Abstract
Purpose
Laser interstitial thermal therapy (LITT) is a minimally invasive cytoreductive treatment option for brain tumors with a risk of vascular injury from catheter placement or thermal energy. This may be of concern with deep-seated tumors that have surrounding end-artery perforators and critical microvasculature. The purpose of this study was to assess the risk of distal ischemia following LITT for deep-seated perivascular brain tumors.
Methods
A retrospective review of a multi-institution database was used to identify patients who underwent LITT between 2013 and 2022 for tumors located within the insula, thalamus, basal ganglia, and anterior perforated substance. Demographic, clinical and volumetric tumor characteristics were collected. The primary outcome was radiographic evidence of distal ischemia on post-ablation magnetic resonance imaging (MRI).
Results
61 LITT ablations for deep-seated perivascular brain tumors were performed. Of the tumors treated, 24 (39%) were low-grade gliomas, 32 (52%) were high-grade gliomas, and 5 (8%) were metastatic. The principal location included 31 (51%) insular, 14 (23%) thalamic, 13 (21%) basal ganglia, and 3 (5%) anterior perforated substance tumors. The average tumor size was 19.6 cm3 with a mean ablation volume of 11.1 cm3. The median extent of ablation was 92% (IQR 30%, 100%). Two patients developed symptomatic intracerebral hemorrhage after LITT. No patient had radiographic evidence of distal ischemia on post-operative diffusion weighted imaging.
Conclusion
We demonstrate that LITT for deep-seated perivascular brain tumors has minimal ischemic risks and is a feasible cytoreductive treatment option for otherwise difficult to access intracranial tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laser interstitial thermal therapy (LITT) is a novel minimally invasive cytoreductive treatment option for both primary and metastatic brain tumors [1,2,3]. LITT offers patients a potentially less morbid means of treating brain tumors when compared to conventional open resection, especially when targeting deep-seated lesions [4]. Given its minimally invasive nature, LITT is an important alternative to consider for difficult-to-access lesions that would otherwise make a patient ineligible or high-risk for a traditional craniotomy [1, 5, 6]. However, inherent to all stereotactic procedures, LITT carries a known risk of direct vascular injury when passing the laser catheter to target. Particular to LITT, there is also potential risk of thermal vascular injury to large vessels or en passant perforating vessels within the ablation zone [3, 7]. Despite the growing use of LITT, the risk of direct or thermal vascular injury to en passant blood vessels leading to distal ischemia has not been previously reported in the literature.
Blood supply via the internal carotid and vertebral arteries provides intracranial circulation through the anterior cerebral, middle cerebral, and posterior cerebral arteries. The deep penetrating branches (such as the recurrent artery of Huebner, anterior choroidal artery, and medial and lateral lenticulostriate arteries), represent terminal branches that supply well-defined deep structures within the brain. Given the vascular nature of their location, lesions that involve the insula, thalamus, basal ganglia, and anterior perforated substance are high risk for open surgical resection due to the significant risk of perforator stroke and hemorrhage during an attempted tumor resection [8, 9].
With LITT, temperatures are monitored via intra-operative MRI thermometry to ensure adequate ablation and minimize over-heating surrounding tissue [1, 2, 10]. When achieving cytoreduction via laser ablation, nearby larger blood vessels and cerebrospinal fluid cisterns act as heat sinks to mitigate the spread of tissue injury [7, 11]. However, the energy emitted and its ablative effects on brain tissue have led to complications, including symptomatic intracerebral hemorrhage, cerebral edema, and new onset seizures in a fraction of cases [10,11,12]. While these complications from LITT have previously been reported for peripheral lesions along the convexity of the brain, the effects of LITT on deep brain structures is yet to be described.
We present the first case series of patients treated with LITT focusing on the ischemic and hemorrhagic complications associated with ablating deep-seated perivascular tumors, namely, within the insula, basal ganglia, thalamus, and anterior perforated substance.
