Abstract
Background
We sought to evaluate the effectiveness of definitive or adjuvant external-beam proton therapy on local control and survival in patients with skull-base chondrosarcoma.
Methods
We reviewed the medical records of 43 patients with a median age of 49 years (range, 23–80 years) treated with double-scattered 3D conformal proton therapy for skull-base chondrosarcomas between January 2007 and February 2016. Proton therapy-related toxicities were scored using CTCAE v4.0.
Results
The median radiotherapy dose was 73.8 Gy(RBE) (range, 64.5–74.4 Gy[RBE]). Thirty-six (84%) and 7 (16%) patients underwent surgical resection or biopsy alone. Tumor grade distribution included: grade 1, 19 (44%) patients; grade 2, 22 (51%); and grade 3, 2 (5%). Forty patients had gross disease at the time of radiotherapy and 7 patients were treated for locally recurrent disease following surgery. The median follow-up was 3.7 years (range, 0.7–10.1 years). There were no acute grade 3 toxicities related to RT. At 4 years following RT, actuarial rates of overall survival, cause-specific survival, local control, and RT-related grade 3 toxicity-free survival were 95%, 100%, 89%, and 95%.
Conclusion
High-dose, double-scattered 3D conformal proton therapy alone or following surgical resection for skull-base chondrosarcoma is an effective treatment with a high rate of local control with no acute grade 3 radiation-related toxicity. Further follow-up of this cohort is necessary to better characterize long-term disease control and late toxicities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chondrosarcomas are a heterogeneous group of slow-growing neoplasms originating from cartilage-producing cells in areas of enchondral ossification that comprise fewer than 0.2% of all skull-base malignancies. While most of these tumors affect the long bones, pelvis, or ribs, a small proportion occur in the head and neck, spine, and skull-base area. Optimal management is maximal safe surgery with the intent of gross total resection or optimization of target geometry for postoperative charged-particle therapy for those with incompletely resectable or unresectable disease, subtotal resection, or positive margins.
As skull-base chondrosarcomas are typically paramedian, arising from the sphenopetroclival synchondrosis of the petroclival fissure, and require radiation doses over 70 Gy(RBE) for disease control, treatment planning can be a challenge when using conventional radiotherapy (RT) because of the proximity to dose-limiting neural structures, such as the brainstem, spinal cord, and optic apparatus [1]. Modern RT techniques, such as static beam angle and rotational intensity-modulated radiotherapy (IMRT), stereotactic radiosurgery (SRS), proton therapy (PT), and carbon therapy (CT) have allowed for RT-dose escalation, which has led to better treatment outcomes [2, 3]. In fact, a recent national cancer database report demonstrated both an overall survival advantage for dose escalation and those treated with proton RT (pubmed ID: 30644538) [4]. Herein we report outcomes of patients treated at a single institution with high-dose conformal PT for skull-base chondrosarcomas.
Methods
Under institutional review board approval, we reviewed the medical records of 43 patients enrolled on an institutional outcomes-tracking protocol (ClinicalTrials.gov Identifier: NCT00797602) who were treated with double-scattered PT at our institution for skull-base chondrosarcomas between January 2007 and February 2016. Statistical analysis was performed with JMP Pro 13.0 (SAS Institute, Cary, NC). Patients with mesenchymal histology were excluded from analysis and pathologic specimens were not routinely re-reviewed at our institution unless the grade or diagnosis from the referring tertiary care center was uncertain. The Kaplan–Meier product limit method provided estimates of local control, distant control, overall survival, and grade 3 toxicity-free survival. PT-related toxicities were scored using the Common Terminology for Criteria for Adverse events, version 4.0.
As our institute is an international referral center, almost all follow-up was conducted by the referring home team. We recommended that participants be followed every 3 months for the first 2 years, every 6 months for the next 5 years, then annually thereafter with routine interval history and physical examinations, imaging as well as annual audiology, ophthalmology, and endocrine testing when indicated based on doses to normal organs. We recommended high-resolution MRI with T1 with and without gadolinium, T2, and T1 fat-suppressed sequences. Our team routinely updated follow-up at least annually according to the duration from treatment and stability of the individual’s toxicity profile and disease status.
