Abstract
Background
Glioma is a challenging malignant tumor with a low survival rate and no effective treatment. Recently, ganciclovir, an antiviral drug, combined with gene therapy and its own antiviral ability, has been proposed as a potential treatment for glioma. However, there are differences in the results of various clinical trials. In this study, we conducted a systematic review and meta-analysis to evaluate the efficacy of ganciclovir in treating glioma.
Methods
We searched databases such as PubMed, EMBASE, and Cochrane Library before March 30, 2023. The search terms included glioma, ganciclovir, valganciclovir and treatment. Calculated 1, 2 and 4-year survival rate by risk difference (RD), and overall survival (OS) by odds ratio (OR).
Results
Five randomized controlled trials (RCTs) with a total of 606 high-grade glioma patients were included. The results showed that ganciclovir can improve 2-yeaer (RD = 0.179, 95% CI 0.012–0.346, P = 0.036) and 4-year survival rate (RD = 0.185, 95% CI 0.069–0.3, P = 0.002) and OS (OR 2.393, 95% CI 1.212–4.728, P = 0.012) compared with the control group.
Conclusions
This meta-analysis showed that ganciclovir significantly improved the prognosis of glioma patients. Therefore, we suggest that more cases of ganciclovir as a glioma treatment can be conducted, or a large clinical trial can be designed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is one of the most aggressive and difficult-to-treat malignant tumors worldwide [1]. This disease originates from neuroglial cells and is classified into grades 1–4 based on the degree of malignancy [2]. Glioblastoma is the most common subtype of high-grade gliomas, which also includes astrocytoma and oligodendroglioma [1, 2]. Currently, there is still a lack of comprehensive understanding of the etiology, diagnosis, and treatment of gliomas, which belongs to the cancer with high recurrence rate and low survival rate [3]. Statistics showed that the median survival time for glioblastoma patients is only 14.4–20.5 months [1], and the 2-year survival rate is even lower than 20% [4]. Moreover, data from the United States between 2000 and 2014 shows that the 5-year survival rate for glioblastoma patients is only 5.8% [3].
The common treatment methods for gliomas are surgery, radiotherapy, and chemotherapy [5], and the first-line chemotherapy drug for gliomas is temozolomide [6]. However, most glioma patients do not have a O6‐methylguanine–DNA methyltransferase promoter which makes the efficacy of temozolomide is not significant [7, 8]. In addition, Scherm et al. conducted a systematic review and meta-analysis on 12 RCTs of targeted drugs in patients with glioma [9], and finally concluded that none of the included targeted drugs provides the improvement of OS. What’s more, because of problems such as drug resistance and safety, cancer patients are not suitable for repeated use of the same prescription [10], which lead to a lack of safe and effective treatment. Although various institutions have been developing new treatments such as Immunotherapy or Oncolytic virus [11, 12], the price of new drugs is relatively high, and their efficacy is still in the research stage, which means it may cause financial toxicity for socioeconomically vulnerable individuals [13]. Currently, drug repurposing in cancer has become more popular [14]. Drugs that have been approved by the Food and Drug Administration (FDA) for other indications are retested to understand if they can be a cancer treatment. Drug repurposing has three major advantages [14], including reducing R&D costs, reducing the financial burden on patients, and discovering new therapeutic targets. Therefore, it allows patients to have other treatment options that are less financially stressful and effective at the same time [14].
