Abstract
Falx and parasagittal meningiomas are common locations for meningiomas of the cranial vault. Many of these tumors are now discovered incidentally during cranial imaging for other reasons. Therefore, in the calculation of the risks and benefits of surgery it behooves the surgeon to do all he/she can to avoid surgical complications. This is a heavily experience based article based off the senior author’s experience with over 1200 intracranial meningiomas. We present three cases to illustrate some of the decision-making and techniques used to reduce complications in the management of these cases treated with an open operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common primary intracranial neoplasm, making up 36 % of all intracranial tumors [1]. They are thought to grow from abnormal proliferation of the arachnoid cap cells [2]. Meningiomas can occur anywhere throughout the central nervous system and are most commonly located along the junction of the convexity and parasagittal dura as well as along the falx [3, 4]. They often invade or involve the major venous sinuses, including the superior sagittal sinus.
Meningiomas are classified as WHO Grade I–III [5]. The majority of meningiomas are WHO Grade I (65–80 %), while atypical, WHO Grade II tumors, make up an additional 20–35 %, and malignant/anaplastic, WHO Grade III meningiomas comprise only 1–3 % [6]. Surgical resection is the standard of care for symptomatic or enlarging tumors, providing definitive diagnosis and treatment for low-grade meningiomas. Stereotactic radiosurgery can also be used as a primary treatment for meningiomas smaller than 2–3 cm in diameter or as an adjuvant for recurrent or higher-grade tumors [7]. Most patients with surgically resected low-grade meningiomas have excellent long-term survival, with >80 % still alive 10-years after surgery [8].
Given that most patients have excellent long-term survival after resection of low-grade meningiomas, complications can be especially devastating and have long-term impact on the patient’s life. A recent series of convexity meningioma resections found the most common surgical complications to be wound infection (3.5 %), cerebral spinal fluid (CSF) leak (1.4 %), and post-operative hematoma (1.4 %) [9]. Medical complications included deep venous thrombosis (4.2 %) and pulmonary embolus (1.4 %) [9]. Another series of convexity meningioma resections found similar results with respect to complications, with infection (3.9 %) being the most common, followed by CSF leak/hydrocephalus (1.8 %) [10]. Complications were higher for parasagittal/falx tumors, where a recent series of 135 tumors found a 19 % complication rate [11]. Surgical complications included wound breakdown/infection (5 %), CSF leak/hydrocephalus (3 %), hematoma (1.5 %), venous infarct/cerebral edema (1.5 %) and air embolism (0.7 %). Medical complications included new seizures (2.2 %), electrolyte disturbance (1.5 %), as well as low rates of pneumonia, urinary tract infection, arrhythmia, idiopathic thrombocytopenia, and deep venous thrombosis (all 0.7 %). Risk factors for medical complications included development of new/worsened neurological deficit, age >65 years, hypertension and being on more than two cardiac medications prior to surgery [12].
One of the most dreaded complications after meningioma resection is venous infarction, which occurs in 2.0–3.3 % of patients, is irreversible, and can cause devastating consequences [12, 13]. Interestingly, one study found the rate to be highest (5.5 %) for superficial meningiomas, specifically convexity and parasagittal/falx tumors [13]. Risk factors include bifrontal craniotomy, tumors >4 cm, tumors with peritumoral edema, and tumor location close to the midline. These risk factors reflect the danger of inadvertently injuring, or purposefully resecting veins that obstruct access to tumors during resection of convexity, falx and parasagittal meningiomas. The superficial venous anatomy encountered by the surgeon as the midline is approached can be very complex, especially with tumors located further posterior and near eloquent brain structures, including the motor cortex [14]. Here, we review some of the peri-operative items to be considered and present three cases that illustrate methods to protect and preserve normal venous anatomy as well as aberrant venous drainage pathways created in response to tumor growth, and thus avoid surgical complications during resection of falx and parasagittal meningiomas.
Pre-operative checklist (Table 1)
Once the decision is made with the patient that surgery will be undertaken, the surgeon should consider a number of items on pre-operative imaging that will influence surgical planning and expectations for extent of planned removal. The status of the superior sagittal sinus (SSS), whether patent or occluded, has a significant impact on surgical decision making. Incomplete obstruction with invasion of the side wall and roof of the sinus (Sindou type II–IV) may prompt the surgeon to select subtotal resection in the middle and posterior thirds of the sinus, reserving radiosurgery to treat the remnants or recurrence [15].
