in neurons could impair mitochondrial functions, potentially leading to apoptosis [79, 80]. Excessive apoptosis can cause certain injuries, including neurological impairment [81]. Hence, we speculated that dysregulation of intracellular Ca2+ regulation and thus excessive mitochondrial apoptosis might be involved in the mechanism underlying LaCl3-induced changes in synaptic plasticity and the ERK/MSK1 signaling pathway. However, the specific mechanisms must be confirmed in future research.
Based on the aforementioned results, we arrive at the conclusions: (1) the spatial learning and memory of young rats was impaired after LaCl3 exposure from birth to 2 month after weaning. (2) Synaptic ultrastructure was negatively affected by LaCl3 exposure. (3) LaCl3 esposure significantly reduced the phosphorylation of MEK1/2, ERK1/2, MSK1, CREB and the expression of c-FOS and BDNF in a dose-dependent manner. The present data demonstrated that the mechanism underlying spatial memory impairment caused by LaCl3 likely involved the p-MEK1/2, p-ERK1/2, p-MSK1, p-CREB, c-FOS, BDNF signalling pathway in the hippocampus of rats.
References
Eliseeva SV, Bunzli JC (2010) Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev 39:189–227
Goll D, Kronmuller H (2000) High-performance permanent magnets. Die Naturwissenschaften 87:423–438
Chen ZYZX (2006) Differences of acceptable daily intake and discussions of agricultural security of rare earths. J Ecol Rural Environ 22:93–96
Guo BS, Zhu, WM, Xiong BK, Liu Z, Wu ZM (1990) Rare earths in agriculture. China Agricultural Science and Technology Press, Beijing, p 11
Lacour B, Lucas A, Auchère D, Ruellan N, de Serre Patey NM, Drüeke TB (2005) Chronic renal failure is associated with increase tissue deposition of lanthanum after 28-day oral administration. Kidney Int 67:1062–1069
D’Haese PC, Spasovski GB, Sikole A et al (2003) A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl (85):S73–S78
Hutchison AJ, Speake M, Al-Baaj F (2004) Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol Dial Transplant 19:1902–1906
Damment S, Secker R, Shen V et al (2011) Long-term treatment with lanthanum carbonate reduces mineral and bone abnormalities in rats with chronic renal failure. Nephrol Dial Transplant 26:1803–1812
Fan G, Yuan Z, Zheng H et al (2004) Study on the effects of exposure to rare earth elements and health-responses in children aged 7–10 years. Wei Sheng Yan Jiu 33:23–28
Zhu WF, Xu SQ, Zhang H, Shao PP, Wu DS, Yang WJ, Feng J (1996) Investigation of children intelligence quotient in REE mining area: bio-effect study of REE mining area in South Jiangxi. Chin Sci Bull 41:914–916
Briner W, Rycek RF, Moellenberndt A et al (2000) Neurodevelopmental effects of lanthanum in mice. Neurotoxicol Teratol 22:573–581
Feng L, He X, Xiao H et al (2007) Ytterbium and trace element distribution in brain and organic tissues of offspring rats after prenatal and postnatal exposure to ytterbium. Biol Trace Elem Res 117:89–104
Che Y, Cui Y, Jiang X (2009) Effects of lanthanum chloride administration in prenatal stage on one-trial passive avoidance learning in chicks. Biol Trace Elem Res 127:37–44
He X, Zhang Z, Zhang H et al (2008) Neurotoxicological evaluation of long-term lanthanum chloride exposure in rats. Toxicol Sci 103:354–361
Feng L, Xiao H, He X et al (2006) Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol Lett 165:112–120
Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323
Kandel ER (1997) Genes, synapses, and long-term memory. J Cell Physiol 173:124–125
Rainbow TC (1979) Role of RNA and protein synthesis in memory formation. Neurochem Res 4:297–312
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
Zheng L, Yang J, Liu Q et al (2013) Lanthanum chloride impairs spatial learning and memory and downregulates NF-kappaB signalling pathway in rats. Arch Toxicol 87:2105–2117
Yang J, Liu Q, Wu S et al (2011) Effects of lanthanum on the phosphorylation of cAMP response element binding protein and expression of immediate early genes in the hippocampal CA3 area of rats. Wei Sheng Yan Jiu 40(299–303):311