Methods
Patient selection
A retrospective review of patients who underwent LITT between 2013 and 2022 were collected from two large-volume, academic brain tumor centers. Patients were included in the study if they had a unifocal non-lobar lesion located in the insula, basal ganglia, thalamus, and anterior perforated substance. For these deep-seated lesions, patients were selected for LITT according to surgeon preference and in accordance with multidisciplinary tumor board recommendations. This retrospective study was approved by the institutional review board (MRI Volumetrics of Patients Treated with Laser Ablation, IRB #9176) with a waiver of patient consent prior to data collection and analysis.
LITT procedure
LITT was performed in a 1.5-T intraoperative-MRI suite. The Neuroblate Laser Ablation System (Monteris Medical Corporation, Plymouth, Minnesota) or Visualase Thermal Therapy System (Medtronic Inc, Minneapolis, Minnesota) were used per surgeon preference and availability. Stereotactic laser placement was done using the BrainLAB VarioGuide system (BrainLab, Munich, Germany) or the Robotic Surgical Assistant (ROSA) ONE Brain robot (MedTech, France). Ablations were performed according to protocol as previously described [13].
Data acquisition
Clinical data was gathered for each patient via independent chart review, including demographic information, medical history, pre-operative coagulation studies, and Karnosfsky performance status (KPS) pre-operatively and at 6-week follow up. Three-dimensional anatomical contouring with BrainLab Elements software (BrainLab, Munich, Germany) was used for volumetric analysis with pre- and post-operative MRI sequences as previously described [13]. All patients had post-operative MRIs obtained within 24-h of LITT. All images were independently reviewed by the primary surgeons, principal authors, and neuroradiology staff.
Outcomes
The primary outcomes of this study were any clinical or radiographic evidence of post-operative non-ablation related ischemia. Clinical ischemic events were defined as any neurological deficit not related to the pathology treated. Radiographic ischemia was defined as any evidence of ischemia beyond the ablation zone on post-operative MRI imaging.
Results
Pre-operative characteristics are shown in Table 1. Between 2013 and 2022, 61 patients (mean age 48 years, 44% female) were treated with LITT for deep brain lesions. Pre-operative comorbidities included seizures (n = 29, 48%), diabetes (n = 8, 13%), hypertension (n = 24, 39%), hyperlipidemia (n = 11, 18%), coronary artery disease (n = 5, 8%), prior stroke (n = 2, 3%), asthma or COPD (n = 8, 13%), prior deep vein thrombosis or pulmonary embolism (n = 4, 7%), and current tobacco use (n = 9, 15%). Coagulation studies were noted to be within normal limits in all patients (PT 13 ± 1, PTT 26 ± 3, INR 1.02 ± 0.09, platelets 225 ± 69). At baseline, most patients (n = 54, 89%) had a favorable KPS ≥ 80 prior to surgery.
Operative characteristics and pathology results are shown in Table 2. Of the patients ablated, the principle pathologic tumor was found in the insula (n = 31, 51%), thalamus (n = 14, 23%), basal ganglia (n = 13, 21%), and anterior perforated substance (n = 3, 5%). The majority of tumors were located on the left side (n = 36, 59%). Most lesions were treated using a single ablation catheter (n = 37, 61%). However, in select cases, two (n = 14, 23%), three (n = 9, 15%), and four (n = 1, 2%) LITT catheters were used based on surgical approach and tumor size. A biopsy was performed prior to ablation in 45 (74%) of patients, with some patients not undergoing a biopsy if they underwent an intended second or third stage and had previously established tissue diagnosis. The mean tumor volume was 19.6 cm3 (range 0.9–93.3) with a median of 13.6 cm3 (IQR 5.6, 20.5). The mean ablation volume was 11.1 cm3 (range 1.0–28.1) with a median of 9.8 cm3 (IQR 5.3, 16.3). The mean extent of ablation was 75.8% ± 29.6% with a median extent of ablation at 91.9% (IQR 29.6, 100.0). Most of the pathology treated were primary central nervous system tumors (n = 56, 91.8%), followed by metastatic brain tumors (n = 5, 8.2%).