One important and often overlooked feature seen on T1 post gadolinium images is the presence of diploic venous channels. These channels can be important pathways for venous drainage for parasagittal draining veins. Coronal images should be reviewed to follow the course of these channels within bone. In Case #1 magnetic resonance (MR) images suggested such a channel as a collateral to SSS venous blood flow that was confirmed on the venous phase of angiography (Fig. 4) and required a more limited unilateral bone flap and acceptance of a lesser extent of resection.
Vasogenic edema seen in the brain adjacent to a meningioma is a clue to the surgeon that pial blood supply has been recruited and therefore the interface between the tumor and brain will be indistinct, i.e. there will be no “arachnoid plane”. Realizing this, and depending on location, will allow the surgeon to decide based on patient and tumor factors whether subpial dissection and a limited area of cortical ischemia will be tolerated. During this dissection the surgeon must try to preserve all sulcal arteries. In peri-central/rolandic locations the concern would be new motor/sensory deficits, which are often mild and recover well. This risk can be discussed with the patient pre-operatively so that for attempts at complete removal along the surface of the brain the expected clinical and imaging findings post-operatively are not a surprise, as illustrated in Case #3. MR imaging will often show a thin rim of restricted diffusion related to sub-pial dissection and in follow-up at 6–12 months post-operatively this rim will enhance as part of the healing process and should not be confused with recurrent tumor.
Extension of tumor across the midline has different implications for falx and parasagittal tumors. For falx tumors this extension may influence patient positioning or head rotation so that the opposite side can more easily be seen from the contra-lateral approach. In addition, falx tumors with extension across the lower edge of the falx may be best handled by positioning with the ipsilateral tumor side down, full lateral position, with head laterally flexed up. For upper edge tumors, 60–90 degrees of rotation for ipsilateral approach with the head laterally flexed towards the floor may be best suited. For parasagittal tumors exposure of the subarachnoid space on both sides may be required. Another option when the SSS is completely occluded is to open the convexity dura only over the involved dura, as in Case #2, and remove the tumor from within.
The risk of operative complications depends on patient and tumor factors as well as location relative to motor, sensory or visual cortex. The position of the central sulcus is easily found by following the superior frontal sulcus back to the pre-central sulcus. If there is too much mass effect then the contralateral side can be used as a reference or diffusion tensor imaging (DTI) with motor fiber tractography is most helpful, as demonstrated in Case #3. The use of tractography also holds true as an excellent adjunct for locating sensory and visual cortex. If there is edema within this cortex then a new post-operative neurologic deficit is pretty much guaranteed and patients should be counseled appropriately.
Intra-operative checklist (Table 2)
Knowledge of the vascular territories of the supraorbital, superficial temporal and occipital artery supply to the scalp is important in deciding on scalp flaps. If prior incisions have been made, extensions to allow for a bigger craniotomy must respect these territories and extensions must always come off prior incisions at right angles. In some cases the surgeon may wish to dissect the subgaleal space and preserve the pericranium as a pedicle based vascularized flap for dural repair or as a flap to lay under the skin incision line to promote wound healing. In patients who have had radiation to the scalp or have been on long-term steroids, we use a combination of vitamin C (500 mg. T.I.D.), vitamin A (10,000 IU), zinc (220 mg) tablets and A&D ointment on the incision (B.I.D.) to assist with collagen formation and cross linking and to counter-act the effect of steroids on wound healing [16, 17]. Pre-operative embolization of scalp vessels that also supply the tumor may increase the risk of wound problems so that routine use of scalp clips might be avoided in these cases.
For all falx and parasagittal tumors the senior author has used the two-part craniotomy to allow full exposure of the midline when venous diploic collateral channels allow [18]. Placing two burr holes in a paramedian position 1.5 cm. from the midline helps avoid large venous lakes and gives enough room to dissect the midline under direct vision. Using the cupped end of the Penfield #1 the SSS can be dissected safely 1 cm. to the opposite side. The author has always been able to control venous bleeding with strips of gelfoam and direct pressure. No lacerations of the sinus have occurred.
