Abstract
BRAFV600E is a common finding in glioma (about 10–60% depending on histopathologic subclassification). BRAFV600E monotherapy shows modest preclinical efficacy against BRAFV600E gliomas and also induces adverse secondary skin malignancies. Here, we examine the molecular mechanism of intrinsic resistance to BRAFV600E inhibition in glioma. Furthermore, we investigate BRAFV600E/MEK combination therapy that overcomes intrinsic resistance to BRAFV600E inhibitor and also prevents BRAFV600E inhibitor induced secondary malignancies. Immunoblotting and Human Phospho-Receptor Tyrosine Kinase Array assays were used to interrogate MAPK pathway activation. The cellular effect of BRAFV600E and MEK inhibition was determined by WST-1 viability assay and cell cycle analysis. Flanked and orthotopic GBM mouse models were used to investigate the in vivo efficacy of BRAFV600E/MEK combination therapy and the effect on secondary malignancies. BRAFV600E inhibition leads to recovery of ERK phosphorylation. Combined BRAFV600E and MEK inhibition prevents reactivation of the MAPK signaling, which correlates with decreased cell viability and augmented cell cycle arrest. Similarly, mice bearing BRAFV600E glioma showed reduced tumor growth when treated with a combination of BRAFV600E and MEK inhibitor compared to BRAFV600E inhibition alone. Additional benefit of BRAFV600E/MEK inhibition was reflected by reduced cutaneous squamous-cell carcinoma (cSCC) growth (a surrogate for RAS-driven secondary maligancies). In glioma, recovery of MAPK signaling upon BRAF inhibition accounts for intrinsic resistance to BRAFV600E inhibitor. Combined BRAFV600E and MEK inhibition prevents rebound of MAPK activation, resulting in enhanced antitumor efficacy and also reduces the risk of secondary malignancy development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRAFV600E mutation, which can constitutively activate the mitogen-activated protein kinase (MAPK) pathway, is one of the most common oncogenic mutations in human cancer, occurring in approximately 67% of melanoma, 36% of papillary thyroid cancer, 10% of colorectal cancer [1–3] and in a significant percentage of pediatric and adult gliomas (Supplementary Table 1). Small molecule kinase inhibitors developed specifically to target BRAFV600E, such as PLX4032 (vemurafenib), have shown significant efficacy against BRAFV600E melanomas [4, 5], raising the possibility that these drugs may show similar efficacy in treating BRAFV600E gliomas.
The efficacy of these drugs, however, may be limited in tumors where receptor tyrosine kinase (RTK) signaling is prominent. Human epidermal growth factor receptor (HER) family member signaling is prominent in BRAFV600E colorectal and thyroid cancer, and the presence of a negative feedback loop between BRAFV600E and EGFR (HER1) or HER3 limits the efficacy of BRAFV600E monotherapy in these tumors [6, 7]. Although EGFR signaling is also prominent in glioma [8, 9], our previous work showed that BRAFV600E monotherapy has efficacy against BRAFV600E glioma [10], and that BRAFV600E inhibitor can be used in combination with a CDK4/6 inhibitor for achieving improved anti-tumor effect [11]. In addition to preclinical results, case reports have shown that BRAFV600E gliomas can respond to BRAFV600E inhibitor (vemurafenib) significantly [12–14]. Moreover, monotherapy against BRAFV600E pediatric gliomas is currently being investigated clinically (ClinicalTrials.gov Identifier: NCT01748149).
Both acquired and intrinsic resistance to BRAFV600E inhibitors has been described in BRAFV600E-mutant malignancies [6, 7, 15, 16], both of which limit the duration of tumor response to inhibitor treatment. An additional concern over the use of BRAFV600E inhibitors is their activation of wild-type RAF proteins, which promotes the development of secondary malignancies [17], and raises concern regarding the safety of BRAFV600E inhibition, especially in young children.
To address these concerns, we investigated the relative efficacy of BRAFV600E inhibition in BRAFV600E gliomas compared to BRAFV600E melanomas. We found that BRAFV600E gliomas showed a relative intrinsic resistance to BRAFV600E inhibition, and that this resistance could be overcome with the addition of a MEK inhibitor. Furthermore, this combination treatment also decreases the growth rate of RAS-driven secondary malignancies.
