Abstract
Background
Craniopharyngioma is a benign tumor that commonly develops within the suprasellar region. The tumor and treatment can have debilitating consequences for pediatric and adult patients, including vision loss and pituitary/hypothalamic dysfunction. Most craniopharyngioma series focus on treatment of the pediatric population. We evaluated the outcomes of all adult craniopharyngioma patients treated at our institution using proton therapy to report outcomes for disease control, treatment-related toxicity, and tumor response.
Methods
We analyzed 14 adult patients (≥ 22 years old). All patients had gross disease at the time of radiotherapy. Five were treated for de novo disease and 9 for recurrent disease. Patients received double-scattered conformal proton therapy to a mean dose of 54 GyRBE in 1.8 GyRBE/fraction (range 52.2–54 GyRBE). Weekly magnetic resonance imaging (MRI) helped to evaluate tumor changes during radiotherapy.
Results
With median clinical and radiographic follow-up of 29 and 26 months, respectively, the 3-year local control and overall survival rates were both 100%. There were no grade 3 or greater acute or late radiotherapy-related side effects. There was no radiotherapy-related vision loss or optic neuropathy. No patients required intervention or treatment replanning due to tumor changes during radiotherapy. Two patients experienced transient cyst expansion at their first post-radiotherapy MRI. Both patients were followed closely clinically and radiographically and had subsequent dramatic tumor/cyst regression, requiring no interventions.
Conclusions
Our data support the safety and efficacy of proton therapy in the treatment of adult craniopharyngioma as part of primary or salvage treatment. We recommend early consideration of radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngioma is a rare benign epithelial tumor that originates along the path of the hypophyseal–pharyngeal duct (craniopharyngeal duct). These tumors commonly occur within the suprasellar region and are believed to arise from remnants of squamous cell rests within Rathke’s pouch, the embryologic precursor to the pituitary gland. They tend to be cystic or have mixed solid and cystic components. The incidence of craniopharyngioma is estimated at ~ 1.3 per million people and, while they occur across all ages, they tend to have a bimodal peak age distribution at 5 to 14 years old and 65 to 74 years old [1]. Although benign in nature, the location of these tumors adjacent to or abutting the optic nerves/chiasm, pituitary gland, and hypothalamus can lead to significant tumor-related morbidity, frequently resulting in vision deficits and endocrinopathies. Surgery remains the mainstay of initial management of craniopharyngioma; however, curative surgery requires gross total resection (GTR), which can be associated with high rates of surgical morbidity including vision impairment, pituitary dysfunction, and hypothalamic injury. Mounting evidence over the past two decades supports the role of minimal resection/surgical decompression followed by postoperative radiotherapy to the residual tumor, which can achieve 10-year local control rates of ~ 90% [2,3,4]. In the pediatric population for whom craniopharyngioma represents the most common non-glial intracranial tumor, there is reluctance to adopt radiotherapy in the upfront management of a benign tumor due to concerns for long-term radiation-related treatment toxicities, including cerebrovascular sequelae, secondary malignancies, and neurocognitive decline in a population that is expected to achieve long-term survival. While the pediatric craniopharyngioma population receives much of the attention for this disease, craniopharyngioma in adults can be equally devastating owing to tumor- and treatment-related complications affecting vision, endocrine function, performance status, and quality of life. Many radiotherapy studies of craniopharyngioma have excluded adults, and studies that have included adults typically have reported outcomes in combination with pediatric patients [4, 5].
With advances in radiotherapy techniques, radiation risks can be minimized to optimize combination therapy and reduce surgical and radiotherapy complications. With proton therapy, specifically, charged particles are utilized to reduce the radiotherapy dose to surrounding normal tissue, including the frontal and temporal lobes and pituitary fossa. The physical properties of the proton beam and the Bragg Peak phenomena in eliminating the radiation beam’s exit dose underlies the potential clinical benefits to proton therapy. The current study evaluates our institutional experience with proton therapy in the treatment of adult craniopharyngioma. We focus on early disease control outcomes, treatment-related complications and toxicity, and response to radiotherapy.