Dural openings for falx and parasagittal meningiomas are traditionally U-shaped for approaches ipsilateral to the side of the dominant tumor mass. However, when one encounters a parasagittal draining vein or venous lake, the dural incision should be altered so as to reduce tension on these structures and the risk of injury or laceration, as illustrated in Case #3. For approaches from the contra-lateral side it is preferred to have the base of the dural flap laterally on the dependent side down so that a smooth rolled edge of dura is against the brain. This may reduce congestion of veins that may occur on the cut linear edge of a dural opening.
Currently MR venogram (MRV) data can be displayed as a colored three-dimensional object on image guided systems that may assist with planning the surgical approach. For small falx meningiomas a contra-lateral approach may be taken if the anatomy is unfavorable on the side of the dominant tumor mass. In these cases the falx is incised anterior to the tumor first, then over the superior pole and then posteriorly to the free edge of the falx. In instances of small amounts of bleeding from parasagittal veins this should be managed with a gelfoam tamponade rather than using bipolar cautery and occluding the vein.
The senior author has not routinely practiced reconstruction of the SSS but has excised a segment of occluded sinus involved by tumor. In such cases the combination of image guidance and hand-held Doppler ultrasonography can be used to define the front and back edges of occluded sinus. Suture ligation can be accomplished with non-absorbable suture beginning on the convexity dura, passing through the falx and back out the convexity dura on the opposite side. In this way the suture cannot slip off the end of the ligated sinus. In some cases where the subarachnoid collateral veins are abundant, a completely intra-dural approach working from inside the tumor out may be taken (Case 2). In Sindou type I and II parasagittal meningiomas dissection of the layers of the lateral wall can be safely performed. After resecting a large parasagittal meningioma that occludes the sinus, a large defect remains (Figs. 1a, 2c). The dura can be reconstructed with dural grafts. However, caution should be taken, because simply measuring the size of the dural graft by laying it across the resection cavity can result in too small of a dural graft being used and subsequent development of an epidural fluid collection at the vertex of the skull (Fig. 1b). This can be prevented by stacking patties in the resection cavity to simulate the shape of the vertex while measuring the size of the dural graft (Fig. 1c). Tack up sutures can then be placed through the bone graft over the vertex (Fig. 1d). An example of this is demonstrated in the case of a large recurrent parasagittal meningioma that completely occluded the venous sinus (Fig. 2).
Convexity dural tack up method to prevent epidural fluid collection at the vertex. Diagram of convexity defect after resection of parasagittal meningioma and SSS (a). Potential space for epidural fluid collection at the vertex if dural graft is flat (b). Placement of patties to allow accurate measurement of dural graft size to fit the convexity of the skull at the vertex (c). End result showing tack up sutures in place with enough graft to prevent potential epidural space from forming (d), and medial/lateral orientation of tack up sutures in relation to previous sinus location (d inset)
Illustrative intraoperative example of the use of vertex convexity tack up method. Pre-operative axial T1 post-contrast MRI showing large parasagittal meningioma with sinus occlusion (a). Pre-operative coronal section (b). Intra-operative photograph of complex skin, bone and dural exposure fro complete removal of multiple recurrent parasagittal meningiomas (c). Post-operative day 1 T1 post-contrast MRI coronal section showing dura tacked up directly to bone with no epidural fluid collection (d)
Post-operatively maintenance of adequate hydration is an important factor in reducing the risk of venous thrombosis. While “keeping the patient dry” is standard practice for intra-axial tumors post-operatively, the reverse is used for meningiomas. We also begin low molecular weight heparin on the second day post-operatively. Cage et al. did not find an increased risk of post-op hematomas using this routinely [19].
Methods
The authors selected clinical case illustrations of complication avoidance in falx and parasagittal meningioma surgery. The details and images for each case were obtained from the electronic medical record. This study was approved by the author’s institutional review board (IRB: 13-12587).