Materials and methods
Cell lines, xenografts, drugs, and primary tumors
Glioma cell lines (AM-38, DBTRG-05MG, NMC-G1) were obtained from ATCC or the Japanese Brain Tumor Repository; Melanoma cell lines (A375, WM793, WM9) were obtained from Dr. Martin McMahon (UCSF); B9 cSCC cell line were obtained from Dr. Alain Balmain (UCSF); BT40 pilocytic astrocytoma chunks were obtained under MTA from Nationwide Children’s Hospital (Dr. Peter Houghton). PLX4720 was obtained from Plexxikon Inc. (Berkeley, CA, USA). PD0325901 was obtained from Pfizer Inc. (New York City, NY, USA). PLX4032 and GDC0973 were obtained from Genentech Inc. (South San Francisco, CA, USA).
Western blot analysis and human phospho-receptor tyrosine kinase (RTK) array analysis
Proteins were extracted using cell lysis buffer (Cell Signaling) supplemented with proteinase inhibitor cocktail (Roche). Western blots were developed as described previously [10]. Antibodies specific for p-ERK, and Beta-Actin were obtained from Cell Signaling Technologies. Antibodies specific for total ERK were purchased from Santa Cruz Biotechnology. The activation of phospho-receptor tyrosine kinase was tested using Human Phospho-RTK Array Kit (R&D Systems, Inc.)
Cell viability assay
Cells were seeded onto 48-well plates at 2500–3000 cells per well and treated with BRAFV600E and/or MEK inhibitors, and media was changed every 3 days. Cell viability was determined by WST-1 assay (Roche), according to manufacturer’s instructions. GI50 value was analyzed using GraphPad Prism5 software.
Cell cycle analysis
Cells were harvested with 0.25% trypsin, washed with PBS, and then fixed with ice-cold 70% ethanol for minimum of 30 min. Cells were then washed with PBS once, and stained with propidium iodide (20 μg/ml) in PBS containing RNaseA (0.4 mg/ml) (Invitrogen). Cells were sorted using FACSCalibur (Becton, Dickinson), and data was analyzed using the ModFit software (Verity).
In vivo experiments using intracranial xenografts models
Five-week-old female athymic mice (nu/nu genotype, BALB/c background) purchased from Simonsen Laboratories were used. Animals were housed under aseptic conditions. The UCSF Institutional Animal Care and Use Committee approved all animal protocols. 1 × 105 modified AM-38 human glioma cells, which were transduced with lentivirus containing firefly luciferase, were intracranially injected into athymic mice as previously described [18]. In vivo bioluminescence imaging (BLI) was carried out using the Xenogen IVIS Lumina System coupled to LivingImage data-acquisition software (Xenogen Corp.). Mice were anesthetized with isoflurane and imaged 10 min after intraperitoneal (i.p.) injection of luciferin (d-luciferin potassium salt, 150 mg/kg, Gold Biotechnology). Signal intensity was quantified using LivingImage software.
Mice implanted with luciferase-modified AM-38 cells were randomized to four groups receiving treatment of vehicle control (DMSO i.p. injection and 0.5% hydroxypropyl methylcellulose plus 0.2% Tween 80 oral gavage), PLX4720 and PD0325901 alone or in combination. PLX4720 treatment was carried out by i.p. injection using a daily dose of 20 mg/kg for consecutive days. PD0325901 treatment was carried out by oral gavage using a daily dose of 5 mg/kg with a schedule of 4 days on treatment and 4 days off. Treatment was initiated at day 7 post-implantation of tumor cells, once tumors were shown to be in log-phase of growth. In addition to monitoring by BLI to detect the luminescence signal, all the mice were checked every day for the development of symptoms related to tumor burden, and euthanized when they exhibited symptoms indicative of significant compromise to neurologic function. In addition to the mice used for survival analysis, two pre-symptomatic mice within each cohort were used for IHC analysis and western blot analysis. These mice were sacrificed 2 h after treatment on the third day, and their brains were resected and either placed in formalin and prepared for IHC, or dissected from surrounding normal brain and snap frozen in liquid nitrogen for western blot analysis. IHC was carried out as previously described [18], using Ki-67 antibody (Ventana Inc) and p-ERK1/2 antibody (Cell Signaling Technologies).
In vivo experiments using subcutaneous xenograft tumor models
Eight-week-old female scid mice (C.B-17 background) were purchased from Taconic Farms, Inc. Animals were housed under aseptic conditions, and UCSF Institutional Animal Care and Use Committee approve all protocols.