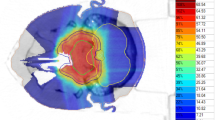
Patient characteristics and presenting symptoms are detailed in Table 1 and treatment and tumor details are shown in Table 2. One patient had Oliver’s disease, of which some reports suggest the lifetime risk of chondrosarcoma is close to 50% [5]. Patients were simulated using a three-dimensional (3D) computed tomography scan with intravenous (IV) contrast (Philips Brilliance, Philips Medical Systems, Madison, WI). Patients were immobilized with a carbon fiber mask with an aquaplast bite plate (Civco Precise-Bite Mouthpiece), which was shaped, anchored, and then attached to a plywood board for mounting with indexing. Before 2013, Eclipse software (Varian Medical Systems, Palo Alto, CA) was used for target delineation and image registration (rather than MIMVista, MIM Software Inc, Cleveland, OH). Target and organ at risk (OAR) volumes were imported into an Eclipse proton treatment planning system (Varian Medical Systems, Palo Alto, CA) and a 3D conformal double-scatter technique. No patients received photon RT as a component of treatment; full details regarding the technical and dosimetric factors of proton treatment planning in this cohort at our institution has been previously described [6].
Treatment planning dose coverage goals and planning constraints were set per institutional protocols, with composite target coverage and heterogeneity goals of planning target volume (PTV) D95 = 100%, PTV D99 ≥ 93%, and PTV V110 ≤ 20%. Treatment volume definitions were defined per our institutional protocol, as outlined in Table 3. Under-coverage of composite target volumes were permitted to meet brainstem, optic chiasm, bilateral optic nerve, and spinal cord absolute constraints. Unilateral optic nerve, temporal lobe, retina, and bilateral cochlear constraints were exceeded on an individual case basis. Target and maximally acceptable dose constraints, respectively, were as follows: brainstem (0.1 cm3) < 55, 64 Gy(RBE); brainstem (Dmax) < 67 Gy(RBE); brainstem core (0.1 cm3) < 50, 60 Gy(RBE); spinal cord (0.1 cm3) < 50, 55 Gy(RBE); Optic chiasm (0.1 cm3) < 55, 60 Gy(RBE); optic nerves (0.1 cc) < 55, 60 Gy(RBE); retina (0.1 cm3) < 50 Gy(RBE); cochlea (mean) < 36, 45 Gy(RBE); temporal lobe (V74) < 2 cm3; hippocampus-head/tail (mean) < 5/20 Gy(RBE); pituitary gland (mean) < 30 Gy(RBE); and lens (mean) < 15 Gy(RBE). The brainstem core was defined as a 3-mm subtraction structure from the surface of the brainstem.
Results
At the time of analysis, three patients were alive with < 1 year of follow-up, 37 had more than 1 year of follow-up, and three were deceased. Seven patients were treated with primary RT after biopsy alone and most underwent fewer than two surgical resections. Only eight patients had three or more procedures, and no patient had more than four surgeries. The oncologic and survival outcomes are shown in Fig. 1. With a median follow-up of 3.7 years, at 4 years local control, overall survival, and toxicity-free survival rates were 89%, 95% and 95%, respectively. Crude events to date are as follows: one patient died with disease more than 5 years after treatment, two died of intercurrent causes 2 years and 8 months after treatment, respectively, and two are alive with disease. Of the two cases of intercurrent deaths, one was unknown with documented stable disease and the other was a suspected stroke. Of the three local failures, two were geographic misses outside of the 80% isodose line, for which the under-coverage is accounted for, as reflected in Table 2. All three patients were treated prior to 2013, two of whom had a grade 2 tumor and one of which had a grade 1. One patient who developed progressive facial weakness and hearing loss, ipsilateral to the initial site of disease, failed within the high-risk CTV. All three underwent unsuccessful surgical salvage.