Case illustrations
Case 1: extensive venous collateralization after sinus occlusion by tumor
A 52-year-old female presented with a focal left lower extremity seizure. Workup with magnetic resonance imaging (MRI) demonstrated a large right parietal parasagittal meningioma (Fig. 3). She was started on levacetiratam and did not have any additional seizures. She also had headaches and left hemi-body paresthesias, but no weakness. She had a history of Her-2 negative, ER/PR positive, breast cancer and had undergone lumpectomy and brachytherapy 3 years prior, and had no detectable residual systemic disease. She had been maintained on Tamoxifen. She did not have a family history of meningioma or personal history of radiation to the head or face as a child. She had no neurological deficits on examination except slightly more brisk reflexes on the left compared to the right.
An MRI with magnetic resonance venography (MRV) was performed during the initial workup. It demonstrated a 4.8 × 3.8 × 3.9 cm parasagittal mass completely occluding the superior sagittal sinus for 2.2 cm. There was peritumoral edema in the surrounding parenchyma (Fig. 3d). A digital subtraction angiogram was performed, which, along with the MRV, revealed prominent cerebral venous cortical collateralization and a diploic channel superior to the occluded sinus where collateralization had occurred through the calvarium (Fig. 4). There were also multiple other diploic channels in the calvarium exiting laterally from the lesion (Fig. 4). Bilateral occipital arteries as well as bilateral middle meningeal arteries provided blood supply to the tumor, all of which were embolized pre-operatively with polyvinyl alcohol particles. A small amount of residual blood supply still fed the tumor, suggesting additional pial blood supply.
Case 1 venography. Oblique MRV demonstrates complete occlusion of the parietal superior sagittal sinus with prominent bilateral collateral cortical and diploic venous channels (a). Lateral right ICA venous phase pre-embolization DSA shows a prominent diploic venous channel superior to the parietal superior sagittal sinus as well as additional diploic channels (b), and zoomed in (d). Right ICA venous phase pre-embolizaion oblique view shows extensive cortical and diploic venous channels (c)
A stereotactic MRI was performed for image guidance and to assist with locating the collateral diploic venous channels. The patient was taken to the operating room and numerous diploic venous channels were encountered when elevating the pericranium. Most were sealed with bone wax, although one channel was unable to be waxed and a small piece of gel foam was placed over the channel and secured in place with a burr hole cover with good control of bleeding. The prominent diploic channel seen on pre-operative imaging was followed and mapped with image guidance and avoided during the craniotomy. In addition, given the large collateral diploic channel seen superior to the occluded cavernous sinus, the craniotomy was kept paramedian, and not extended over the midline. The dura was then opened and reflected medially. The tumor was debulked and the margins dissected, which required bipolar cauterization of pial blood supply to the tumor. The tumor was resected to the falx, and then the falx was cut and resection continued onto the left (contralateral) side. A near total, Simpson IV resection, was performed. The dural leaflet was cut just adjacent to the superior sagittal sinus and a pericranial graft was sewn in place. The craniotomy was then closed.
The patient recovered well and post-operative MRI showed a near total resection (Fig. 5). Pathology was a WHO grade II, atypical meningioma. She has been followed with serial scans for four years without tumor progression.
Case 2: internal debulking prior to dissecting margins
A 46 year old female presented with a mild traumatic brain injury. She developed a complex post-traumatic disability syndrome. As part of her subsequent workup she had a non-contrast MRI, which revealed a 1.6 cm mass consistent with meningioma within the middle third of the superior sagittal sinus. She was lost to neurosurgical follow up for 7 years. Upon return to care, repeat MRI with contrast showed enlargement of the parasagittal parietal tumor, which now measured 3.4 × 3.0 cm (Fig. 6). There was no surrounding edema. She had weekly headaches, but no other consistent neurological symptoms. She had no risk factors for meningioma, including no family history of meningioma and no personal history of radiation to the head/face, breast cancer or thyroid cancer. She had no other past medical history. She had no focal neurological deficits on examination, but was unable to cooperate with motor testing due to breakaway weakness. She did have an impaired tandem gait. Given the growth of the tumor, it was deemed too large for radiosurgery by the radiation oncology team, so she elected to undergo surgical resection.