To investigate the efficacy of RO5185426 (PLX4032) and GDC0973 combined treatment against BRAFV600E-mutant glioma, BT40 chunks were subcutaneously implanted into the right flank of scid mice. These mice were randomized to four groups receiving treatment of vehicle control (2% Hydroxypropylcellulose, and 0.5% hydroxypropyl methylcellulose plus 0.2% Tween 80 oral gavage), PLX4032 and GDC0973 alone or in combination. PLX4032 treatment was carried out by oral gavage using a dose of 25 mg/kg twice daily for 16 days with a schedule of 6 days on treatment and 2 days off. GDC0973 treatment was carried out by oral gavage using a daily dose of 5 mg/kg for 16 days with a schedule of 4 days on treatment and 4 days off. Two additional mice within each cohort were used for IHC analysis and western blot analysis. These mice were sacrificed 2 h after treatment on the fourth day in order to collect BT40 tumors. IHC and western blot analysis were carried out as those for intracranial BRAFV600E MA xenografts above. Mice were monitored every two days and euthanized due to significant tumor burden (length >15 mm), weight loss >15%, or symptoms related to tumor burden.
To investigate the efficacy of PLX4720 and PD0325901 combined treatment against RAS-driven secondary malignancies, BT40 chunks were subcutaneously implanted into the right flank of scid mice. Then, after 10 days, 1 × 106 RAS-mutant B9 cutaneous squamous cells were subcutaneously injected to the left flank of scid mice (Fig. 5c). These mice were randomized to four groups receiving treatment of vehicle control (DMSO i.p. injection and 0.5% hydroxypropyl methylcellulose plus 0.2% Tween 80 oral gavage), PLX4720 and PD0325901 alone or in combination. PLX4720 treatment was carried out by i.p. injection using a daily dose of 10 mg/kg for 16 consecutive days. PD0325901 treatment was carried out by oral gavage using a daily dose of 5 mg/kg for 16 days with a schedule of 4 days on treatment and 4 days off. Mice were monitored every two days and euthanized due to significant tumor burden (length >20 mm), weight loss >15%, or symptoms related to tumor burden such as skin ulcer.
All of these treatments were initiated when tumor volume reached at 100 mm3. The maximum longitudinal diameter (length) and the maximum transverse diameter (width) of tumors were measured by caliper every 2 days, and tumor volume was calculated using the equation 1/2 × (length × width2).
Statistical analysis
A 2-tailed unpaired t test was used for statistical comparison, with p values of <0.05 considered significant. A log-rank (Mantel-Cox) test was employed for survival analysis (GraphPad Prism5).
Results
BRAFV600E inhibitor suppression of MEK-ERK activity in glioma cell lines is transient
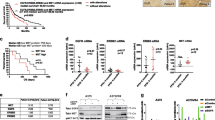
We compared effects of PLX4720 (BRAFV600E inhibitor, a tool compound analog of PLX4032) treatment in BRAFV600E glioma (AM-38, DBTRG-05MG and NMC-G1) and melanoma (A375, WM793 and WM9) cell lines. Genetic characteristics of the cell lines tested are described in Supplementary Table 2 [19–23]. While phosphorylation of ERK was initially suppressed by PLX4720 in all cells, this phosphorylation partially recovered in glioma lines, but not in melanoma lines for the entire 24-h period of sample collection (Fig. 1a). This recovery was also observed functionally, as PLX4720 induced less inhibition of cell viability in glioma cell lines compared to melanoma cell lines (p = 0.0082—Fig. 1b, Supplementary Fig. 1). While G1 phase arrest induced by PLX4720 was observed in all lines, arrest was less prominent in glioma cells compared to melanoma cells (p = 0.0216—Fig. 1c).