Postoperative characteristics are shown in Table 3. No areas of distal ischemia were seen on diffusion weighted imaging (DWI) sequences within the entire cohort. An example of a right sided insular LITT ablation with pre-, intra-, and post-operative imaging is shown in Figs. 1 and 2. In total, nine patients had post-operative complications requiring intensive care management: two patients had focal weakness after a clinically significant hematoma, two patients had obstructive hydrocephalus requiring external ventricular drains and shunt placement, three patients had expressive aphasia from cerebral edema requiring hyperosmolar therapy, and two patients had a decline in mental status from hyponatremia secondary to cerebral salt wasting. Cumulatively, most patients (n = 49, 82%) had a favorable post-operative KPS ≥ 80 at 6-week follow-up. A decline in KPS—10 was seen in 8 patients, and—20 in 3 patients on 6-week follow-up. 7 patients were readmitted to the hospital in less than 30 days for progression of disease and 2 required a return to OR. The median length of stay was 2 days with 82% of patients being discharged home, 16% to rehab, and 2% to hospice.
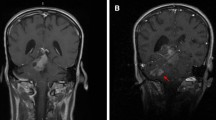
An example of a right sided insular glioma that underwent a three catheter LITT ablation. Pre-operative axial T1-sequence (A), axial T2-sequence (B), axial FLAIR sequence (C), and sagittal, axial, and coronal T1-weighted (D–F) imaging is shown. Intra-operative sagittal, axial, and coronal T1-weighted imaging demonstrates the extent of LITT ablation. Note the critical vasculature at risk of injury near the site of the insular tumor during ablation, such as the M2 perforators and lenticulostriate arteries
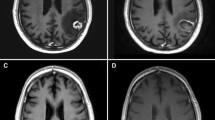
Sequential diffusion weighted imaging of the same patient as shown in Fig. 1. No sites beyond the area of ablation demonstrate any ischemic changes
Discussion
In our experience, LITT for deep-seated brain tumors in anatomic areas with critical perforating blood vessels did not result in any evidence of post-operative distal ischemia. All patients included in our study had deep-seated non-lobar lesions that were involving perforating vessels, all of which supplied critical functional networks that would invariably be at risk during open surgical resection—a risk repeatedly described in the literature. For example, Hou et al. reported that of their patient cohort undergoing a craniotomy for insular glioma resections, 58.7% showed acute ischemic changes on DWI sequences, with 12% developing new motor deficits [9]. This is in stark contrast to the lack of ischemic events seen in our cohort. Even with artery-preserving strategies, vasospasm and ischemic events may still occur during open resection for deep-seated tumors [8, 14,15,16]. Although the presence of CSF spaces and large blood vessels are known to act as heat sinks for thermal spread during LITT, it has been unclear whether peri-tumor microvasculature would be able to safely tolerate ablation [7, 10, 11, 17, 18]. Our study is the first to report that perforating vasculature can safely be preserved when ablating deep-seated tumors within the basal ganglia, thalamus, insula, and anterior perforated substance.
However, our study does show that there is a risk of hemorrhage associated with LITT when targeting deep-seated tumors. Lesion characteristics, such as size, geometry and location play a significant role in LITT complications—which is pertinent to this study, given our heterogeneous collection of tumors of significantly different sizes that underwent either LITT alone or, for larger tumors, LITT combined with surgical resection [19].For example, the first case of hemorrhage was a 62-year-old female who underwent a left thalamic LITT ablation and was noted to have right sided hemiparesis post-operatively; imaging demonstrated an ablation tract hemorrhage that extended into the internal capsule. The second case of hemorrhage was a 62-year-old male who underwent a left insular LITT ablation combined with surgery with no evidence of stroke or hemorrhage on post-operative imaging. However, the patient’s hospital course was complicated by a gastrointestinal bleed requiring blood transfusions and management of uncontrolled hypertension—on post-operative day seven, the patient had a decline in mental status and imaging demonstrated a large intracerebral hemorrhage at the site of ablation. Complications such as cerebral hemorrhage, cerebral edema, cerebral spinal fluid leak, and neurologic deficits are associated with LITT, albeit at a significantly reduced rate compared to open craniotomy [10, 12, 20, 21]. In our cohort, additional complications requiring further management included obstructive hydrocephalus, aphasia, and cerebral salt wasting. Nonetheless, the median length of stay was 2 days with 82% of patients being discharged home. These results suggest that LITT is a feasible cytoreductive treatment option for deep-seated perivascular brain tumors.