Davis S, Laroche S (2006) Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav 5(Suppl 2):61–72
Peng S, Zhang Y, Zhang J et al (2010) ERK in learning and memory: a review of recent research. Int J Mol Sci 11:222–232
Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317
Rosen LB, Ginty DD, Weber MJ et al (1994) Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12:1207–1221
Brami-Cherrier K, Roze E, Girault JA et al (2009) Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem 108:1323–1335
Deak M, Clifton AD, Lucocq LM et al (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J 17:4426–4441
Heldt SA, Stanek L, Chhatwal JP et al (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12:656–670
Hennigan A, Callaghan CK, Kealy J et al (2009) Deficits in LTP and recognition memory in the genetically hypertensive rat are associated with decreased expression of neurotrophic factors and their receptors in the dentate gyrus. Behav Brain Res 197:371–377
Guzowski JF (2002) Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus 12:86–104
Morris RG, Garrud P, Rawlins JN et al (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Guldner FH, Ingham CA (1980) Increase in postsynaptic density material in optic target neurons of the rat suprachiasmatic nucleus after bilateral enucleation. Neurosci Lett 17:27–31
Jones DG, Devon RM (1978) An ultrastructural study into the effects of pentobarbitone on synaptic organization. Brain Res 147:47–63
Ploski JE, Newton SS, Duman RS (2006) Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. J Neurochem 99:1122–1132
Xiao H, Li F, Zhang Z et al (2005) Distribution of ytterbium-169 in rat brain after intravenous injection. Toxicol Lett 155:247–252
Yang J, Liu Q, Zhang L et al (2009) Lanthanum chloride impairs memory, decreases pCaMK IV, pMAPK and pCREB expression of hippocampus in rats. Toxicol Lett 190:208–214
Zheng L, Yang J, Liu Q et al (2013) Lanthanum chloride impairs spatial learning and memory and downregulates NF-kappaB signalling pathway in rats. Arch Toxicol 87:2105–2117
Poucet B, Save E, Lenck-Santini PP (2000) Sensory and memory properties of hippocampal place cells. Rev Neurosci 11:95–111
Yang J, Liu Q, Wu S et al (2013) Effects of lanthanum chloride on glutamate level, intracellular calcium concentration and caspases expression in the rat hippocampus. Biometals 26:43–59
Cortés-Mendoza J, Díaz de León-Guerrero S, Pedraza-Alva G et al (2013) Shaping synaptic plasticity: the role of activity-mediated epigenetic regulation on gene transcription. Int J Dev Neurosci 31:359–369
Pang PT, Lu B (2004) Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3:407–430
Silva AJ, Kogan JH, Frankland PW et al (1998) CREB and memory. Annu Rev Neurosci 21:127–148
Barco A, Marie H (2011) Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol Neurobiol 44:330–349
Benito E, Barco A (2010) CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33:230–240
Zagaar M, Dao A, Levine A et al (2013) Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampus. Sleep 36:751–761
Mouravlev A, Dunning J, Young D et al (2006) Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc Natl Acad Sci USA 103:4705–4710
Restivo L, Tafi E, Ammassari-Teule M et al (2009) Viral-mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. Hippocampus 19:228–234
Guzowski JF, McGaugh JL (1997) Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA 94:2693–2698
Pittenger C, Huang YY, Paletzki RF et al (2002) Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34:447–462
Davis S, Vanhoutte P, Pages C et al (2000) The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci 20:4563–4572
Impey S, Obrietan K, Wong ST et al (1998) Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21:869–883
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183
Valjent E, Caboche J, Vanhoutte P (2001) Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol 23:83–99