Efficacy of PLX4720 against BRAFV600E melanoma and glioma cells. BRAFV600E-mutant glioma cells (DBTRG-05MG, AM-38 and NMC-G1) and BRAFV600E-mutant melanoma cells (A375, WM9 and WM793) were used. a Cells were cultured in 10% serum media, and treated with 2 μM PLX4720 for 0 h, 15 m, 1, 6, and 24 h. Cell lysates were analyzed using western blot. Phosphorylation of ERK was inhibited by PLX4720 initially in all cell lines, but partially recovered in glioma cell lines compared to in melanoma cell lines. b Cells were treated with DMSO (control) or 2 μM PLX4720 for 6 days. Media was changed once every 3 days. Cell viability was measured using WST-1 assay. Error bars indicate the variation between triplicate measurements. PLX4720 induced suppression of cell viability in all cell lines. But more cell viability was observed in glioma cell lines compared to in melanoma cell lines (p = 0.0082). c Cells were treated with DMSO (control) or 2 μM PLX4720 for 24 h for cell cycle analysis. PLX4720 induced G1 phase arrest in all cell lines. But less G1 phase arrest was observed in glioma cell lines compared to in melanoma cell lines (p = 0.0216)
Co-inhibition of BRAFV600E and MEK prevents recovery of MAPK signaling
Given the results above, we hypothesized that the combination of BRAF and MEK inhibitors may increase and/or extend the anti-tumor effect of small molecule inhibitors on glioma cell lines, relative to that of inhibitor monotherapy. The results show that combined use of PLX4720 and PD0325901 (MEK inhibitor) significantly reduced activation of ERK in both AM-38 and DBTRG-05MG glioma cells (Fig. 2a). The effect on ERK was accompanied by a more pronounced growth inhibitory activity of combination treatment (Fig. 2b), and by modest, yet discernable increases in G1 phase cells in cultures treated with both inhibitors (all p values >0.05—Fig. 2c, Supplementary Fig. 2).
Combined BRAFV600E and MEK inhibition prevented ERK recovery, decreased cell viability, and increased G1 phase arrest in BRAFV600E glioma cell lines. All treatments used DMSO (control), 2 μM PLX4720, 10 nM PD0325901 and their combination. a AM-38 and DBTRG-05MG cells were cultured in 10% serum media overnight, then treated for 0, 1, 6 and 24 h. Cell lysates were analyzed by western blot. In both AM-38 and DBTRG-05MG cell lines, combined treatment decreased the recovery of p-ERK induced by PLX4720. b AM-38 and DBTRG-05MG cells were treated for 5 days. Media was changed once every 3 days. Cell viability was measured using WST-1 assay. Error bars indicate the variation between triplicate measurements. PLX4720 and PD0325901 alone or in combination reduced cell viability significantly (p value of control vs. PLX4720 in AM-38 = 3.1339E-05, in DBTRG-05MG = 4.0725E-05; p value of control vs. PD0325901 in AM-38 = 1.5265E-05, in DBTRG-05MG = 9.2805E-06; p value of control vs. combo in AM-38 = 5.5372E-07, in DBTRG-05MG = 5.2469E-06). However, combined therapy led to the most significant cell viability reduction compared to either monotherapy in both AM-38 and DBTRG-05MG cell lines (p value of combo vs. PLX4720 in AM-38 = 0.0015, in DBTRG-05MG = 0.0005; p value of combo vs. PD0325901 in AM-38 = 0.0237, in DBTRG-05MG = 0.0005). c AM-38 and DBTRG-05MG cells were treated for 24 h for cell cycle analysis. In both of AM-38 and DBTRG-05MG cell lines, all three therapies increased G1 population cells, among which modest but discernable increases of G1 phase cells were induced by combined treatment compared to monotherapy (all p values >0.05)
Combined BRAFV600E and MEK blockade shows increased anti-tumor efficacy against BRAFV600E-mutant glioma in vivo
As the combination of BRAFV600E and MEK inhibitors shows increased efficacy against cultured glioma cell lines, we next attempted to extend in vitro observations to in vivo intracranial BRAFV600E glioma xenograft models. Athymic mice were injected intracranially with AM-38 cells modified with a luciferase reporter, and then treated with PLX4720 or PD0325901 or both. Treatment with PLX4720 and PD0325901 alone reduced the growth of intracranial tumors, with further growth reduction evident from combination treatment (Fig. 3a). Consistently, all three therapies significantly prolonged the survival of mice compared to control, while combined treatment showed more benefit (statistically significant compared to PLX4720; modest but discernable compared to PD0325901) (Fig. 3b). We also examined treatment effects on Ki-67 staining (used to indicate proliferating cells) and phosphorylation of ERK, in tumors obtained from mice euthanized while on therapy. Singular PLX4720 and PD0325901 treatments decreased Ki-67 positivity in AM-38 tumors, and combination therapy resulted in further reduced staining (Fig. 3c). Similarly, we found that the combination of PLX4720 and PD0325901 resulted in a more substantial blockade of MAPK signaling than either drug alone (Fig. 3d, Supplementary Fig. 3).