Ganciclovir and its derivative valganciclovir [15] have been used in clinical trials to be the treatment of glioma since 2000 [10, 16,17,18,19,20]. Recently, because of the rapid development in biotechnology and bioinformatics, this drug is once again receiving attention in the field of cancer treatment [21, 22]. Ganciclovir is an antiviral drug and therefore has anti cytomegalovirus (CMV) properties [23]. Because over 90% of cancer patients have been found to have CMV nucleic acid and protein in their bodies [24], CMV is becoming a new target in cancer treatment. Some studies have indicated that glioma patients with low-level CMV infection would have longer median survival than those with high-level CMV infection (33 vs. 13 months, P = 0.036), as well as higher two-year survival rates (63.6 vs. 17.2%, P = 0.003) [25, 26]. Furthermore, ganciclovir was also used to combine with gene therapy to treat glioma. Gene therapy implants target gene is into the body to achieve the goal of killing cancer cells [27, 28]. The mechanism of ganciclovir combined with gene therapy is clear, and there are many clinical cases demonstrating its safety and efficacy as a treatment method [29]. Its primary mechanism involves the introduction of a virus thymidine kinase gene (Vtk), also known as a suicide gene, into the target cells via a viral vector. After the gene is expressed and produces thymidine kinase, the enzyme will phosphorylate ganciclovir into a toxic substance that inhibits DNA synthesis and induces apoptosis in the cells [30]. Moreover, phosphorylated ganciclovir can diffuse through gap junctions to the neighboring cells that have not been implanted with the Vtk gene, inducing death in the surrounding cells. This bystander effect further strengthens the therapeutic effect of the ganciclovir-Vtk system [29, 31].
Although multiple trials have been conducted to investigate the use of ganciclovir in the treatment of gliomas, there are still some differences in treatment efficacy among these studies. Since no one has integrated and discussed the results of randomized controlled trials so far, we decided to conduct a systematic review and meta-analysis to know the improvement of prognosis of ganciclovir in glioma. The focus will be on survival indicators, including OS and 1-, 2-, and 4-year survival rates, and to objectively describe the overall efficacy and evaluation of ganciclovir in the treatment or adjuvant therapy of gliomas.
Methods
This systematic review and meta-analysis have been registered in the prospective registration protocol available online (PROSPERO identifier CRD42023407070) [32], and the reporting will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33]. (For the PRISMA checklist, see Supplementary Figs. S1 and S2 online).
Study selection
In this meta-analysis, we systematically searched four databases including PubMed (MEDLINE), Cochrane Library, Embase, and ClinicalTrials.gov from the start of the studies to March 30th, 2023. During the search process, we did not consider the language or publication year of the articles and used the following keywords: “Ganciclovir” or “Valganciclovir,” “Cancer” or “Tumor,” and “Glioma” or “Glioblastoma” or “Brain Cancer.” If needed, the articles would be filtered by “Clinical Trial” and “Randomized Controlled Trial”. Additionally, we manually searched the reference lists of all included articles to ensure that all studies meeting the criteria were included. Two reviewers (NWH and HHC) independently reviewed and selected papers for study inclusion. Any discrepancies or conflicts were adjudicated by a third reviewer (CCT).
Eligibility criteria
This study will select the RCTs that meet all of the following criteria: (1) the trial includes adult patients with a confirmed diagnosis of high-grade glioma; (2) one of the groups in the trial receives treatment with either ganciclovir or valganciclovir; (3) the study provides data on at least one of the following: OS and 1-, 2-, or 4-year survival rates; (4) the original report of the trial must be in English; (5) the full text of the trial is obtainable and contains sufficient information; (6) in cases where two trials include overlapping patient populations, the most recent and complete report will be used. This study excludes single-arm trials, case reports, animal or in vitro experiments, non-English publications, reports with insufficient information, and other studies that do not involve treatment of glioma. All studies that meet the inclusion criteria will be independently reviewed by two other reviewers, and any screening disagreements will be resolved through discussion.
Data extraction
We extracted the following information from studies that met the above criteria: author, publication year, country of study, participant age, gender distribution, glioma type, follow-up period, sample size, and treatment type. When the necessary data was provided in figures or tables, we extracted the required survival indicators, including overall survival data, hazard ratio with its 95% confidence interval, and survival rates (1-, 2-, and 4-year). All data will be independently extracted by two reviewers (NWH and HHC) to ensure the certainty of the values. The results of data extraction will be available in Supplementary Table S1 online.
Quality appraisal
The quality of each article was scored by two of us (CTC and HHC) using Oxford Centre for EBM Levels of Evidence (OCEBM) and selected Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) to measure risk of bias [34]. RoB 2 is a risk of bias measurement tool specific to randomized controlled trials. After being assessed against five different domains, the results will indicate that the risk of bias of each included study is judged as “low risk of bias”, “some concerns” or “high risk of bias” [34].