Acute post-surgical complications included the following: 3 (7%) cases of complete hearing loss, 2 (5%) cerebrovascular events, 1 (2%) patient with damage to an internal carotid artery requiring stenting, 1 (2%) patient with craniospinal fluid leak requiring endoscopic repair, and 1 (2%) patient with a pulmonary embolism and respiratory distress requiring tracheostomy and dysphagia requiring a temporary percutaneous feeding tube. One patient had a cerebrovascular event during an attempted salvage surgery after RT.
RT-related toxicities were divided between acute (during RT) and late events (following RT). All patients had varying degrees and combinations of acute grade 1 and 2 toxicities during RT, which included fatigue, radiation dermatitis, alopecia, and mucositis but there were no grade 3 acute toxicities attributed to RT. In terms of late toxicity, there have been 6 grade 3 events to date. One patient had a grade 2 temporal lobe and brainstem radiation necrosis, and another had had a grade 2 temporal lobe necrosis within 2 years of treatment. For the patient with both a grade 2 temporal lobe and brainstem necrosis, the temporal lobe V74Gy(RBE) was 2.1 cm3 and the brainstem (Dmax to 0.1 cm3) received was 64 Gy(RBE). The patient who experienced a grade 2 ipsilateral temporal necrosis had a V74Gy(RBE) of 5.5 cm3. Lastly, one patient developed bilateral grade 3 temporal lobe necrosis requiring surgical resection for epilepsy 4 years from completion of treatment; the V74GyRBE in this case was less than 2 cm3. Another ten patients who received a V74GyRBE of at least 4 cm3, and eight who had a brainstem Dmax (0.1 cm3) of 64 GyRBE, did not demonstrate any clinical or radiographic evidence of radionecrosis. At least seven patients had or required intervention for middle ear effusions, of which there were 4 (9.0%) who experienced expected grade 3 hearing loss. One patient developed grade 2 peripheral vision loss following surgery that progressed to grade 3 by 10 years after treatment. While 4 (9%) patients had pituitary dysfunction prior to RT, 1 (2%) patient had hormone deficiency that warranted replacement with testosterone, cortisol, and thyroid medications following treatment. We do not have sufficient laboratory follow-up to report detailed information regarding untreated hormone deficiencies following treatment. In summary, while the radiation-related grade 3 toxicity was 95% (n = 2) at 4 years, most events occurred later (n = 4) and were related to expected hearing loss (n = 4).
Discussion
Low-grade, asymptomatic skull base chondrosarcomas for which treatment would cause clinically significant morbidity can be observed with close surveillance. Otherwise, maximal safe surgery to relieve symptoms related to mass effect or to improve the target geometry of highly conformal RT is the preferred initial approach to skull-base chondrosarcomas. Maximal safe surgery can provide a histologic diagnosis, relieve compression of critical structures, and optimize target geometry for postoperative RT [7].
Because gross total resection of these tumors is rare, the ability to deliver conformal high-dose RT is essential for local control. Bolch et al. performed a meta-analysis of over 500 patients with cranial chondrosarcoma and found that, while the 5-year recurrence rate among all patients was 22%, the recurrence rate was highest in patients who underwent surgery alone compared with surgery and RT (44% vs 9%; p < 0.0001) or RT alone (19%; p = 0.036) [8]. Given the tendency of these tumors to originate in paramedian bony sites, surgeons have approached these tumors using a craniotomy, endoscopic or combined approach. These techniques resulted in improved recurrence-free survival, and the shift in surgical management to maximal safe resection parallels technological advancements in the field of radiation oncology and a greater ability to provide highly-conformal adjuvant treatment [9, 10].
For patients with gross residual disease and optimal displacement from critical normal structures, dose intensification with modern RT techniques, such as PT, has been used in the treatment of skull-base chondrosarcomas for local control. Since the dose tolerances of the brainstem, spinal cord, and optic OAR are below the dose required to control gross disease, a minimum separation of at least 1 to 2 mm between the residual disease and the OARs is necessary for adequate target coverage [3, 11,12,13,14,15,16,17,18,19]. Having a radiation oncologist involved early in the surgical planning can assist with the defining areas of interest that achieve the largest benefit from post-surgical target geometry optimization.