Combined treatment with BRAFV600E and MEK inhibitors suppressed tumor growth in intracranial BRAFV600E AM-38 xenografts model. Mice intracranially injected with modified AM-38 cells were treated with vehicle (control), PLX4720 and PD0325901 alone or in combination. Tumor tissues were harvested for subsequent analysis. a All three therapies suppressed the growth of intracranial tumor, but combined therapy led to the most suppression (22 days post treatment, p value of combo vs. PLX4720 = 0.0317, combo vs. PD0325901 = 0.0265). b All three therapies prolonged the survival of mice, and combined treatment showed more benefit (statistically significant compared to PLX4720; modest but discernable compared to PD0325901) (p value of control vs. PLX4720 = 0.0003, control vs. PD0325901 < 0.0001, control vs. combo = 0.0002, combo vs. PLX4720 = 0.0388, combo vs. PD0325901 = 0.1612). c Ki-67 expression was suppressed by all three therapies (p value of control vs. PLX4720 = 0.0151, control vs. PD0325901 = 0.0001, control vs. combo = 2.6741E-05). Combined treatment suppressed Ki-67 the most significantly (p value of PLX4720 vs. combo = 1.1237E-05, PD0325901 vs. combo = 0.0017). d Western blot analysis revealed that phosphorylation of ERK was inhibited by all three types of treatments, among which the combined treatment was the most effective to down-regulate ERK activity
In an additional experiment with compounds currently in being used to treat cancer patients (BRAFV600E inhibitor PLX4032 and MEK inhibitor GDC0973: currently being tested in patients with BRAFV600E melanoma), we administered mono and combination therapy to mice with subcutaneous BRAFV600E BT40 xenografts. As shown in Fig. 4a, PLX4032 therapy cause tumor growth stasis, whereas GDC0973 therapy promoted tumor regression that was made even more substantial by including PLX4032. Consistently, PLX4032 and GDC0973 alone or in combination significantly increased mice survival compared to control, while combined treatment has even better efficacy (statistically significant compared to PLX4032; modest but discernable compared to GDC0973) (Fig. 4b). Immunohistochemical (IHC) analysis showed that, compared to PLX4032 and GDC0973 monotherapies, combined treatment resulted in the largest decrease in tumor Ki-67 staining (Fig. 4c). Similarly, our data showed combination treatment as being most effective in suppressing ERK activation (Fig. 4d, Supplementary Fig. 4).
PLX4032 and GDC0973 combined treatment reduced the growth of BRAFV600E BT-40 astrocytoma xenografts. BT40 chunks were subcutaneously implanted into flank of scid mice. Mice were treated with vehicle (control), PLX4032 and GDC0973 alone or in combination. Tumor tissues were harvested for subsequent analysis. a All three therapies suppressed the growth of BT-40 tumors (p value of control vs. PLX4032 = 0.0001, control vs. GDC0973 = 0.0002, control vs. combo = 0.0002). Mice treated with PLX4032 alone showed slow-down tumor growth, but mice treated with GDC0973 alone and combined with PLX4032 showed tumor shrink, in which, combined treatment led to the most significant tumor regressions (p value of GDC0973 vs. PLX4032 = 0.0053, combo vs. PLX4032 = 0.0034, combo vs. GDC0973 = 0.0028). b All three therapies prolonged the survival of mice, and combined treatment showed more benefit (statistically significant compared to PLX4720; modest but discernable compared to PD0325901) (p value of control vs. PLX4032 = 0.0044, control vs. GDC0973 = 0.0018, control vs. combo = 0.0018, combo vs. PLX4032 = 0.0018, combo vs. GDC0973 = 0.0768). c Compared to control, all three therapies inhibited Ki-67 expression significantly (p value of control vs. PLX4032 = 0.0024, control vs. GDC0973 = 0.0001, control vs. combo = 1.1711E-05), among which, combined treatment induced the most inhibition (p value of combo vs. PLX4032 = 0.0235, combo vs. GDC0973 = 0.0268). d Phosphorylation of ERK was suppressed by all three therapies, while combined therapy inhibited the most ERK activity
Combined BRAFV600E and MEK inhibition decreases cutaneous squamous-cell carcinoma growth
cSCC can be an alarming consequence of vemurafenib monotherapy for melanoma patients [4, 24]. Both preclinical and clinical studies have shown that BRAFV600E inhibition has the potential to accelerate the progression of cSCC, and that the addition of MEK inhibitors can inhibit the development of cSCC in patients [17, 25, 26].