Given that our multi-institutional cohort was made up of lesions with differing pathology, size and extent of treatment that were treated by a number of surgeons using two different LITT systems, we do not believe there was a common LITT strategy among the treatments that explains our findings. In general, each LITT ablation targeted the area of tumor that would provide the most cytoreductive benefit and highest pathology yield—for contrast enhancing lesions, ablation of contrast enhancement was prioritized; for non-contrast enhancing lesions, safe maximum ablation of FLAIR abnormality was prioritized. Ablation strength and timing was at the discretion of the treating surgeon with no suspected commonality leading to better outcomes. Although we believe that no particular ablation strategy was responsible for our findings, there are general principles of LITT treatment that should be followed. Given the potential risk for vascular injury from laser fiber placement and ablation, principles of safe stereotaxis apply. For example, Pruitt et al. described their lessons learned with complication avoidance by co-registering a contrasted MRI with CT angiography to reduce the risk of intracranial hemorrhage [7]. The caveat is that the small perforating vessels are generally not visible on MRI or CTA. By planning out the trajectory so that it is not crossing any nearby blood vessels or crossing multiple pial planes, there is an inherent risk reduction in vascular complications (Fig. 3). With neuronavigational software, our institutions rely on the use of the “probe’s eye view” to follow the barrel of the laser down to target and directly visualize any surrounding vasculature. Whenever possible, the use of fewer laser fibers also decreases the chance for vascular injury.
Three-dimensional anatomical contouring with BrainLab Elements software (BrainLab, Munich, Germany), including overlayed tumor sectioning and trajectory planning. Pre-operative axial FLAIR with tumor delinated in red (A), three-dimensional LITT trajectory overview (B), pre-operative axial T1-weighted contrast enhanced MRI with overlayed trajectories (each color representing a different LITT ablation plan) (C), sagittal T1-weighted MRI with frontal trajectories shown (D) and post-operative DWI imaging showing ablation directly adjacent to large MCA and microvascular lenticulostriate vessels, both of which were undamaged and without evidence of distal microvasculature ischemia (E)
With LITT as a viable treatment option, it is imperative to study the overall survival (OS) and progression free survival (PFS) in patients with these lesions. Unfortunately, limited data is available with two notable studies by Schwarzmair et al. and Sloan et al. reporting increased OS of patients who had undergone LITT for recurrent GBMs [3, 22]. A systematic review by Montemurro et al. pooled data from 17 studies on OS and PFS in patients with recurrent glioblastomas who underwent LITT. The review spanned 2000–2020 and included 219 patients. Their study on LITT treatment of recurrent GBM showed a slightly decreased OS and PFS as compared with similar studies. This was suggested to be caused by a higher number of patients in their cohort with deep-seated lesions (in the thalamus, basal ganglia, and midbrain), where it may be harder to achieve gross total resection during first surgery [23]. Although the pooled data from 17 studies highlighted LITT as a promising intervention for increasing OS and PFS, these studies did not focus on the location and accessibility of the lesions.
On the other hand, Mohammadi et al. conducted a series on 34 patients with difficult-to-access high grade gliomas to determine PFS post-LITT treatment. Tumor locations varied between lobar, thalamic, insular, and corpus collosum. Outcomes of their study indicated progression in 71% of the cohort and a median PFS of 5.1 months. Despite this, increased extent of ablation coverage was identified as a positive prognostic factor for PFS in tumors with a volume less than 10 cm [3, 4]. Another study by Shah et al. comprised a cohort of six patients who had LITT on deep brain legions, where mean PFS was found to be an encouraging 14.3 months. The median extent of ablation was reported at 98%. A literature review done by Shah and colleagues on patients with deep brain legions who had LITT (from 2012 to 2016) showed a mean PFS of 4.2 months (2–11.5 months), although data is sparse regarding survival post-LITT and more extensive follow-up is needed to determine the efficacy of LITT on prolonged OS and/or PFS [24].