Although early reports are promising that PT provides advantages over photon-based RT in delivering an adequate radiation dose to the tumor while reducing the dose to normal tissue and may even provide a survival advantage with dose escalation, there is a relative scarcity of data on the long-term effectiveness of particle therapy [4]. As demonstrated in Table 4, the 3- to 5-year local control ranges from approximately 85% to over 95%, with minimal acute and acceptable late toxicity [11,12,13,14,15,16,17,18, 20, 21]. For instance, the Massachusetts General Hospital (Boston) experience reported on 165 patients treated to a median dose of 72 Gy(RBE) with combined photon and proton RT. They observed an excellent 5-year local control rate of 98% [18]. Investigators from the Switzerland, Japan and France have similarly shown promising results [12,13,14].
In a outcomes study collaboration between Paul Scherrer Institut and the Institut Curie Proton Therapy center-Orsay, Weber et al reported long-term outcomes in 251 patients with skull-base chondrosarcoma, of whom 135 were treated with protons alone. The purpose to evaluate for prognostic factors, and with a median follow-up of over 7 years, they reported a 95% local control rate, a 94% overall survival rate, and an 84% toxicity-free survival rate. A univariate analysis demonstrated that a gross tumor volume greater than or equal to 25 mL was associated with both a statistically significant increased risk of failure and decreased overall survival [16]. Gross residual disease > 25 mL at the time of RT has been a known prognostic indicator for inferior local control [15, 17]. The PSI-ICOP analysis showed that age > 40 at the start of RT, > 1 surgery and a gross tumor volume > 25 mL to be significant for overall survival on univariate analysis, which are consistent with other reports [8, 13, 20, 22, 23]. We did not observe a relationship between recurrence and tumor volume or whether the patient was treated with definitive versus adjuvant RT, which may be attributable to the small sample size. Uhl et al. reported that tumors with boost volumes of up to 55 mL can be treated without an increased risk of local failure [21]. Lastly, while there are few reports investigating the use of carbon therapy in the treatment of skull-base chondrosarcomas, its continued study will determine the role of other forms of heavy-ion particle therapy [20, 24,25,26,27].
Because the chance of salvage after a recurrence is remote, the ability to deliver an adequate RT dose to these skull base chondrosarcomas is crucial to improving local tumor control and overall prognosis. In our series, none of the three local recurrences were surgically salvaged. Only 1 of the local recurrences occurred within the 80% isodose line. The other two were marginal, with the 80% isodose line traversing gross residual disease with a steep dose gradient. This observation connotes the importance of re-evaluating high-risk CTV margins, high-quality pre- and postoperative imaging review within a multidisciplinary team, with an understanding of the potential benefits and limitations of certain surgical techniques and the uncertainties related to sharp-dose fall off, which is often desired when the tumor is adjacent to a critical nerve and optic structures [28].
This approach is echoed in a report from MD Anderson Cancer Center (Houston, TX). Although not specific for skull-base tumors, of the 2830 cases they presented for peer review, changes were recommended in over 10%; 28% were categorized as a dose change, but almost 70% were a target change of which 3% were considered major changes. Of the sites modified, head-and-neck tumors accounted for the majority of changes made [29]. The importance of target delineation, precise image registration, and sub-site chart review cannot be overstated in the era of highly-conformal treatment.
With improved image fusion software and modifications in our treatment planning algorithm using physician-directed image registration of 3D MRI sequence of ≤ 1-mm thick slices and high spatial resolution, we have not documented any geographic failures. We have also implemented a protocol requiring physician-radiologist review for each case and for standardization of our planning process regarding surgical hardware [28]. While no patient in the present series required surgical stabilization, if a patient is to undergo surgical stabilization that will be dispositioned to postoperative RT, the use of carbon-fiber-reinforced polyetheretherketone materials an emerging tool that can mitigate hardware artifacts. This has the added benefit of reducing range uncertainties, and clarity of tumor bed for treatment, surveillance and follow-up [30].