To investigate whether the combination of BRAFV600E and MEK inhibitors could reduce cSCC in our experiments, we used the RAS-mutant B9 cSCC cell line [17] to model secondary skin cancer. PLX4720 and PD0325901 alone or in combination were used to treat mice bearing B9 cSCC xenografts on one flank and BRAFV600E BT40 xenografts on the other. Our results show that combination treatment caused the most substantial regression of BT40 xenografts (Fig. 5a, c), and that PD0325901, alone or in combination with PLX4720, was effective in preventing the growth of B9 cSCC xenografts (Fig. 5b, c).
Combined BRAFV600E and MEK inhibition reduced the risk of secondary skin malignancies. BT40 chunks were subcutaneously implanted into the right flank of scid mice. After 10 days, 1 × 106 B9 cutaneous squamous cells were subcutaneously injected to the left flank of scid mice. Mice were treated with vehicle (control), PLX4720 and PD0325901 alone or in combination. a All three therapies suppressed the growth of BT40 tumors compared to control (p value of control vs. PLX4720 = 0.0014, control vs. PD0325901 = 0.0003, control vs. combo = 9.1724E-05), among which combined treatment showed the most suppression (p value of combo vs. PLX4720 = 1.0885E-05, combo vs. PD0325901 = 0.0189). b Compared to control and PLX4720 alone, PD0325901 alone or combined with PLX4720 repressed the growth of B9 tumors significantly (p value of PD0325901 vs. control = 0.0134, PD0325901 vs. PLX4720 = 0.0197; p value of combo vs. control = 0.0165, combo vs. PLX4720 = 0.0231). (c) Images of mice carrying BT40 and B9 xenografts. In each group, images of one mouse (day 0, 6, and 10) were shown
Discussion
BRAFV600E tumors targeted therapy using small molecule kinase inhibitors produces varying extent of responses in diverse BRAFV600E tumors, from pronounced response in melanoma to limited response in colon and thyroid cancers [4, 6, 7]. Our previously published results show only modest pre-clinical effect of PLX4720 against BRAFV600E gliomas [10], which contrast with the robust preclinical efficacy observed when used in treating melanoma [27]. This disparity in anti-tumor activity prompted the current study, where we illustrate transient MEK-ERK blockade by BRAFV600E inhibitor that results in only modest cell viability loss and cell cycle impairment in BRAFV600E glioma (Fig. 1).
While BRAFV600E is a potent oncogene and promotes increased MAPK pathway signal transduction [28], its expression also results in a significant activation of proteins whose role is to attenuate pathway signaling [29, 30]. These negative regulators act at various points along the RTK/RAS/MEK/MAPK axis [29, 31]. In BRAFV600E tumors where RTK signaling is prominent, a recovery of RTK signaling is promoted by BRAFV600E inhibition because of the inhibitor’s repressive effect on negative regulators of RTK activity. As demonstrated in BRAFV600E colon cancer and thyroid cancer, activation of HER family members was induced by BRAFV600E inhibition [6, 7]. RTK activation is known to promote RAS activation, which recruits and activates wild-type RAF promoters. We believe this series of inhibitor responses is responsible for the recovery of the MAPK pathway in BRAFV600E glioma cells treated with BRAFV600E inhibitor. As demonstrated in our previous work [16], BRAFV600E inhibition leads to MEK/ERK signaling suppression in BRAFV600E glioma, which in turn down-regulates the EGFR phosphatase PTPN9. Inhibition of PTPN9 in turn results in sustained EGFR phosphorylation and enhanced EGFR activity, which promote the activation of RAS, then further activates c-RAF/MEK/ERK signaling. However, targeting single RTKs in gliomas can result in tumor cells adjusting by activating alternative RTKs and leave downstream signaling little affected [32, 33]. Our Phospho-RTK Array data also showed that besides EGFR, another RTK, Axl was activated in the presence of PLX4720 (Supplementary Fig. 5). Thus, we combined a downstream MEK inhibitor with BRAFV600E inhibitor to treat BRAFV600E gliomas.