Statistical analysis
The meta-analysis software used in this study was Comprehensive Meta-Analysis (CMA) version 2.0, developed by Biostat. All survival indicators were integrated analyzed based on sufficient data. OS data were extracted according to the method of McGrath et al., where necessary data were obtained from the figures in the articles and converted to mean and standard deviation for analysis [35, 36]. Hazard ratios (HR) and survival rates (1-, 2-, and 4-year) were directly extracted from the articles or Kaplan–Meier plots. The integration results of OS were presented as odds ratios (OR), and the risk difference (RD) was used to present the survival rates for each year [37]. If the heterogeneity among studies was low, a fixed-effect model was used; if the heterogeneity was high, a random-effect model was used to evaluate the effect size [38,39,40]. The heterogeneity evaluation methods are I2 test and Cochran’s Q test, where I2 statistic > 50% and P < 0.05 indicated high heterogeneity among studies. Then, we used funnel plots and Egger’s test to detect publication bias [41, 42]. The standard for statistical significance in all statistical tests was set at P < 0.05 (two tailed).
Other analysis
Other analysis was conducted by the methods we previously mentioned. We conducted subgroup analysis on OS according to glioma were newly diagnosed or recurrent and sensitivity analysis according to glioma subtype proportion.
Results
Study selection and included studies
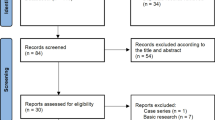
The detailed process of the PRISMA standard flowchart was illustrated in Fig. 1, According to our search strategy, a total of 1842 articles were collected in the initial database search and reference screening, and 52 duplicate articles were removed. Finally, after assessment by reviewers, we identified five RCTs [10, 17,18,19,20] published from 2000 to 2016, involving a total of 606 patients with glioma. 304 patients who received ganciclovir or valganciclovir as the experimental group; in addition, 302 patients who received placebo or first-line (standard) treatment regimens served as the control group. Studies that might appear to meet the inclusion criteria, but which did not contain the control group (one arm study) were be excluded[43,44,45,46]. Table 1 shows the basic data of 5 RCTs. Three studies included patients with newly diagnosed glioma [17, 19, 20], and two studies included patients with recurrent glioma [10, 18]. In the subtypes of glioma, almost 90% of patients are diagnosed with glioblastoma. Among the five studies included, one was conducted in Asia (China), while the remaining four were conducted in Europe (Germany, Sweden, Finland). In terms of drug use, four studies employed ganciclovir [10, 17, 18, 20], while one study employed valganciclovir [19]. Only two literatures [17, 19] had experimental groups receiving standard therapy in addition to ganciclovir or valganciclovir, while the other three [10, 18, 20] did not have additional standard therapy. Data on OS and 1-year survival rate were provided in all five RCTs, 2-year survival rate was reported in four RCTs [10, 18,19,20], 4-year survival rate was reported in three RCTs [10, 18, 19]. The quality of each study is rated as the high quality. For the risk of bias, only one study with “some concerns” and other studies are all “low risks of bias”. (See Supplement Fig. S3 online).
Survival rate
In the experimental group, the overall mean 1-year survival rate was 59.2% (180/304) compared with 46.2% in the control group (140/302). The integrated results of RD using a random-effect model (Fig. 2a and Table 2) were 0.112 (95% CI − 0.076 to 0.3, P = 0.244), with an I2 of 80.5% (P = 0.0004). The overall mean 2-year survival rate in the experimental group was 30% (54/180), while in the control group it was 11.8% (21/178). The integrated results of RD using a random-effect model (Fig. 2b and Table 2) were 0.179 (95% CI 0.012–0.346, P = 0.036), with an I2 of 66.8% (P = 0.029). When the observation time was extended to 4-year (as there was a lack of literature tracking beyond the 5th year, the 4th year was selected), the overall mean 4-year survival rate in the experimental group was 21.3% (13/61), while in the control group it was 3.3% (2/61). The integrated results of RD using a fixed-effect model (Fig. 2c and Table 2) were 0.185 (95% CI 0.069–0.3, P = 0.002), with an I2 of 0% (P = 0.508). One study that tracked patients until the 5th year also showed only patients in the experimental group survived [19]. Therefore, it can be inferred that ganciclovir has a better effect on 2-year and 4-year survival rates.