In regards to systemic therapy, no patient in the present series received upfront induction or adjuvant chemotherapy as those with mesenchymal disease were excluded from analysis. Chemotherapy was reserved for those with recurrent disease. Emerging data, however, demonstrate improved local control and survival in patients with mesenchymal tumors who receive induction systemic treatment [31]. While data analyzing recurrent and metastatic disease are beginning to emerge, future research should help determine which agents are the most efficacious [32].
The main limitations of this study are those inherent to any outcomes study for which most of the primary follow-up and toxicity assessment has been performed by an outside institution. Nevertheless, direct contact with both patients and referring physicians continued throughout the follow-up care, including toxicity and outcomes assessments, using an electronic patient portal; relevant imaging was reviewed by the study team. While we were limited in our ability to report grade 1 and 2 toxicity, the high rate of follow-up compliance allowed for us to report oncologic and severe grade 3 + long-term toxicity. Additionally, such limitations are also intrinsic to studies of rare diseases when patients are referred to an international center to which travel for continued follow-up care is preclusive to the patient for various logistical or financial reasons.
Conclusions
High-dose, conformal PT alone or following surgical resection for skull base chondrosarcomas is an effective treatment with a high rate of local control and a relatively low grade 3 toxicity profile within 4 years of treatment completion. Further follow-up of this cohort is necessary to better characterize long-term disease control and late toxicities.
References
Harsh GR, Vaz-Guimaraes F (eds) (2017) Chordomas and chondrosarcomas of the skull base and spine, 2nd edn. Academic Press, Cambridge, MA
Fossati P, Vavassori A, Deantonio L, Ferrara E, Krengli M, Orecchia R (2016) Review of photon and proton radiotherapy for skull base tumours. Rep Pract Oncol Radiother 21(4):336–355
Sahgal A, Chan MW, Atenafu EG, Masson-Cote L, Bahl G, Yu E et al (2015) Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro-Oncology 17(6):889–894
Palm RF, Oliver DE, Yang GQ, Abuodeh Y, Naghavi AO, Johnstone PAS (2019) The role of dose escalation and proton therapy in perioperative or definitive treatment of chondrosarcoma and chordoma: an analysis of the National Cancer Data Base. Cancer 125(4):642–651
Verdegaal SH, Bovee JV, Pansuriya TC, Grimer RJ, Ozger H, Jutte PC et al (2011) Incidence, predictive factors, and prognosis of chondrosarcoma in patients with ollier disease and maffucci syndrome: an international multicenter study of 161 patients. Oncologist 16(12):1771–1779
Deraniyagala RL, Yeung D, Mendenhall WM, Li Z, Morris CG, Mendenhall NP et al (2014) Proton therapy for skull base chordomas: an outcome study from the university of Florida proton therapy institute. J Neurol Surg B 75(1):53–57
Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH et al (2008) The clinical approach towards chondrosarcoma. Oncologist 13(3):320–329
Bloch OG, Jian BJ, Yang I, Han SJ, Aranda D, Ahn BJ et al (2010) Cranial chondrosarcoma and recurrence. Skull Base 20(3):149–156
Tzortzidis F, Elahi F, Wright DC, Temkin N, Natarajan SK, Sekhar LN (2006) Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chondrosarcomas. Neurosurgery 58(6):1090–1098
Gay E, Sekhar LN, Rubinstein E, Wright DC, Sen C, Janecka IP et al (1995) Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 36(5):887–896.