Early melanoma studies demonstrated that near-complete target inhibition of the MAPK pathway was crucial to have a clinical effect, which reveals the prominent role of MAPK signaling in BRAFV600E tumors [34]. In our experiments, we observed that, compared with BRAFV600E inhibition only, combination of BRAFV600E and MEK inhibition provides more substantial and durable anti-tumor effect in BRAFV600E glioma (Figs. 2, 3, 4). This means that the MAPK signaling can also be a main contributor to BRAFV600E glioma resistance when it is reactivated by BRAFV600E inhibition. Therefore, combined treatment in our experiments is more effective because of additional MEK inhibition preventing MAPK reactivation. This is also confirmed by a variety of reports. In BRAFV600E resistant glioma cells, our previous work did not find any obvious MAPK inhibition induced by BRAFV600E inhibitor, which is related to the insensitivity of cell response to BRAFV600E inhibitor [35]. In BRAFV600E melanoma, Basile et al. found that the MAPK reactivation, as results of HRAS mutation or BRAFV600E variants induced by long-term BRAFV600E inhibition, is the main reason for tumor resistance to BRAFV600E inhibitors [36]. Co-therapy using BRAFV600E and MEK inhibitors has also been studied in BRAFV600E melanoma patients, and early results show improved efficacy in treating BRAF-mutant melanoma [25, 26, 37]. In BRAFV600E colon cancer, Corcoran et al. confirmed that the inefficiency of BRAFV600E inhibition is caused by EGFR-mediated reactivation of MAPK signaling, which can be overcome by combining with EGFR or MEK inhibitors [38].
Based on our in vitro and in vivo results, we believe that therapy with BRAFV600E and MEK co-inhibition is a promising strategy for clinical trials against BRAFV600E glioma treatment. In addition, BRAFV600E inhibitors are known to promote the development of RAS-driven secondary malignancies, which is a particular concern when treating pediatric patients. Reports have shown that 20–30% of melanoma patients treated with BRAFV600E inhibitors develop keratoacanthomas/cutaneous squamous-cell carcinoma (cSCC) [4, 24, 37]. Preclinical results have shown that BRAFV600E inhibition accelerates the progression of cSCC, whereas combined BRAF/MEK inhibition can prevent this [17]. Furthermore, clinical trial results have shown that combined treatment with BRAF and MEK inhibitors improves progression-free survival of patients with melanoma, and, as well, decreases the risk of cutaneous squamous-cell carcinoma [25, 26, 37]. Our in vivo results show that while BRAFV600E inhibitor decreases the growth of BRAFV600E gliomas, the combination of BRAFV600E and MEK inhibitors not only suppresses BRAFV600E glioma growth, but also inhibits the development of secondary skin malignancies (Fig. 5).
Initial clinical trials with MEK inhibitors showed significant toxicity, with rash and ocular reactions being dose-limiting [39]. For this reason, we used an intermittent dosing strategy of MEK inhibitors in this study, which has proven to be better tolerated by patients in contemporary clinical trials. It will be interesting to follow clinical trial progress for pediatric BRAFV600E glioma, and observe what approaches are used for extending benefit to patients from inhibiting this activated oncogene.
References
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954. doi:10.1038/nature00766
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454–1457
Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S et al (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712. doi:10.1200/JCO.2008.18.0786
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K et al (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819. doi:10.1056/NEJMoa1002011
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. doi:10.1056/NEJMoa1103782
Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R (2012) Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483:100–103. doi:10.1038/nature10868
Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA (2013) Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 3:520–533. doi:10.1158/2159-8290.CD-12-0531
Huang PH, Xu AM, White FM (2009) Oncogenic EGFR signaling networks in glioma. Sci Signal 2:re6. doi:10.1126/scisignal.287re6
Hatanpaa KJ, Burma S, Zhao D, Habib AA (2010) Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 12:675–684
Nicolaides TP, Li H, Solomon DA, Hariono S, Hashizume R, Barkovich K, Baker SJ, Paugh BS, Jones C, Forshew T et al (2011) Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res 17:7595–7604. doi:10.1158/1078-0432.CCR-11-1456
Huillard E, Hashizume R, Phillips JJ, Griveau A, Ihrie RA, Aoki Y, Nicolaides T, Perry A, Waldman T, McMahon M et al (2012) Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc Natl Acad Sci USA 109:8710–8715. doi:10.1073/pnas.1117255109
Robinson GW, Orr BA, Gajjar A (2014) Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer 14:258-2407-14-258. doi:10.1186/1471-2407-14-258
Bautista F, Paci A, Minard-Colin V, Dufour C, Grill J, Lacroix L, Varlet P, Valteau-Couanet D, Geoerger B (2014) Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer 61:1101–1103. doi:10.1002/pbc.24891