Overall survival
Only two studies provided HR data, and the integrated results (See Supplementary Fig. S4 online) for forest plot) showed that the HR was 0.513 (95% CI 0.093–2.818, P = 0.442), calculated using a random-effect model (I2 = 94.1%, P = 0.00004). Due to high heterogeneity and insufficient number of studies, we instead adopted the approach used by McGrath et al. [35] to extract survival data from each study and calculate the mean and standard deviation [35]. The OS results (Fig. 3a and Table 3) was OR 2.393 (95% CI 1.212–4.728, P = 0.012), calculated using a random-effect model (I2 = 74.9%, P = 0.003). The results showed that the use of ganciclovir has a positive effect on the treatment of glioma. For the subgroup analysis, we found that the patients belonged to newly diagnosed glioma with lower OS, so we took out 2 studies[10, 18] which contain the patients belonged to recurrent glioma to test the effect of meta-analysis (Fig. 3b, c and Table 3). The subgroup analysis result for newly diagnosed group was OR 1.481 (95% CI 1.084–2.023, P = 0.014), calculated by fixed-effect model (I2 = 59.1%, P = 0.087). It can be seen that the result of ganciclovir in newly diagnosed glioma is slightly worse than that in recurrent glioma, but the positive result still exists. For the recurrence group, the result calculated by the fixed effect model was OR 6.51 (95% CI 2.786–15.215, P = 0.000015, I2 = 0%, P = 0.376), which represented a very strong positive correlation between ganciclovir and the OS of recurrent glioma.
Sensitivity analysis
Although the proportion of patients with glioblastoma (GBM) in this meta-analysis exceeded 90%, we found that two studies in the original text did not allocate the proportion of GBM patients well in the experimental and control groups [10, 18]. Compared with the control group, the proportion of GBM patients in the experimental group was 63.6% vs. 81.8% and 76.4% vs. 94.7%, respectively. Fortunately, Immonen et al.[18] provided additional comparisons of GBM patients in which the treatment effect in the experimental group was still better and there was a significant difference (median survival of 55.3 weeks vs. 37.0 weeks, P = 0.02).
Safety
Among the five selected articles, three of them reported that there were no severe adverse events (grade 3 or higher according to Common Terminology Criteria for Adverse Events; CTCAE) observed in either the experimental or control groups, but there were occasional mild to moderate common adverse reactions such as fever, vomiting, leukopenia, and rash [10, 19, 20]. On the other hand, the other two phase III clinical trials mentioned more information on severe adverse events [17, 20]. Westphal et al. [20] reported that the experimental group had more cases of two severe adverse events compared to the control group, which were cerebral hemorrhage (8 cases vs.1 case) and thrombosis (16 cases vs.13 cases). Rainov et al. [17], on the other hand, indicated that severe adverse events were more common in the experimental group than in the control group, with 81 patients (65%) and 66 patients (52%), respectively. However, both of these phase III clinical trials explained that these severe adverse events rarely led to death and were difficult to confirm a direct relationship with either ganciclovir.
Publication bias
Funnel charts showed no significant publication bias in all outcomes (Fig. 4). Egger’s test also indicated no significant publication bias in all outcomes. (See Supplement Table S2) This finding suggested that no serious publication bias affected the results of this meta-analysis.
Discussion
Main findings and interpretation of the evidence
Ganciclovir and its derivative valganciclovir are considered to have two approaches for the treatment of glioma: one is based on the ability to resist viruses [16], and the other one is to combine with gene therapy [30]. Many studies have indicated that cancer patients infected with cytomegalovirus generally have a poorer prognosis [25, 26]. Therefore, cytomegalovirus may be an important target in the treatment of cancer, and valganciclovir is usually chosen in the treatment of antiviral ability, because valganciclovir is relatively convenient, and patients can take it orally medication [15, 47]. In contrast, ganciclovir is currently used in combination with gene therapy and administered intravenously due to its poor oral availability [15, 47]. The purpose of gene therapy is to use a viral vector to deliver the thymidine kinase gene into the body, which activates ganciclovir, allowing it to inhibit DNA synthesis and suppress rapidly proliferating cancer cells [48]. Nowadays, ganciclovir has received more attention in the treatment of glioma, and some researchers have published updated clinical trials or retrospective studies [8, 49], but there are differences in survival outcomes, so it is necessary to conduct a detailed systematic review and meta-analysis on this topic.