Methods
Patients
We retrospectively reviewed the medical records of 14 adult craniopharyngioma patients who received proton therapy between March 1, 2012, and July 23, 2018. Patients were enrolled on an Institutional Review Board-approved outcomes tracking protocol (IRB201802930). Patients below 22 years old at the time of radiotherapy were excluded. No patient had received prior radiotherapy. Thirteen patients had pathologic confirmation of craniopharyngioma and 1 patient had a radiographic diagnosis. The date of diagnosis was the date of the first confirmatory imaging study identifying the craniopharyngioma.
Patient and disease characteristics are detailed in Table 1. Presenting symptoms are described in Table 2.
Radiotherapy treatment
Patients were simulated using a 3-dimensional (3D) computed tomography (CT) scan. Patients were immobilized with a thermoplastic mask with a bitepiece. Target delineation was informed by the co-registration of contrast-enhanced, pre- and postoperative magnetic resonance imaging (MRI) to the radiation planning CT scan. Target volumes were generated as follows: The gross tumor volume (GTV) included the tumor resection bed and gross residual tumor. The clinical target volume (CTV) was generated by adding a 5-mm isotropic expansion to the GTV. The CTV extending into uninvolved brain parenchyma or barriers of tumor spread was limited to 2 to 3 mm. The planning target volume (PTV) was generated by adding a 3-mm isotropic expansion to CTV. Proton therapy was prescribed to the PTV to a dose of 54 GyRBE over 30 fractions (1.8 GyRBE per fraction). One patient received 52.2 GyRBE in 1.8 GyRBE/fraction. One patient received 12 of 30 fractions (21.6 GyRBE of 54 GyRBE) delivered with intensity-modulated radiotherapy (IMRT) before completing the radiotherapy course with protons (32.4 GyRBE). All treatment plans utilized double-scattered protons. Proton plans generally consisted of three fields including right and left superior oblique fields and a superior anterior oblique field (or, infrequently, a posterior–anterior field). Beam angles were selected to avoid proton fields with an end-of-range in the brainstem. One patient was treated with a 4-field plan and received treatment with two fields daily with alternating field sets. Individualized brass apertures and Lucite™ compensators were generated for each patient. Treatment planning goals included 99% of the CTV volume covered by 100% of the prescription dose and 100% of the CTV covered by 99% of the prescription dose. The following organ-at-risk (OAR) dose limits were met for all patients: brainstem maximum dose of 0.1 cm3 < 56 GyRBE; brainstem maximum point dose < 60 GyRBE; optic nerve maximum dose of 0.1 cm3 < 56 GyRBE; and optic chiasm maximum dose of 0.1 cm3 < 56 GyRBE. Supplementary Table 1 shows detailed dosimetry data for organs at risk for all treatment plans of patients included in the series.
Daily orthogonal radiographic imaging based on bony anatomy was used for daily image guidance. Thirteen patients underwent weekly MRI evaluation of the target volume during radiotherapy, while 1 patient received biweekly MRI during radiotherapy. Patients underwent imaging on a 0.23 T Open Bore MRI (Philips Panorama MR Scanner, USA) to assess for tumor/cyst changes. THRIVE (T1-weighted high-resolution isotropic volume examination) and 3D B-FFE (balanced fast-field echo) sequences were obtained without contrast to assess both solid and cystic components of the GTV.
Patient follow-up
Patients typically underwent follow-up clinical examination and imaging 1–3 months after completing radiotherapy. Clinical and imaging follow-up was recommended at 3- to 6-month intervals thereafter until 2 to 3 years following radiotherapy, at which time clinical and imaging follow-up could be extended at the discretion of the treating team. Pituitary function labs were obtained at 6- to 12-month intervals. Ophthalmology evaluation with visual field testing was performed before beginning radiotherapy. Annual post-radiotherapy ophthalmology follow-up was recommended.