Limitations
This study has inherent limitations due to its retrospective nature and reliance on available, previously documented records. Given the selective patient population with deep-brain lesions, there is a small sample size included in the study especially for the anterior perforated substance tumors. Key statistics and comparison groups, such as cortical lesions that underwent LITT, were not included in the study. Certainly, there are differences between each patient’s vascular anatomy and location of each lesion, rendering generalizations and comparisons amongst individual patients difficult. Nevertheless, the overall trend of using LITT for these deep-seated lesions has shown to be minimal risk for causing distal ischemic stroke. Further prospective studies and larger cohorts are needed to verify our findings.
Conclusion
This study suggests that MRI-guided LITT is a feasible procedure for deep-seated pathology in which small perforating vessels may be at risk for causing distal ischemia. Recognizing the limitations of the data, it seems that lesions that would be at elevated risk for stroke during resection due to injury to perforating vessels can be ablated with minimal risk of distal ischemia. In this multi-center retrospective case series, patients were able to achieve high rates of extent of ablation (median 92%). Whether or not this translates to increased OS and PFS as is seen in patients undergoing resection remains to be seen. Future studies could prospectively compare open resection to laser ablation both in terms of survival and post-operative complications. For these difficult-to-reach tumors, the inherent risks of craniotomy with surgical resection render the procedure high risk or impossible. LITT may serve as a viable alternative to achieve meaningful cytoreduction. Further research efforts should continue to focus on the safety profile of LITT in deep-seated tumors as well as the impact on OS and PFS following LITT ablation compared to craniotomy alone. In addition, studies evaluating neurocognitive outcomes and quality of life, especially compared to traditional open resection, are sorely needed.
Data availability
The dataset utilized during this study is available from the corresponding author upon reasonable request.
Abbreviations
- CSF:
-
Cerebral spinal fluid
- KPS:
-
Karnofsky performance status
- LITT:
-
Laser interstitial thermal therapy
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion weighted imaging
- OS:
-
Overall survival
- PFS:
-
Progression free survival
References
Kamath AA, Friedman DD, Akbari SHA et al (2019) Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery 84(4):836–843. https://doi.org/10.1093/neuros/nyy375
Patel P, Patel NV, Danish SF (2016) Intracranial MR-guided laser-induced thermal therapy: single-center experience with the visualase thermal therapy system. J Neurosurg 125(4):853–860. https://doi.org/10.3171/2015.7.JNS15244
Sloan AE, Ahluwalia MS, Valerio-Pascua J et al (2013) Results of the neuroblate system first-in-humans phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg 118(6):1202–1219. https://doi.org/10.3171/2013.1.JNS1291
Mohammadi AM, Hawasli AH, Rodriguez A et al (2014) The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med 3(4):971–979. https://doi.org/10.1002/cam4.266
Thomas JG, Rao G, Kew Y, Prabhu SS (2016) Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus 41(4):E12. https://doi.org/10.3171/2016.7.FOCUS16234
Willie JT, Laxpati NG, Drane DL et al (2014) Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery 74(6):569–584. https://doi.org/10.1227/NEU.0000000000000343
Pruitt R, Gamble A, Black K, Schulder M, Mehta AD (2017) Complication avoidance in laser interstitial thermal therapy: lessons learned. J Neurosurg 126(4):1238–1245. https://doi.org/10.3171/2016.3.JNS152147
Gempt J, Förschler A, Buchmann N et al (2013) Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg 118(4):801–808. https://doi.org/10.3171/2012.12.JNS12125
Hou Z, Huang Z, Li Z et al (2022) Incidence of ischemic complications and technical nuances of arteries preservation for insular gliomas resection. Front Surg 9:956872. https://doi.org/10.3389/fsurg.2022.956872