In this meta-analysis, we identified five eligible RCTs with a total of 606 patients included for the analysis. The integrated results showed that ganciclovir had a positive effect on survival outcomes of glioma patients, including 2-year survival rate, 4-year survival rates and OS. However, the statistical difference in 1-year was not significant. In terms of OS, due to only two studies that provided HR and the high heterogeneity (I2 = 94.1%), we calculated odds ratio (OR) by survival time data. After integrating the results of the five RCTs, we confirmed that ganciclovir provided benefits to the OS of glioma patients, with an OR of 2.393 (P = 0.012).
In order to clarify the differences in treatment effects among the five RCTs, we conducted a more in-depth discussion on the survival data. Among them, three studies conducted by Westphal et al. [20], Rainov et al. [17], and Stragliotto et al. [19], in the original records, the median survival of patients in the experimental group and control group were 497 vs. 452 days [20], 365 vs. 354 days [17], and 17.9 vs. 17.4 months [19], respectively. From the perspective of a single original study, there was no significant survival benefit in the experimental group. However, Ji et al. [10] and Immonen et al. [18] reported significant survival benefits with a median survival of 29.6 vs. 8.4 weeks [10] and 62.4 vs. 37.7 weeks [18], respectively.
In our sensitivity analysis, we considered whether the subtype of glioma, specifically glioblastoma, could lead to differences in treatment effect. This is because the two studies with significant survival benefits had a lower proportion of patients with glioblastoma in the treatment group. However, in the original study by Immonen et al. [18], a comparison was made only among patients with glioblastoma, and a significant difference was found (median survival of 55.3 vs. 37.0 weeks, P = 0.0214). Therefore, we speculate that the true reason for the difference in treatment efficacy may lie in the type of glioma, whether it is a “newly diagnosed” or “recurrent”. Immonen et al. [18] also pointed out that the efficacy of ganciclovir in newly diagnosed glioblastoma only tended to be significant (54.4 vs. 42.8 weeks).
The patients with newly diagnosed glioma [17, 19, 20] had no significant survival benefit in original studies, so we removed two articles that included patients with recurrent glioma and had significant survival benefit and conducted subgroup analysis. The subgroup analysis result showed a slight decrease in OR (from 2.393 to 1.481), but there was still a statistically significant difference (P = 0.014). Notably, if we only conducted meta-analysis by two recurrent glioma studies, the result would be OR 6.51 (95% CI 2.786–15.215, P = 0.000015). Therefore, we consider that there is an extremely positive relationship between ganciclovir and recurrent glioma. This is important for recurrent patients because they are often already treated with standard therapy and therefore are in great need of a different treatment to suppress recurrent cancer cells [50, 51]. We also recommend that ganciclovir should be used in combination with standard therapy in newly diagnosed glioma to avoid ignoring the contribution of standard therapy. Based on the above discussion, we have a clearer understanding of the efficacy of ganciclovir in patients with glioma, and also found the target patients who have the most chance to benefit from it. In terms of safety, the common side effects of ganciclovir are blood type side effects such as anemia, neutropenia or thrombocytopenia [52]. In addition, because the gene therapy will inject the viral vector into the human body, it may also cause some patients to have a short-term fever. However, in all the studies included in this article, it was not mentioned that ganciclovir or valganciclovir directly caused any serious and fatal side effects, which is also in line with the previous studies mentioned that ganciclovir is a safe and feasible treatment [46].