Radiographic tumor assessment
Tumor progression was evaluated per two previously described criteria for assessing craniopharyngioma response following radiotherapy: the first was established by The Pediatric Brain Tumor Consortium (see PBTC-039 Trial). The second was established by St. Jude Children’s Hospital (Memphis, TN) for use in pediatric patients, which considered progression if the solid portion of the tumor progressively grew by a measurable amount or if the cystic portion of the tumor complex continued to progress beyond 3 years after treatment [6].
Volumetric tumor response was calculated using contrast-enhanced diagnostic MRIs using MIM™ software (MIM Software, Inc., Cleveland, OH, USA) by a board-certified neuroradiologist (DR) with a certificate of added qualification. Gross tumor, including the cystic and solid tumor components, were delineated on pre- and post-radiotherapy MRIs for each patient when available. The extent of tumor regression was calculated as a ratio of pre-radiotherapy tumor volume to post-radiotherapy tumor volume.
Statistical analysis
Basic descriptive statistics are provided for this series. Medians were used to estimate the center of a continuous distribution rather than to determine outliers overly influencing the estimate.
Results
Fourteen adult patients with craniopharyngioma were treated with proton therapy and were included in this study. The median age at diagnosis was 26 years old (range 19–53 years). The median age at the time of treatment was 28 years old (range 22–53 years). The most common presenting symptoms were vision changes (affecting 71% of patients), headaches, and endocrinopathies (both affecting 50%; Table 2). All patients had gross disease at the time of radiotherapy, although 2 had only small-volume residual disease along the optic chiasm. Of the 13 patients with pathologic confirmation of craniopharyngioma, 11 patients (85%) had the adamantinomatous subtype and 2 patients (15%) had the papillary subtype. Both tumors of papillary subtype contained the BRAF V600E mutation. Five patients (36%) were treated at initial presentation and 9 were treated for recurrent disease. No patient received prior radiotherapy. Among the 9 patients treated for recurrent disease, 7 received surgical salvage followed by postoperative radiation and 2 were treated with salvage radiotherapy alone (Table 3). The median time to recurrence from prior surgery for the 9 patients undergoing salvage treatment was 6 months (range 1.2–39.6 months).
Surgery
Thirteen of the 14 patients had surgery prior to receiving radiotherapy. Of these, 11 patients received postoperative (adjuvant) radiotherapy (5 after surgery at initial presentation and 6 after salvage surgery), and 2 received definitive radiotherapy for salvage after recurrence following a prior resection. One patient did not have surgery and received definitive radiotherapy. Including surgeries for initial treatment and salvage therapy, 8 patients (62%) had at least 2 surgeries before receiving radiotherapy and 3 patients had three surgeries prior to radiotherapy (Table 1). Ten of the 13 patients (77%) who underwent surgery had a craniotomy as part of their surgical management, while 3 patients (23%) had only endonasal endoscopic surgeries.
Radiotherapy
Eleven patients received adjuvant radiotherapy and 3 patients received definitive radiotherapy (1 at initial presentation and 2 for salvage). The median radiotherapy dose was 54 GyRBE (range 52.2–54 GyRBE). The median number of elapsed treatment days was 43 (range 39–45 days). Among the 11 patients who received adjuvant radiotherapy, the median time interval from surgery to the start of radiotherapy was 2.3 months (range 1.1–5.1 months).
Imaging and tumor assessment during radiotherapy
Weekly MRI was performed during radiotherapy for each patient. No patient had tumor or cyst changes that necessitated surgical intervention or treatment replanning. All lesions remained stable during radiation, including in one patient with an external ventricular drain with an Ommaya reservoir placed prior to radiotherapy who did not require reservoir access/drainage during radiotherapy.
Local control and survival outcomes
The median clinical follow-up was 2.4 years (range 1.4–7.1 years) and the median radiographic follow-up was 2.2 years (range 0.9–5.3 years) for the entire cohort. With a median follow-up of 2.4 years, there have been no local recurrences or patient deaths. No patient had radiographic tumor progression by either PBTC or St. Jude criteria (see “Radiographic Tumor Assessment” above).