Zhang F, Zhu Q, Xue Q et al (2013) Extra-cellular signal-regulated kinase (ERK) is inactivated associating hippocampal ARC protein up-regulation in sevoflurane induced bidirectional regulation of memory. Neurochem Res 38:1341–1347
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev MMBR 75:50–83
Hardingham GE, Arnold FJ, Bading H (2001) A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci 4:565–566
Blum S, Moore AN, Adams F et al (1999) A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci 19:3535–3544
Arthur JS, Cohen P (2000) MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett 482:44–48
Yang J, Cai Y, Liu Q et al (2009) Effects of lanthanum on memory and expression of c-fos mRNA and c-Fos protein of cerebral cortex in rats. Wei Sheng Yan Jiu 38:348–351
Balamotis MA, Tamberg N, Woo YJ et al (2012) Satb1 ablation alters temporal expression of immediate early genes and reduces dendritic spine density during postnatal brain development. Mol Cell Biol 32:333–347
Abraham WC, Mason SE, Demmer J et al (1993) Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience 56:717–727
Fleischmann A, Hvalby O, Jensen V et al (2003) Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci 23:9116–9122
Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246
Lanahan A, Worley P (1998) Immediate-early genes and synaptic function. Neurobiol Learn Mem 70:37–43
Tischmeyer W, Grimm R (1999) Activation of immediate early genes and memory formation. Cell Mol Life Sci CMLS 55:564–574
Radwanska K, Valjent E, Trzaskos J et al (2006) Regulation of cocaine-induced activator protein 1 transcription factors by the extracellular signal-regulated kinase pathway. Neuroscience 137:253–264
Salzmann J, Marie-Claire C, Le Guen S et al (2003) Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol 140:831–838
Vanhoutte P, Barnier JV, Guibert B et al (1999) Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol 19:136–146
Mattson MP, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27:589–594
Ferreira AG, Scherer EB, da Cunha MJ et al (2011) Physical exercise reverses cognitive impairment in rats subjected to experimental hyperprolinemia. Neurochem Res 36:2306–2315
Alder J, Thakker-Varia S, Black IB (2002) Transcriptional analysis in the brain: trophin-induced hippocampal synaptic plasticity. Neurochem Res 27:1079–1092
Yamada K, Mizuno M, Nabeshima T (2002) Role for brain-derived neurotrophic factor in learning and memory. Life Sci 70:735–744
Mizuno M, Yamada K, He J et al (2003) Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem 10:108–115
Patterson SL, Pittenger C, Morozov A et al (2001) Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron 32:123–140
Ying SW, Futter M, Rosenblum K et al (2002) Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22:1532–1540
Das T, Sharma A, Talukder G (1988) Effects of lanthanum in cellular systems. Rev Biol Trace Elem Res 18:201–228
Lettvin JY, Pickard WF, McCulloch WS et al (1964) A theory of passive ion flux through axon membranes. Nature 202:1338–1339
Duan W, Rangnekar VM, Mattson MP (1999) Prostate apoptosis response-4 production in synaptic compartments following apoptotic and excitotoxic insults: evidence for a pivotal role in mitochondrial dysfunction and neuronal degeneration. J Neurochem 72:2312–2322
Rapizzi E, Pinton P, Szabadkai G et al (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159:613–624
Springer JE (2002) Apoptotic cell death following traumatic injury to the central nervous system. J Biochem Mol Biol 35:94–105
Acknowledgments
This study was funded by the National Natural Science Foundation of China and Science Project of Education Department in Province Liaoning, China. The project numbers are 81072316, 81273117, 81373024, L2010702 and L2012290.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Yang, J., Liu, Q. et al. Lanthanum Chloride Impairs Spatial Memory Through ERK/MSK1 Signaling Pathway of Hippocampus in Rats. Neurochem Res 39, 2479–2491 (2014). https://doi.org/10.1007/s11064-014-1452-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1452-6