Patel B, Yang PH, Kim AH (2020) The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int J Hyperth 37(2):35–43. https://doi.org/10.1080/02656736.2020.1783461
Salem U, Kumar VA, Madewell JE et al (2019) Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 19(1):65. https://doi.org/10.1186/s40644-019-0250-4
Holste KG, Orringer DA (2020) Laser interstitial thermal therapy. Neuro-Oncol Adv 2(1):vdz035. https://doi.org/10.1093/noajnl/vdz035
Fadel HA, Haider S, Pawloski JA et al (2022) Laser interstitial thermal therapy for first-line treatment of surgically accessible recurrent glioblastoma: outcomes compared with a surgical cohort. Neurosurgery 91(5):701–709. https://doi.org/10.1227/neu.0000000000002093
Loit MP, Rheault F, Gayat E et al (2019) Hotspots of small strokes in glioma surgery: an overlooked risk? Acta Neurochir (Wien) 161(1):91–98. https://doi.org/10.1007/s00701-018-3717-3
Schaller C, Zentner J (1998) Vasospastic reactions in response to the transsylvian approach. Surg Neurol 49(2):170–175. https://doi.org/10.1016/s0090-3019(97)00283-8
Gempt J, Krieg SM, Hüttinger S et al (2013) Postoperative ischemic changes after glioma resection identified by diffusion-weighted magnetic resonance imaging and their association with intraoperative motor evoked potentials. J Neurosurg 119(4):829–836. https://doi.org/10.3171/2013.5.JNS121981
Hwang BY, Eremiev A, Palla A et al (2021) Association of intraoperative end-tidal carbon dioxide level with ablation volume during magnetic resonance–guided laser interstitial thermal therapy for mesial temporal lobe epilepsy. J Neurosurg 137(2):427–433. https://doi.org/10.3171/2021.9.JNS211554
Paul A, Paul A (2018) Computational study of photo-thermal ablation of large blood vessel embedded tumor using localized injection of gold nanoshells. J Therm Biol 78:329–342. https://doi.org/10.1016/j.jtherbio.2018.10.021
Beechar VB, Prabhu SS, Bastos D et al (2018) Volumetric response of progressing Post-SRS lesions treated with laser interstitial thermal therapy. J Neurooncol 137(1):57–65. https://doi.org/10.1007/s11060-017-2694-3
Zeller S, Kaye J, Jumah F et al (2021) Current applications and safety profile of laser interstitial thermal therapy in the pediatric population: a systematic review of the literature. J Neurosurg Pediatr 1(aop):1–8. https://doi.org/10.3171/2021.2.PEDS20721
Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D (2006) Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods 3(2):99–108. https://doi.org/10.1038/nmeth844
Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W et al (2006) MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol 59(2):208–215. https://doi.org/10.1016/j.ejrad.2006.05.010
Montemurro N, Anania Y, Cagnazzo F, Perrini P (2020) Survival outcomes in patients with recurrent glioblastoma treated with laser interstitial thermal therapy (LITT): a systematic review. Clin Neurol Neurosurg 195:105942. https://doi.org/10.1016/j.clineuro.2020.105942
Shah AH, Burks JD, Buttrick SS, Debs L, Ivan ME, Komotar RJ (2019) Laser interstitial thermal therapy as a primary treatment for deep inaccessible gliomas. Neurosurgery 84(3):768–777. https://doi.org/10.1093/neuros/nyy238
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Each author listed contributed to the study design. H.F., J.P., M.S. and S.H. each contributed to data collection, analysis, and draft revisions. R.K, E.L, A.M, M.I, A.R., S.K. and I.L provided commentary on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This retrospective study was approved by the institutional review board (MRI Volumetrics of Patients Treated with Laser Ablation, IRB #9176) with a waiver of patient consent prior to data collection and analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reese, J.C., Fadel, H.A., Pawloski, J.A. et al. Laser interstitial thermal therapy for deep-seated perivascular brain tumors is not associated with distal ischemia. J Neurooncol 166, 265–272 (2024). https://doi.org/10.1007/s11060-023-04546-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04546-6