Tumor response to radiotherapy
Thirteen of the 14 patients in our series had measurable volumetric tumor regression following treatment (a minimum of 18.5% volume). One patient had stable disease on imaging 5 years after completing treatment. Seven patients with ≥ 1cm3 of gross tumor at the time of radiotherapy had volumetric imaging follow-up available to assess the response rate to radiotherapy. At the time of last radiographic follow-up, the median volumetric response was 83% (range 18.5–100%) and all 7 had measurable tumor regression. Three patients (30%) had a complete radiographic response, 3 others had > 80% volumetric regression, and 1 patient had 18.5% regression.
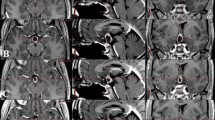
No patient required cyst aspiration or decompression during or after radiotherapy. Two patients experienced asymptomatic transient cyst expansion shortly after completing radiotherapy. One of these had tumor cyst enlargement on 3-month post-radiotherapy MRI. The patient remained asymptomatic and underwent close clinical and radiographic monitoring. Repeat MRI 2 months later (5 months after RT) showed significant regression of both solid and cystic components of the tumor. The tumor continued to regress and, at last follow-up (17 months after radiotherapy), the patient had a near complete radiographic response with only minimal residual tumor (Fig. 1). A second patient had tumor cyst enlargement on the 1-month post-treatment MRI after undergoing postoperative radiotherapy. The patient remained asymptomatic and a repeat MRI 1 month later (2 months after RT) showed significant tumor regression. The tumor continued to regress and on the most recent follow-up over 3 years after RT, the patient had a near complete response.
Transient post-radiotherapy craniopharyngioma cyst expansion with subsequent regression. Postoperative and pre-radiotherapy T1 + C (left column) and T2 (right column) coronal magnetic resonance images shown in row 1 depict residual cystic and solid portions of tumor (identified by crosshairs and arrow). Three-month post-radiotherapy MRI in row 2 shows transient cyst expansion. MRI 2 months later (5 months after radiotherapy; row 3) depicts solid and cystic tumor regression; at 17 months after radiation (row 4) there remains very small-volume residual enhancing tumor
Treatment-related toxicity
Surgery: Two of 13 patients (15%) experienced new postoperative vision deficits, while 7 of the 11 patients (64%) with preoperative vision deficits had postoperative vision improvement. Ten patients (77%) experienced new postoperative endocrine deficits, including 7 (54%) who developed pan-hypopituitarism. Eight patients (62%) experienced postoperative diabetes insipidus. Surgical complications are detailed in Table 4.
Radiotherapy: The most common grade 1/2 radiotherapy acute complications included alopecia, headaches, and fatigue. No ≥ grade 3 acute complications occurred. Four patients (29%) experienced new post-radiotherapy endocrinopathies (grade 2). Three patients (21%) developed growth hormone deficiency and 1 patient (7%) developed post-radiotherapy central adrenal insufficiency. One patient developed late asymptomatic (grade 1) orbitofrontal lobe encephalomalacia secondary to radiotherapy. No other late ≥ grade 2 complications occurred as a result of radiotherapy. No patient reported vision loss or worsening visual acuity following radiotherapy (Table 5). Eight of the 14 patients in our series have continued regular ophthalmology follow-up, 6 of whose records were available for review. With a median post-radiotherapy ophthalmology follow-up time of 22 months (range 1–40 months) there have been no ophthalmology reported cases of radiation-related optic neuropathy, optic neuritis, or visual acuity/visual field deterioration.
Discussion
While histologically benign, craniopharyngioma can be devastating due to complications of the disease and/or treatment. Although the majority of the literature focuses on the treatment of childhood craniopharyngioma, the overall incidence of craniopharyngioma is higher among adults [7, 8]. To date, there is one report that includes adult patients treated with proton therapy for craniopharyngioma [9]. The current study represents a larger, adult-only cohort treated with contemporary proton therapy for craniopharyngioma.
