Abstract
The aim of the present study is to determine new positron emission tomography (PET) imaging-related factors predictive of progression-free survival as well as survival in patients with recurrent malignant glioma (MG) prior to and after re-irradiation. Fifty-six patients with recurrent MG who underwent re-irradiation treatment and pretherapeutic dynamic [18F]-fluoroethyl-l-tyrosine (FET)–PET scan were retrospectively analyzed. The prognostic value of different parameters, such as biological tumor volume, maximal tumor uptake (SUVmax/BG), mean tumor uptake (SUVmean/BG), as well as uptake kinetics, was evaluated. [18F]FET uptake kinetics was classified according to a five-point rating as category G1–2 (strongly/mainly increasing kinetics), G3 (mixed 1:1), or G4–5 (mainly/strongly decreasing kinetics). Patients within the pretherapeutic kinetic group G4–5 had significantly worse survival than the other two groups (p = 0.01). Multivariate analysis revealed that histologic grade, Karnofsky Performance Score (KPS), and kinetic group were independent significant predictors for survival after re-irradiation. The uptake kinetics of [18F]FET–PET is an independent determinant of overall and to a lesser extent also progression-free survival. Thus, [18F]FET–PET kinetics may provide valuable additional prognostic information for treatment decisions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prognosis of patients with high-grade glioma is limited by a high rate of local failure [1]. Although addition of temozolomide (TMZ) increases local control and survival, 72.8 % of patients still die within 24 months [2].
In selected patients, a second course of radiotherapy represents a reasonable treatment option [3, 4], whereas besides molecular markers [5, 6] several imaging-related markers may also provide information to personalize the corresponding treatment approach: Conventional magnetic resonance imaging (MRI) mainly provides information on the size and localization of the tumor and delineates secondary phenomena such as hemorrhage, edema, and mass effect; positron emission tomography (PET) with diverse radiolabeled compounds has been proposed to characterize the tumor at metabolic and molecular level [7]. PET studies using [18F]-fluorodeoxyglucose (FDG), which is widely used in oncology settings, have been performed for gliomas as well, e.g., for estimation of patient prognosis and treatment response evaluation [8, 9]. Due to their higher tumor-to-brain contrast, use of amino acid analogs, especially [18F]FET which does not require an on-site cyclotron, has attracted increasing interest.
At present, the clinical value of [18F]FET–PET in the context of human glioma has been documented for the following situations: improved planning of surgery, biopsy guidance, and radiation therapy [10, 11], treatment response [12], as well as differentiation of tumor recurrence from unspecific posttherapeutic tissue changes [13, 14]. Only a few studies have investigated the value of [18F]FET–PET as a predictive or prognostic marker [12, 15–17]. Thus, the aim of the current study is to evaluate the prognostic value of dynamic [18F]FET–PET, when performed prior to and after a second irradiation in patients suffering from malignant glioma recurrence.
Patients and methods
Patient selection
All patients re-irradiated for recurrent malignant glioma (MG) at the Department of Radiation Oncology, University Hospital Munich from 5/2004 until 5/2011 were identified using the department database. Re-irradiation was only performed in patients with macroscopic tumor in the brain (MRI proven), if recurrence was proven by biopsy/surgery (61 %), or definitely by Revised Assessment in Neuro-Oncology (RANO) criteria and previous radiotherapy was >0.5 years ago. Only patients with initially available [18F]FET–PET were analyzed. Altogether, 56 patients were identified.
Treatment follow-up
Baseline evaluation included gadolinium-enhanced brain MRI as well as [18F]FET–PET examination, complete physical and neurological examination, as well as blood tests before treatment. Treatment outcome was evaluated on a regular basis by brain MRI every 3 months, [18F]FET–PET (only in 67.9 % of patients), and RANO criteria for neurological status.