Study limitations
The main limitation of this article is the small number of included literature, which will be reflected in all results [53]. The incidence of glioma is relatively low, so the total number of patients included in this study is limited, which also leads to a slight gap between this meta-analysis and the most real situation, and also reduces the statistical effect. Finally, the results included in this study only include survival rate and OS, and there is still a lack of other efficacy indicators such as remission rate and progression-free survival (PFS). Another potential issue is the limited discussion of ganciclovir and valganciclovir together in glioma treatment due to uncertain mechanisms. Although these two drugs have gradually been considered equivalent [54], we think that intravenous ganciclovir injection only contributes a little to countering CMV, particularly considering that ganciclovir is typically administered in the short-term [10, 17, 18, 20]. In contrast, valganciclovir is often used for long-term treatment [19], and one study also demonstrated that survival is correlated with the duration of valganciclovir treatment (r = 0.815, P < 0.0001) [19]. Patients receiving valganciclovir for at least 6 months had a longer median survival compared to those with short-term or no valganciclovir treatment (24.1 vs. 13.1 months, P < 0.0001) [19]. Notably, Hossain et al. [55] found that long-term treatment with valganciclovir could improve the effectiveness of gene therapy, consequently leading to an improved survival in rats afflicted with glioblastoma (gene therapy combined with 3-month valganciclovir treatment has a significant survival benefit compared with gene therapy only, P = 0.008) [55]. This finding suggests that combining these two distinct drug forms could be a novel strategy for glioma treatment, as long-term valganciclovir not only addresses CMV infection but also extends the effects of gene therapy.
In summary, considering the current clinical results, it seems that relying solely on a single therapeutic mechanism may not maximize the efficacy of ganciclovir in glioma treatment. Therefore, we hope that future clinical trials will consider the combination of both mechanisms, specifically continuing long-term valganciclovir treatment for an extended period following the completion of gene therapy in the initial weeks.
Currently, the newer studies include two retrospective studies on the adjuvant treatment of glioma with valganciclovir published by Stragliotto et al. in 2020 [8, 49], and an ongoing clinical trial [56]. These two retrospective controlled studies also suggested that patients with glioma who received adjuvant valganciclovir had better survival compared with the control group. In particular, one of the patients recruited belonged to secondary (metastatic) glioma [49], and the outcome of valganciclovir treatment was still better than the control group, which means that valganciclovir may be effective for both primary and secondary glioma patient. This is a rare phenomenon [56]. The ongoing clinical trial is VIGAS2 (NCT04116411), which is a multi-center randomized double-blind controlled phase II study. This phase II study recruits 220 people and is expected to be completed in 2024. It mainly evaluates the efficacy of long-term valganciclovir (2 years) as an adjuvant therapy in glioblastoma patients.
Conclusion
The results of this meta-analysis confirmed that ganciclovir can increase the 2-year survival rate, 4-year survival rate and OS of glioma patients. However, the number of clinical studies on this topic is still very small, so there are some publication biases. Therefore, we expect further large-scale clinical RCTs to verify the authenticity of this systematic review and meta-analysis.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Hamad A, Yusubalieva GM et al (2023) Recent developments in glioblastoma therapy: oncolytic viruses and emerging future strategies. Viruses. https://doi.org/10.3390/v15020547
Louis DN, Perry A et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Tan AC, Ashley DM et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70:299–312. https://doi.org/10.3322/caac.21613
Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM (2020) Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10(1):11622. https://doi.org/10.1038/s41598-020-68011-4
Davis ME (2016) Glioblastoma: overview of disease and treatment. Clin J Oncol Nursing 20:S2-8. https://doi.org/10.1188/16.CJON.S1.2-8