Similar to childhood craniopharyngioma, the disease can be debilitating in adults, with high rates of vision injury, endocrinopathies, and sleep and eating disturbances. GTR provides good tumor control, but yields high rates of surgical complications unless cases are carefully selected [10, 11]. Limited resection, on the other hand, yields high rates of local recurrence, which can result in additional surgical interventions and increased treatment morbidity [11]. Limited resection with adjuvant radiotherapy provides comparable tumor control to GTR with the benefits of surgical debulking/decompression but reduced surgical morbidity [12, 13]. While a combined approach includes the added risks of radiotherapy, these can be lowered with advances in radiotherapy treatment techniques. Improvements in target delineation and localization, and more conformal radiotherapy techniques, enable safer and more effective treatment. Proton therapy represents one such advance, which compared to advanced photon radiation techniques (e.g. IMRT) reduces radiation dose to the hippocampus, dentate gyrus, subventricular zone, carotid arteries, and anterior circle of Willis. Protons also reduce integral dose to non-target supratentorial and infratentorial brain [14,15,16].
While several studies describe clinical outcomes after proton therapy in pediatric patients [9, 17,18,19], few adult patients are included in the reported outcomes. The current series represents the largest adult craniophayngioma cohort treated with proton therapy to date and is the first contemporary proton therapy series on the subject. Our results demonstrate excellent treatment tolerance. There were no significant acute radiotherapy-related complications. Four patients (29%) experienced post-radiotherapy endocrinopathies. Three of 4 endocrinopathies were growth hormone deficiency and 1 was central adrenal insufficiency. Of note, no vision complications occurred as a result of proton therapy in our series.
While our series will benefit from additional follow-up time to provide more meaningful disease control outcomes, our early results are encouraging with 100% local control and overall survival at 3 years. Longer follow-up will likewise provide additional treatment-related toxicity data; however, our acute and early-late toxicity profiles for proton therapy are excellent.
Several series have reported rates of transient cyst expansion during radiotherapy for craniopharyngioma. The rate of cyst/tumor expansion during photon-based radiotherapy for childhood craniopharyngioma ranges from ~ 11 to 64% [15, 20]. Similarly, cyst expansion during proton therapy for childhood craniopharyngioma has been reported. Winkfield et al. reported 6 of 17 children (35%) who had significant cystic changes during proton therapy necessitating either treatment plan modifications or cyst drainage (1 patient) [21]. Bishop et al. reported cyst growth during proton therapy in 19% of pediatric craniopharyngioma patients, half of whom required treatment replanning [17]. Ajithkumar et al. described cyst expansion in 31% of childhood patients during proton therapy, 12.5% (2 of 5) of whom required replanning or cyst aspiration [19]. Of note, cyst contraction also occurs during conventional radiotherapy and can require treatment plan modifications [17]. In our series, patients were rigorously monitored with weekly MRI with image fusion of all weekly images (including pre-RT imaging) to assess for tumor or cyst changes requiring treatment replanning or intervention. There were no cases of tumor/cyst changes during treatment that necessitated treatment planning changes or intervention.
Post-radiotherapy cyst expansion is also a well-documented phenomenon. Rates of early cyst expansion within 6 months of completing treatment have been reported at ~ 40–60% [17, 20, 22, 23]. Post-radiotherapy craniopharyngioma cyst expansion is usually transient and most cases require only close clinical and imaging follow-up without surgical intervention, provided the patient remains asymptomatic [20, 22, 23]. Such was the case with our 2 patients who experienced asymptomatic transient post-radiotherapy cyst enlargement shortly after completing post-operative proton therapy. Both patients continued close imaging and clinical follow-up and ultimately had dramatic tumor regression and near-complete radiographic responses without need of further intervention.
Whether differences exist in tumor cyst dynamics during or after radiotherapy between adult and pediatric craniopharyngioma remains unclear. Similarly, whether histologic subtype (adamantinomatous versus papillary) influences radiotherapy-related cyst dynamics remains unknown. One pediatric series comparing photon and proton outcomes suggested lower rates of cyst changes during and after radiotherapy with protons [17]. While our proton series shows a low rate of tumor/cyst changes during and after radiotherapy, the small patient cohorts and wide variability across series precludes any meaningful conclusions. That both patients in our series who experienced transient post-radiotherapy cyst expansion had subsequent rapid and dramatic tumor responses (see Fig. 1) is particularly interesting. Perhaps a significant post-radiotherapy inflammatory response as evidenced by cyst expansion portends a favorable treatment response.