[18F]FET–PET image acquisition and evaluation
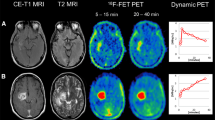
PET data were acquired with a Siemens ECAT EXACT HR+ scanner. After a 15-min transmission scan (68Ge sources), approximately 180 MBq [18F]FET was injected intravenously. Dynamic acquisition was performed from 0 to 40 min postinjection (128 × 128 matrix, 3D acquisition). Images were reconstructed by filtered backprojection using a Hann filter and were corrected for scatter and attenuation. Data were transferred to a HERMES workstation (Hermes Medical Solutions, Sweden). For quantitative evaluation, the maximal/mean tumoral [18F]FET uptake (SUVmax and SUVmean) was determined from a sum image (20–40 min p.i.) and corrected for the mean background (BG) uptake of a large region of interest (ROI) placed in the contralateral hemisphere (ratios SUVmax/BG, SUVmean/BG). Biological tumor volume (BTV) was assessed by means of a semiautomatic threshold-based calculation of a volume of interest (VOI; SUV/BG ≥1.8).
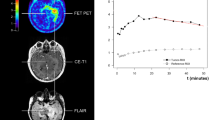
To extract time–activity curves (TACs) for each individual slice with suspicious [18F]FET uptake, 90 % isocontour threshold ROIs were defined on a summed image (10–30 min p.i.) and applied to the dynamic PET data (Fig. 1). According to the kinetic analysis, the lesions were classified into the following five groups: (1) homogeneously increasing TACs, (2) predominantly increasing TACs, (3) 50 % increasing and 50 % decreasing TACs, (4) predominantly decreasing TACs, and (5) homogeneously decreasing TACs.
Radiotherapy and treatment schedule
Radiotherapy was performed as described before in detail [18]. In brief, the planning target volume (PTV) was typically defined as gross tumor volume (GTV) + 10 mm (GTV normally not exceeding 5 cm in diameter); a dose of 36 Gy in 2-Gy single fractions was applied. Whenever bevacizumab was administered, patients received 10 mg/kg on days 1 and 15 during radiotherapy (RT). Maintenance therapy was selected based on individual considerations, and mainly bevacizumab-containing regimens were chosen.
Statistics
We performed all analyses using the Statistical Package for Social Sciences (SPSS version 19.0; SPSS Inc., Chicago, IL). Outcome measures of this study were (postrecurrence) survival (measured from the first day of re-irradiation until death or last follow-up) and progression-free survival. Demographic data as well as treatment response were analyzed using descriptive statistics (including pre- and posttherapeutic SUVmax/BG, SUVmean/BG, biological tumor volume, and kinetics). Comparative analysis was performed using the paired Wilcoxon signed-rank test. Survival analyses were based on Kaplan–Meier estimates; uni- and multivariate modeling were performed using Cox proportional-hazards analyses. Two-tailed p value ≤0.05 was considered significant.
Results
Patient characteristics
The patients’ baseline characteristics are listed in Table 1. Fifty-six patients with malignant glioma were included in this retrospective analysis; median age was 50 years (range 18–68 years), and 33.9 % of patients were younger than 60 years.
Median radiotherapy dose of re-irradiation was 36 Gy in 2-Gy single fractions; the major part of this patient population had undergone resection prior to initial radiotherapy (80.4 %). The male-to-female ratio was 1.5:1, and 76.8 % of the patients were shown to suffer from glioblastoma WHO grade IV at recurrence. Median pretherapeutic Karnofsky Performance Score (KPS) of this patient population was 80 (range 50–100), and 73.2 % of patients had KPS ≥70. O(6)-Methylguanine-DNA methyltransferase (MGMT) methylation status was available in 47 cases (83.9 %), being unmethylated in 26 of them (46.4 % of the entire cohort). Median follow-up time was 209 days including dead and censored patients (range 5–715 days).
Descriptive statistics
Concerning PET analysis, all 56 patients had received one pretherapeutic [18F]FET–PET examination and 38 patients had received PET imaging as early follow-up modality (after median 2 months, interquartile range 1–3 months, and in one case up to 12 months). Of the patients, 53.6 % had decreasing kinetics (4–5), 25 % an intermediate profile (3), and 21.4 % increasing kinetics (1–2). Median pretherapeutic SUVmax/BG was 3.3 (SUVmean/BG 2.2). After the end of re-irradiation, the first follow-up examination revealed a median SUVmax/BG of 2.6 (SUVmean/BG 2.3). The median pretherapeutic BTV was 13.7 cc, and the median posttherapeutic BTV was 7.3 cc.