Rush S, Foreman N, Liu A (2013) Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol 31:e159–e160. doi:10.1200/JCO.2012.44.1568
Spagnolo F, Ghiorzo P, Queirolo P (2014) Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget 5:10206–10221
Yao TW, Zhang J, Prados M, Weiss WA, James CD, Nicolaides T (2015) EGFR blockade prevents glioma escape from BRAFV600E targeted therapy. Oncotarget 6:21993–22005
Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT et al (2012) RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 366:207–215. doi:10.1056/NEJMoa1105358
Hashizume R, Gupta N, Berger MS, Banerjee A, Prados MD, Ayers-Ringler J, James CD, VandenBerg SR (2010) Morphologic and molecular characterization of ATRT xenografts adapted for orthotopic therapeutic testing. Neuro Oncol 12:366–376. doi:10.1093/neuonc/nop033
Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, Ozawa T, James CD, Waldman T (2010) Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 70:3228–3238. doi:10.1158/0008-5472.CAN-09-4559
Silva JM, Bulman C, McMahon M (2014) BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Mol Cancer Res 12:447–463. doi:10.1158/1541-7786.MCR-13-0224-T
Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB, Gallardo H, Liu C, Merghoub T, Hefter B et al (2012) Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene 31:446–457. doi:10.1038/onc.2011.250
Meyer P, Klaes R, Schmitt C, Boettger MB, Garbe C (2003) Exclusion of BRAFV599E as a melanoma susceptibility mutation. Int J Cancer 106:78–80. doi:10.1002/ijc.11199
Levesque MJ, Ginart P, Wei Y, Raj A (2013) Visualizing SNVs to quantify allele-specific expression in single cells. Nat Methods 10:865–867. doi:10.1038/nmeth.2589
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT et al (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366:707–714. doi:10.1056/NEJMoa1112302
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ et al (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888. doi:10.1056/NEJMoa1406037
Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L et al (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371:1867–1876. doi:10.1056/NEJMoa1408868
Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK et al (2008) Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 105:3041–3046. doi:10.1073/pnas.0711741105
Tsavachidou D, Coleman ML, Athanasiadis G, Li S, Licht JD, Olson MF, Weber BL (2004) SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res 64:5556–5559. doi:10.1158/0008-5472.CAN-04-1669
Lito P, Rosen N, Solit DB (2013) Tumor adaptation and resistance to RAF inhibitors. Nat Med 19:1401–1409. doi:10.1038/nm.3392
Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N (2009) (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA 106:4519–4524. doi:10.1073/pnas.0900780106
Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, Huang A, Wong WL, Callahan MK, Merghoub T et al (2012) Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22:668–682. doi:10.1016/j.ccr.2012.10.009
Martinho O, Zucca LE, Reis RM (2015) AXL as a modulator of sunitinib response in glioblastoma cell lines. Exp Cell Res 332:1–10. doi:10.1016/j.yexcr.2015.01.009
Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C et al (2007) Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318:287–290
Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G et al (2010) Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467:596–599. doi:10.1038/nature09454
Nicolaides T, Yao TW, Yoshida Y, Zhang J, Ozawa T, James D (2014) Targeting resistance pathways in BRAF-mutant pediatric gliomas. Neuro. Oncol 16:iii27–iii28
Basile KJ, Abel EV, Dadpey N, Hartsough EJ, Fortina P, Aplin AE (2013) In vivo MAPK reporting reveals the heterogeneity in tumoral selection of resistance to RAF inhibitors. Cancer Res 73:7101–7110. doi:10.1158/0008-5472.CAN-13-1628
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N et al (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694–1703. doi:10.1056/NEJMoa1210093
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE, et al (2012) EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2:227–235. doi:10.1158/2159-8290.CD-11-0341
Zhao Y, Adjei AA (2014) The clinical development of MEK inhibitors. Nat Rev Clin Oncol 11:385–400. doi:10.1038/nrclinonc.2014.83
Acknowledgements
This study was supported by grants from the St. Baldrick’s Foundation (TN), UCSF Resource Allocation program (TN), the Frank A Campini Foundation (TN), the National Institute for Neurological Disorders and Stroke (K08NS065268: TN; R01NS080619: CDJ), the National Cancer Institute (P50CA097257: TN, MP, CDJ; P30CA82103: WAW; U01CA176287: WAW; U54CA163155: WAW), the Pediatric Brain Tumor Foundation (WAW; TN) and the Samuel Waxman Cancer Research Foundation (WAW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Yao, TW., Hashizume, R. et al. Combined BRAFV600E and MEK blockade for BRAFV600E-mutant gliomas. J Neurooncol 131, 495–505 (2017). https://doi.org/10.1007/s11060-016-2333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2333-4