Age has frequently been reported to impact survival in craniopharyngioma patients, with increasing age portending decreased overall survival [3, 5, 24]. Yet local control and disease-specific survival do not seem to worsen with age [4, 5, 24]. The survival differences observed between age groups are likely related to other age-related causes of mortality [8]. As with many benign tumor types, the competing risk of death from non-tumor factors is high among adult craniopharyngioma patients. However, the impact on health-related quality of life of craniopharyngioma or craniopharyngioma recurrence on the adult population should not be understated.
Numerous reports suggest no overall survival or cause-specific survival detriment to salvage radiotherapy compared to adjuvant radiotherapy [3, 4, 24,25,26]. Nevertheless, recurrent tumor and surgical salvage are associated with increased risks of surgical complications that may be avoided with early radiotherapy [10, 27, 28]. Moon et al. compared cohorts of adult and pediatric craniopharyngioma patients treated with either adjuvant or salvage radiotherapy (with or without salvage surgery) [2]. There were no overall survival or progression-free survival differences between the patients treated with adjuvant radiotherapy versus salvage therapy; however, the adjuvant radiotherapy cohort had lower rates of diabetes insipidus, visual acuity loss, and visual field deficits compared to the salvage therapy group [2]. Muller et al. followed a cohort of 102 craniopharyngioma survivors longitudinally and found through multivariate analysis that tumor relapse and progression had a negative impact on quality of life [27].
Notably, in our series, the median interval to recurrence (prior to undergoing radiotherapy) after subtotal resection was 6 months (range 1.2–39.6), which is consistent with other series that have reported median times to local recurrence between 3.5 and 10 months following surgery [29, 30]. It appears that delaying adjuvant radiotherapy unlikely results in a meaningful time interval before salvage therapy is required. Unlike pediatric patients, among adults there is no developmental benefit to delaying radiotherapy, and the risks of tumor recurrence and additional surgery likely outweigh any severe risks from radiotherapy. With highly advanced radiotherapy techniques, like proton therapy, especially, our findings suggest a benefit to postoperative (adjuvant) radiotherapy over salvage radiotherapy to reduce the risk of complications from tumor recurrence and salvage surgery.
As regards adjuvant radiotherapy for craniopharyngioma, the balance between appropriate treatment versus over-treatment rests in the safe and accurate delivery of adjuvant radiotherapy to achieve tumor control (and reduce the risk of subsequent tumor recurrence, surgical salvage, and associated complications) while minimizing the risks of radiotherapy. With radiotherapeutic advances, like proton therapy, the therapeutic ratio can shift towards favoring adjuvant radiation.
The expected benefits of proton therapy among adult craniopharyngioma patients are primarily attributable to a reduction in integral dose around normal brain tissue that is unique to protons. This dose reduction likewise reduces the risk of radiation-associated malignancies, and, by sparing substructures of the brain involved in cognition from radiation dose (e.g., hippocampus, dentate gyrus, subventricular zone), neurocognitive function may be better preserved. In comparison to photons, with protons, most of the dose reduction in the surrounding uninvolved brain comes in the form of reduced low and intermediate dose, as demonstrated in Supplementary Fig. 1, which is a planning comparison between IMRT (photons) and 3D conformal passively scattered proton radiotherapy (as utilized in the current series). Radiation planning studies in childhood craniopharyngioma comparing IMRT and proton therapy have shown that proton therapy reduces the radiation dose to the temporal lobes and brain substructures involved in cognition [14, 31]. In adults, as in children, reduced doses to these brain substructures may improve neurocognitive preservation. A dramatic example of how low/intermediate dose sparing to brain substructures can benefit patients is demonstrated in the recently reported phase III trial, NRG CC001, comparing whole-brain radiotherapy with memantine to whole-brain radiotherapy with a reduced dose to the bilateral hippocampi (i.e. hippocampal-avoidance whole-brain radiotherapy) with memantine for patients with brain metastases. The rate of neurocognitive function decline at 4 and 6 months among patients in the latter arm was significantly lower [32]. Given the expected long-term survival in craniopharyngioma patients, it is critically important to preserve neurocognitive function to maintain quality of life and productivity. With ongoing advances in proton radiotherapy delivery techniques (such as intensity-modulated proton therapy, or IMPT) additional normal tissue avoidance can be achieved, thereby further reducing the risk to uninvolved brain [16, 31].