Schaff LR, Mellinghoff IK (2023) Glioblastoma and other primary brain malignancies in adults: a review. JAMA 329:574–587. https://doi.org/10.1001/jama.2023.0023
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003. https://doi.org/10.1056/NEJMoa043331
Stragliotto G, Pantalone MR, Rahbar A, Bartek J, Söderberg-Naucler C (2020) Valganciclovir as add-on to standard therapy in glioblastoma patients. Clin Cancer Res 26(15):4031–4039. https://doi.org/10.1158/1078-0432.Ccr-20-0369
Scherm A, Ippen FM, Hau P et al (2023) Targeted therapies in patients with newly diagnosed glioblastoma—a systematic meta-analysis of randomized clinical trials. Int J Cancer 152(11):2373–2382. https://doi.org/10.1002/ijc.34433
Ji N, Weng D et al (2016) Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 7:4369–4378. https://doi.org/10.18632/oncotarget.6737
Wang JL, Scheitler KM, Wenger NM, Elder JB (2021) Viral therapies for glioblastoma and high-grade gliomas in adults: a systematic review. Neurosurg Focus 50(2):E2. https://doi.org/10.3171/2020.11.Focus20854
Carpenter AB, Carpenter AM, Aiken R, Hanft S (2021) Oncolytic virus in gliomas: a review of human clinical investigations. Ann Oncol 32(8):968–982. https://doi.org/10.1016/j.annonc.2021.03.197
Smith GL, Banegas MP, Acquati C et al (2022) Navigating financial toxicity in patients with cancer: a multidisciplinary management approach. CA Cancer J Clin 72(5):437–453. https://doi.org/10.3322/caac.21730
Rodrigues R, Duarte D et al (2022) Drug repurposing in cancer therapy: influence of patient’s genetic background in breast cancer treatment. Int J Mol Sci 23:4280. https://doi.org/10.3390/ijms23084280
Curran M, Noble S (2001) Valganciclovir. Drugs 61(8):1145–1150. https://doi.org/10.2165/00003495-200161080-00013
Yang T, Liu D et al (2022) Cytomegalovirus and glioblastoma: a review of the biological associations and therapeutic strategies. J Clin Med 11:5221. https://doi.org/10.3390/jcm11175221
Rainov NG (2000) A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Therap 11:2389–2401. https://doi.org/10.1089/104303400750038499
Immonen A, Vapalahti M et al (2004) AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Therap 10:967–972. https://doi.org/10.1016/j.ymthe.2004.08.002
Stragliotto G, Rahbar A et al (2013) Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int J Cancer 133:1204–1213. https://doi.org/10.1002/ijc.28111
Westphal M, Ylä-Herttuala S et al (2013) Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 14:823–833. https://doi.org/10.1016/S1470-2045(13)70274-2
Sherin DR, Manojkumar TK (2021) Exploring the selectivity of guanine scaffold in anticancer drug development by computational repurposing approach. Sci Rep 11(1):16251. https://doi.org/10.1038/s41598-021-95507-4
Karjoo Z, Chen X, Hatefi A (2016) Progress and problems with the use of suicide genes for targeted cancer therapy. Adv Drug Deliv Rev 99(Pt A):113–128. https://doi.org/10.1016/j.addr.2015.05.009
Boeckh M, Ljungman P (2009) How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 113:5711–5719. https://doi.org/10.1182/blood-2008-10-143560
Stragliotto G, Pantalone MR et al (2020) Valganciclovir as add-on to standard therapy in glioblastoma patients. Clin Cancer Res 26:4031–4039. https://doi.org/10.1158/1078-0432.CCR-20-0369
Rahbar A, Orrego A et al (2013) Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol 57:36–42. https://doi.org/10.1016/j.jcv.2012.12.018
Rahbar A, Stragliotto G et al (2012) Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; —a case-control study. Herpesviridae 3:3. https://doi.org/10.1186/2042-4280-3-3
Culver KW, Ram Z, Wallbridge S et al (1992) In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 256(5063):1550–1552. https://doi.org/10.1126/science.1317968
Ram Z, Culver KW, Oshiro EM et al (1997) Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3(12):1354–1361. https://doi.org/10.1038/nm1297-1354
Amano S, Gu C, Koizumi S, Tokuyama T, Namba H (2011) Tumoricidal bystander effect in the suicide gene therapy using mesenchymal stem cells does not injure normal brain tissues. Cancer Lett 306(1):99–105. https://doi.org/10.1016/j.canlet.2011.02.037
Oishi T, Ito M, Koizumi S et al (2022) Efficacy of HSV-TK/GCV system suicide gene therapy using SHED expressing modified HSV-TK against lung cancer brain metastases. Mol Ther Methods Clin Dev 26:253–265. https://doi.org/10.1016/j.omtm.2022.07.001
Li S, Gu C, Gao Y et al (2012) Bystander effect in glioma suicide gene therapy using bone marrow stromal cells. Stem Cell Res 9(3):270–276. https://doi.org/10.1016/j.scr.2012.08.002