Case 2: pre-operative T1 non-contrast sagittal image demonstrates 1.6 cm parietal parasagittal tumor 7 years prior to surgery (a). Pre-operative T1 post-contrast MRI reveals near doubling of tumor size and significant mass effect on the falx and superior sagittal sinus (b sagittal; c axial; d coronal). Post-operative T1 post-contrast MRI shows no residual disease, resolution of mass effect, and flow through the superior sagittal sinus at the site of the prior tumor (e axial; f coronal)
A pre-operative stereotactic MRI was performed and she was taken to the operating room for resection. A linear incision was made over the tumor. The tumor was mapped with the intraoperative navigation. A two part craniotomy was performed [18]. First, two burr holes were made on the left side lateral to the incision and a bone flap was elevated. Then the dura was dissected free across midline and a second craniotomy flap was elevated. The tumor was debulked internally and then approached around the margins and removed. Small margins of tumor were intentionally left where it bordered the anterior and posterior borders with the superior sagittal sinus.
Immediately post-operatively the patient awoke with left lower extremity weakness. She had some movement in the leg, but could not lift it anti-gravity. An urgent post-operative CT was done, but there was no evidence of hemorrhage or infarct. On post-operative day 1, an MRI was done that showed no residual detectable tumor and no infarctions. There was no thrombosis within either the remaining anterior or posterior portions of the superior sagittal sinus. The strength in her left leg slowly improved and she was discharged to acute rehab. Final pathology was WHO Grade 1, fibrous meningioma. At 6 month follow up she was ambulating independently.
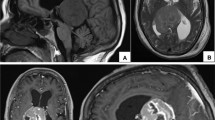
Case 3: large draining veins laying over the tumor
A 72 year old active male began to have left leg weakness that was slowly progressive over 3 months. He had no headaches, seizures, paresthesias or neglect. He was referred to a neurologist, and brain and spine MRI were performed, which demonstrated a 4.5 × 3.0 × 3.6 cm right falx parasagittal meningioma (Fig. 7). There was no surrounding peritumoral edema. He had no history of face/head radiation. His past medical history was notable for recurrent sinusitis, gastroesophageal reflux disease, chronic kidney disease, hypothyroidism and prostate cancer. He had prior prostatectomy, tonsillectomy, sinus surgery and eye surgery. On physical examination he had full strength in the right arm and leg and left arm. His left leg had minimal force against resistance at the hip, and was less than anti-gravity in the foot. He had a drop-foot gait and hyperreflexia at the left patella and ankle.
Case 3: pre-operative T1 post-contrast MRI shows the large right parasagittal tumor based on the falx (a axial; b coronal). Diffusion tensor tractography was used to overlay the right motor pathways (a, c) that can be seen coursing around the tumor. Post-operative diffusion weighted imaging shows two small areas of diffusion restriction posterior to the resection cavity (d). Post-operative T1 post-contrast MRI shows gross total resection of the tumor with decreased mass effect on the surrounding parenchyma (e axial; f coronal)
A stereotactic MRI was performed and the patient was taken to the operating room for resection. The patient was positioned left semi-lateral with the right side up. A two part craniotomy was performed [18]. First, two right paramedian and one lateral burr holes were drilled. The craniotomy flap was elevated. The dura was dissected off over the superior sagittal sinus under direct visualization and the foot-plate was then used to elevate the second part of the craniotomy, extending over the midline. The dura was then opened and reflected medially. Two large parasagittal draining veins drained into a large venous lake directly overlying the tumor. Thus, the dura had to be opened in multiple sections in order to preserve the dural entry for these large draining veins. A small corticectomy was made posterior to the posterior parasagittal draining vein, within the post-central gyrus, posterior to the tumor and motor cortex, to create a window to gain access to the tumor underneath the large draining vein. The tumor margin was then quickly encountered. The margin was followed and the tumor was detached from midline and debulked through the small cortical opening with an ultrasonic aspirator and carbon dioxide laser. The margins were followed along the falx. Small pial feeders were coagulated as well as a small branch of the anterior cerebral artery that directly supplied the tumor, but no sub-pial dissection was needed. The rest of the tumor was removed, and the craniotomy was closed.
Post-operatively, the patient had no movement in his left leg, but intact sensation. He regained proximal strength at least anti-gravity, but remained with limited distal movement. Post-operative MRI showed a gross total resection and a very small region of cytotoxic edema around the posterior margin of the tumor where the corticectomy had been made to access the tumor and preserve the large draining veins. He also developed new focal left lower extremity seizures post-operatively. Neurology was consulted and he was started on levacetiratam, and he had no additional seizures. Final pathology was WHO Grade 1, fibroblastic meningioma. He was stable and discharged to acute rehab and at 1 month follow up had marked improvement in his left lower extremity strength, with ankle dorsiflexion now able to flex against resistance.