Interest is mounting in the use of targeted agents to manage craniopharyngioma, specifically the papillary subtype with a BRAF V600E mutation. Targeted inhibition of the BRAF/MEK pathway has shown promise in individual cases and is the subject of an ongoing clinical trial [33, 34]. Targeted agents may someday provide non-surgical, non-radiotherapy treatment options in select populations.
While the current series suffers from the weaknesses inherent to any retrospective review, a major strength is in the uniform treatment of the patients included. All patients had pre-radiotherapy MRI imaging with uniform target delineation (uniform CTV and PTV expansions) and on-treatment MRI imaging to assess target changes, which is in contrast to the wide variability reported in adult craniopharyngioma treatment [4, 5, 24, 26]. Additionally, no patient has been lost to follow-up.
Conclusions
Our early clinical outcomes, including side effects, disease control, and survival, are excellent for adult craniopharyngioma patients treated with proton therapy. Radiotherapy should be considered in patients with residual disease after surgical resection.
Change history
22 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11060-021-03740-8
References
Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM (1997) The descriptive epidemiology of craniopharyngioma. Neurosurg Focus 3(6):e1
Moon SH, Kim IH, Park SW et al (2005) Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas—a study in single institute. Childs Nerv Syst 21(8–9):799–807
Harrabi SB, Adeberg S, Welzel T et al (2014) Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol 9:203
Lo AC, Howard AF, Nichol A et al (2014) Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int J Radiat Oncol Biol Phys 88(5):1011–1018
Regine WF, Mohiuddin M, Kramer S (1993) Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 27(1):13–21
Merchant TE, Kun LE, Hua CH et al (2013) Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 85(4):e187–e192
Zhang C, Verma V, Lyden ER et al (2018) The role of definitive radiotherapy in craniopharyngioma: a SEER analysis. Am J Clin Oncol 41(8):807–812
Rao YJ, Hassanzadeh C, Fischer-Valuck B et al (2017) Patterns of care and treatment outcomes of patients with craniopharyngioma in the national cancer database. J Neurooncol 132(1):109–117
Fitzek MM, Linggood RM, Adams J, Munzenrider JE (2006) Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys 64(5):1348–1354
Puget S, Garnett M, Wray A et al (2007) Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 106(1 Suppl):3–12
Muller HL (2015) Craniopharyngioma: long-term consequences of a chronic disease. Expert Rev Neurother 15(11):1241–1244
Schoenfeld A, Pekmezci M, Barnes MJ et al (2012) The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol 108(1):133–139
Clark AJ, Cage TA, Aranda D et al (2013) A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst 29(2):231–238
Boehling NS, Grosshans DR, Bluett JB et al (2012) Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys 82(2):643–652
Beltran C, Roca M, Merchant TE (2012) On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys 82(2):e281–e287
Yeung D, McKenzie C, Indelicato DJ (2014) A dosimetric comparison of intensity-modulated proton therapy optimization techniques for pediatric craniopharyngiomas: a clinical case study. Pediatr Blood Cancer 61(1):89–94
Bishop AJ, Greenfield B, Mahajan A et al (2014) Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 90(2):354–361
Luu QT, Loredo LN, Archambeau JO, Yonemoto LT, Slater JM, Slater JD (2006) Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J 12(2):155–159
Ajithkumar T, Mazhari AL, Stickan-Verfurth M et al (2018) Proton therapy for craniopharyngioma—an early report from a single European centre. Clin Oncol (R Coll Radiol) 30(5):307–316
Lamiman K, Wong KK, Tamrazi B et al (2016) A quantitative analysis of craniopharyngioma cyst expansion during and after radiation therapy and surgical implications. Neurosurg Focus 41(6):E15