Page MJ, Shamseer L, Tricco AC (2018) Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev 7(1):32. https://doi.org/10.1186/s13643-018-0699-4
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A (2020) Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 29(9):2520–2537. https://doi.org/10.1177/0962280219889080
Cai S, Zhou J, Pan J (2021) Estimating the sample mean and standard deviation from order statistics and sample size in meta-analysis. Stat Methods Med Res 30(12):2701–2719. https://doi.org/10.1177/09622802211047348
Sitch A (2020) Six of one, half a dozen of the other? Testing differences in proportions. BJOG 127(7):811. https://doi.org/10.1111/1471-0528.15570
Ioannidis JP, Patsopoulos NA, Evangelou E (2007) Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335(7626):914–916. https://doi.org/10.1136/bmj.39343.408449.80
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Germano IM, Fable J, Gultekin SH, Silvers A (2003) Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol 65(3):279–289. https://doi.org/10.1023/b:neon.0000003657.95085.56
Prados MD, McDermott M, Chang SM et al (2003) Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol 65(3):269–278. https://doi.org/10.1023/b:neon.0000003588.18644.9c
Voges J, Reszka R, Gossmann A et al (2003) Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54(4):479–487. https://doi.org/10.1002/ana.10688
Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C (2003) Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther 7(6):851–858. https://doi.org/10.1016/s1525-0016(03)00100-x
Paya C, Humar A, Dominguez E et al (2004) Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4(4):611–620. https://doi.org/10.1111/j.1600-6143.2004.00382.x
Barese CN, Krouse AE, Metzger ME et al (2012) Thymidine kinase suicide gene-mediated ganciclovir ablation of autologous gene-modified rhesus hematopoiesis. Mol Ther 20(10):1932–1943. https://doi.org/10.1038/mt.2012.166
Stragliotto G, Pantalone MR, Rahbar A, Söderberg-Nauclér C (2020) Valganciclovir as add-on to standard therapy in secondary glioblastoma. Microorganisms. 8(10):1471. https://doi.org/10.3390/microorganisms8101471
Zhao B, Wu J, Xia Y et al (2022) Comparative efficacy and safety of therapeutics for elderly glioblastoma patients: a Bayesian network analysis. Pharmacol Res 182:106316. https://doi.org/10.1016/j.phrs.2022.106316
Schritz A, Aouali N, Fischer A et al (2021) Systematic review and network meta-analysis of the efficacy of existing treatments for patients with recurrent glioblastoma. Neurooncol Adv. 3(1):vdab052. https://doi.org/10.1093/noajnl/vdab052
Cochrane AB (2006) Antiviral dosing and efficacy for prophylaxis of cytomegalovirus disease in solid organ transplant recipients. Am J Health Syst Pharm 63(19 Suppl 5):S17-21. https://doi.org/10.2146/ajhp060379
Gurevitch J, Koricheva J, Nakagawa S, Stewart G (2018) Meta-analysis and the science of research synthesis. Nature 555(7695):175–182. https://doi.org/10.1038/nature25753
Haverkos BM, Alpdogan O, Baiocchi RA et al (2023) Targeted therapy with nanatinostat and valganciclovir in recurrent Epstein-Barr virus-positive lymphoid malignancies: a phase 1b/2 study. Blood Adv. https://doi.org/10.1182/bloodadvances.2023010330
Hossain JA, Latif MA, Ystaas LAR et al (2019) Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro Oncol 21(7):890–900. https://doi.org/10.1093/neuonc/noz060
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19(4):764–772. https://doi.org/10.1158/1078-0432.Ccr-12-3002
Acknowledgements
We would like to thank our lab peers, Yi-Ning Chou, Fang-Chi Hsu and Yu-Hsuan Lin, for their assistance with the database search.
Funding
No specific funding sources were used for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study design and Drafting of Manuscript were performed by CTC and NWH. Conception and Design of Experiment were performed by CTC, SHC and NWH. Data Collection was performed by CTC, HHC and CCC. Data Analysis and Interpretation were performed by CTC, HHC, CCC and SHC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
There is not any conflict of interest in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, CT., Chen, HH., Chuang, CC. et al. Ganciclovir as a potential treatment for glioma: a systematic review and meta-analysis. J Neurooncol 165, 399–411 (2023). https://doi.org/10.1007/s11060-023-04503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04503-3