Rosenberg AE, Nielsen GP, Keel SB, Renard LG, Fitzek MM, Munzenrider JE et al (1999) Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol 23(11):1370–1378
Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP et al (2009) Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol, Biol, Phys 75(4):1111–1118
Feuvret L, Bracci S, Calugaru V, Bolle S, Mammar H, De Marzi L et al (2016) Efficacy and safety of adjuvant proton therapy combined with surgery for chondrosarcoma of the skull base: a retrospective, population-based study. Int J Radiat Oncol, Biol, Phys 95(1):312–321
Fuji H, Nakasu Y, Ishida Y, Horiguchi S, Mitsuya K, Kashiwagi H et al (2011) Feasibility of proton beam therapy for chordoma and chondrosarcoma of the skull base. Skull Base 21(3):201–206
Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA et al (1999) Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 91(3):432–439
Weber DC, Murray F, Combescure C, Calugaru V, Alapetite C, Albertini F et al (2018) Long term outcome of skull-base chondrosarcoma patients treated with high-dose proton therapy with or without conventional radiation therapy. Radiother Oncol 129:520–526
Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M et al (2016) Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 120(1):169–174
Munzenrider JE, Liebsch NJ (1999) Proton therapy for tumors of the skull base. Strahlentherapie und Onkologie 175(Suppl 2):57–63
Grosshans DR, Zhu XR, Melancon A, Allen PK, Poenisch F, Palmer M et al (2014) Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol, Biol, Phys 90(3):540–546
Mattke M, Vogt K, Bougatf N, Welzel T, Oelmann-Avendano J, Hauswald H et al (2018) High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer 124(9):2036–2044
Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD et al (2014) High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer 120(10):1579–1585
Li D, Weng JC, Zhang GJ, Hao SY, Tang J, Zhang LW et al (2018) Proposed treatment paradigm for intracranial chondrosarcomas based on multidisciplinary coordination. World Neurosurg 109:e517–e530
Bohman LE, Koch M, Bailey RL, Alonso-Basanta M, Lee JY (2014) Skull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis: a SEER analysis. World Neurosurg 82(5):806–814
Uhl M, Herfarth K, Debus J (2014) Comparing the use of protons and carbon ions for treatment. Cancer J (Sudbury, Mass). 20(6):433–439
Combs SE, Nikoghosyan A, Jaekel O, Karger CP, Haberer T, Munter MW et al (2009) Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 115(6):1348–1355
Schulz-Ertner D, Nikoghosyan A, Hof H, Didinger B, Combs SE, Jakel O et al (2007) Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol, Biol, Phys 67(1):171–177
Rieken S, Habermehl D, Nikoghosyan A, Jensen A, Haberer T, Jakel O et al (2011) Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol, Biol, Phys 81(5):e793–e801
Terezakis SA, Heron DE, Lavigne RF, Diehn M, Loo BW (2011) Jr. What the diagnostic radiologist needs to know about radiation oncology. Radiology 261(1):30–44
Ballo MT, Chronowski GM, Schlembach PJ, Bloom ES, Arzu IY, Kuban DA (2014) Prospective peer review quality assurance for outpatient radiation therapy. Pract Radiat Oncol 4(5):279–284
Li CS, Vannabouathong C, Sprague S, Bhandari M (2015) The use of carbon-fiber-reinforced (CFR) PEEK material in orthopedic implants: a systematic review. Clin Med Insights Arth Musculoskelet Disord 8:33–45
Frezza AM, Cesari M, Baumhoer D, Biau D, Bielack S, Campanacci DA et al (2015) Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European musculoskeletal oncology society study. Eur J Cancer (Oxford, England: 1990) 51(3):374–381
van Maldegem A, Conley AP, Rutkowski P, Patel SR, Lugowska I, Desar IME et al (2018) Outcome of first-line systemic treatment for unresectable conventional, dedifferentiated, mesenchymal, and clear cell chondrosarcoma. Oncologist 24:110–116
Acknowledgements
The authors would like to acknowledge Robin Cacchio, and Amanda Prince for research assistance; the CNS cancer nursing team, including Michelle Wear, Lauren Mynatt, and Michelle Myers; and Jessica Kirwan and Christopher Stich for editorial assistance.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work (ALH, RR, WMM); acquisition, analysis, or interpretation of data for the work (all authors); drafting the work (ALH); revising it critically for important intellectual content (all authors); final approval of the version to be published (all authors).
Corresponding author
Ethics declarations
Conflict of interest
Educational grant from Ion Beam Applications (DI).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holtzman, A.L., Rotondo, R.L., Rutenberg, M.S. et al. Proton therapy for skull-base chondrosarcoma, a single-institution outcomes study. J Neurooncol 142, 557–563 (2019). https://doi.org/10.1007/s11060-019-03129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03129-8