Conclusions
Falx and parasagittal meningiomas are common locations for meningiomas of the cranial vault, and their resection is fraught with pitfalls that can lead to complications. Here, we present both a pre-operative checklist and an intra-operative checklist that address issues that arise during planning and resection of falx and parasagittal meningiomas. We also present case examples to illustrate these surgical pearls. These insights should aid surgeons and patients as they determine whether to operate and subsequently how to safely resect falx and parasagittal meningiomas.
References
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17(Suppl 4):iv1–iv62
Yamashima T, Kida S, Yamamoto S (1988) Ultrastructural comparison of arachnoid villi and meningiomas in man. Mod Pathol 1:224–234
Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, Berger MS, Parsa AT (2011) Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117:1272–1278
Sughrue ME, Rutkowski MJ, Shangari G, Fang S, Parsa AT, Berger MS, McDermott MW (2011) Incidence, risk factors, and outcome of venous infarction after meningioma surgery in 705 patients. J Clin Neurosci 18:628–632
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, Schiff D, Weber DC, Wen PY, Vogelbaum MA (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 122:4–23
Kondziolka D, Madhok R, Lunsford LD, Mathieu D, Martin JJ, Niranjan A, Flickinger JC (2009) Stereotactic radiosurgery for convexity meningiomas. J Neurosurg 111:458–463
Perry A (2006) Meningiomas. Russell and Rubinstein’s pathology of tumors of the nervous system, 7th edn. Hodder Arnold, London
Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW (2010) Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg 112:913–919
Nanda A, Bir SC, Konar S, Maiti TK, Bollam P (2015) World Health Organization grade I convexity meningiomas: study on outcomes, complications and recurrence rates. World Neurosurg. doi:10.1016/j.wneu.2015.11.050
Sughrue ME, Rutkowski MJ, Shangari G, Parsa AT, Berger MS, McDermott MW (2011) Results with judicious modern neurosurgical management of parasagittal and falcine meningiomas. Clinical article. J Neurosurg 114:731–737
Sughrue ME, Rutkowski MJ, Shangari G, Chang HQ, Parsa AT, Berger MS, McDermott MW (2011) Risk factors for the development of serious medical complications after resection of meningiomas. Clinical article. J Neurosurg 114:697–704
Jang W-Y, Jung S, Jung T-Y, Moon K-S, Kim I-Y (2012) Predictive factors related to symptomatic venous infarction after meningioma surgery. Br J Neurosurg 26:705–709
Rhoton AL (2002) The cerebral veins. Neurosurgery 51:S159–S205
Sindou MP, Alvernia JE (2006) Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg 105:514–525
Adjepong M, Agbenorku P, Brown P, Oduro I (2016) The role of antioxidant micronutrients in the rate of recovery of burn patients: a systematic review. Burn Trauma 4:18
Talas DU, Nayci A, Atis S, Comelekoglu U, Polat A, Bagdatoglu C, Renda N (2003) The effects of corticosteroids and vitamin A on the healing of tracheal anastomoses. Int J Pediatr Otorhinolaryngol 67:109–116
Burke J, Han SJ, Han JH, McDermott MW (2014) Two-part parasagittal craniotomy: technical note. Cureus 6:e193
Cage TA, Lamborn KR, Ware ML, Frankfurt A, Chakalian L, Berger MS, McDermott MW (2009) Adjuvant enoxaparin therapy may decrease the incidence of postoperative thrombotic events though does not increase the incidence of postoperative intracranial hemorrhage in patients with meningiomas. J Neurooncol 93:151–156
Acknowledgments
Authors have no conflicts of interest concerning the materials and methods used in this study or the findings specified in this paper. This manuscript has not been previously published in whole or in part or submitted elsewhere for review. No funding sources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Magill, S.T., Theodosopoulos, P.V. & McDermott, M.W. Resection of falx and parasagittal meningioma: complication avoidance. J Neurooncol 130, 253–262 (2016). https://doi.org/10.1007/s11060-016-2283-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2283-x