Winkfield KM, Linsenmeier C, Yock TI et al (2009) Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys 73(3):716–721
Constine LS, Randall SH, Rubin P, McDonald J (1989) Craniopharyngiomas: fluctuation in cyst size following surgery and radiation therapy. Neurosurgery 24(1):53–59
Shi Z, Esiashvili N, Janss AJ et al (2012) Transient enlargement of craniopharyngioma after radiation therapy: pattern of magnetic resonance imaging response following radiation. J Neurooncol 109(2):349–355
Masson-Cote L, Masucci GL, Atenafu EG et al (2013) Long-term outcomes for adult craniopharyngioma following radiation therapy. Acta Oncol 52(1):153–158
Kim T, Flynn MR (1991) Airflow pattern around a worker in a uniform freestream. Am Ind Hyg Assoc J 52(7):287–296
Pemberton LS, Dougal M, Magee B, Gattamaneni HR (2005) Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol 77(1):99–104
Muller HL, Bruhnken G, Emser A et al (2005) Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv Syst 21(11):975–980
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97(1):3–11
Stripp DC, Maity A, Janss AJ et al (2004) Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys 58(3):714–720
Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH (2003) Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol 40(4):214–218
Toussaint L, Indelicato DJ, Muren LP et al (2019) Temporal lobe sparing radiotherapy with photons or protons for cognitive function preservation in paediatric craniopharyngioma. Radiother Oncol 142:140–146
Gondi V, Deshmukh S, Brown PD et al (2019) NRG Oncology CC001: a phase III trial of hippocampal avoidance (HA) in addition to whole-brain radiotherapy (WBRT) plus memantine to preserve neurocognitive function (NCF) in patients with brain metastases (BM). J Clin Oncol 37(15 Suppl):2009
Brastianos PK, Shankar GM, Gill CM et al (2016) Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv310
Alliance for Clinical Trials in Oncology. Vemurafenib and cobimetinib in treating patients with BRAF V600E mutation positive craniopharyngioma. https://clinicaltrials.gov/ct2/show/NCT03224767. ClinicalTrials.gov Identifier: NCT03224767. Accessed 3 Dec 2019
Author information
Authors and Affiliations
Contributions
Study design, MSR; Data Collection, MSR; Statistical Analysis, CGM; Data Interpretation, MSR, RLR, DR, ALH, DJI, SH, WMM; Drafting Manuscript, MSR; Final Approval of Manuscript, MSR, RLR, DR, ALH, DJI, SH, WMM.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article has been revised: The Abstract has been corrected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2020_3432_MOESM2_ESM.tif
Supplementary file2 (TIF 475 kb) Supplementary Fig. 1. Intensity-modulated radiation therapy (IMRT) and 3-dimensional (3D) conformal proton radiotherapy treatment plan comparison for adult craniopharyngioma. A representative case of treatment planning comparison between 7-field IMRT (left images) and 3D conformal passively scattered proton radiotherapy (right images). The planning target volume (PTV) delineated by the green line and PTV coverage is the same for both plans. Shaded colorwash shows doses ranging from 10 GyRBE to the maximum dose for each plan. Low dose (10 GyRBE in blue) and intermediate dose (30 GyRBE in teal) spread far beyond the target volume and extend further into normal brain and eyes with the IMRT plan compared to the proton plan. The prescription dose and high dose radiation (yellow and red) are similar between both treatment modalities
Rights and permissions
About this article
Cite this article
Rutenberg, M.S., Rotondo, R.L., Rao, D. et al. Clinical outcomes following proton therapy for adult craniopharyngioma: a single-institution cohort study. J Neurooncol 147, 387–395 (2020). https://doi.org/10.1007/s11060-020-03432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03432-9