Comparative analysis
Using the Wilcoxon signed-rank test, a significant decrease of both SUVmax/BG (p < 0.001) and BTV (p = 0.006) was found after re-irradiation, whereas the therapy-related change of SUVmean/BG did not reach significance (p = 0.13).
Survival and univariate analysis
Median survival of the entire patient cohort was 270 days, and median progression-free survival (PFS) was 204 days. The following factors were included in univariate testing: age, sex, previous initial surgery, WHO grade at relapse, MGMT methylation status, KPS, BTV, SUVmax/BG and SUVmean/BG (pre- and posttherapeutic), different ratios (BTV post- and pretherapeutic, SUVmax/BG post- and pretherapeutic), kinetics (G1–2, G3, G4–5), use of bevacizumab, and uni- versus multifocal recurrence.
Endpoints were survival after re-irradiation and PFS. The overall results are presented in Table 2. Several variables were tested as both nominal and continuous, such as SUVmax/BG pre re-RT (<3.3; ≥3.3), BTV pre re-RT (<13.7; ≥13.7 cc), and different post-/pretherapeutic ratios, with the median values as threshold parameters.
No significant results were derived for age, sex, previous surgery, MGMT methylation status, BTV, posttherapeutic kinetics, SUVmax/BG and SUVmean/BG (pre- and posttherapeutic), bevacizumab, and all associated categories/ratios as well as continuous versions (for both PFS and survival). Significant univariate factors for PFS after re-irradiation were WHO grade at relapse (p = 0.04) and KPS (p = 0.04). Median PFS was shorter for patients with WHO grade III compared with grade IV (145 versus 211 days). Median PFS for patients with KPS <70 was 169 days and 211 days for KPS ≥70 (Fig. 2a). Pretherapeutic kinetics G1–2 revealed median PFS of 234 days, kinetics G3 showed 236 days, and G4–5 showed 169 days (p = 0.14) (Fig. 3a).
Concerning survival, the factors WHO grade (p = 0.009), KPS (p = 0.01), and pretherapeutic kinetics (p = 0.01) were significant prognostic factors. Median survival for WHO grade III patients was 229 versus 298 days (grade IV). Patients with KPS <70 survived 189 days compared with 308 days for KPS ≥70 (Fig. 2b). For kinetics, G1–2 revealed a median survival of 352 days, kinetics G3 showed 298 days, and G4–5 showed 214 days (Fig. 3b).
Multivariate analysis
On multivariate analysis, the factors WHO grade, KPS, kinetics, MGMT methylation status, and use of bevacizumab were included (see also Table 2). For PFS, no significant independent prognostic factors could be determined. For survival, KPS, kinetics, and WHO grade turned out to be significant independent prognostic factors. The hazard ratios were 6.29 for WHO grade [95 % confidence interval (CI) 1.65–23.94, p = 0.007], 5.79 for KPS (95 %CI 1.82–18.43, p = 0.003), and 2.64 for kinetics (95 %CI 1.28–5.43, p = 0.008).
Discussion
Metabolic imaging with radiolabeled amino acids has gained increasing importance for diagnostics of glioma.
Additional kinetic analysis of dynamic [18F]FET–PET was shown to enable delineation of the most malignant tumor parts within heterogeneous gliomas and to be highly superior to conventional [18F]FET–PET analysis for differentiation between low- and high-grade glioma: although the pathophysiological mechanisms are not yet clarified, less aggressive low-grade tumor tissue seems to be characterized by continuously increasing TACs in dynamic analysis, whereas aggressive high-grade tumor tissue typically exhibits decreasing TACs. In this regard, additional analysis of [18F]FET kinetics might in the same way provide valuable information on pre- and posttherapeutic tumor aggressiveness.
In this study, we aimed to investigate the value of dynamic [18F]FET–PET in patients with re-irradiated malignant glioma in order to identify a new predictive tool. Our data suggest that patients with lower KPS (<70) at the time of re-irradiation exhibited significantly earlier tumor progression and had shorter posttreatment survival than those with higher KPS. Interestingly, patients with WHO grade IV tumors had significantly longer PFS and survival after re-irradiation compared with patients with WHO grade III tumors. This might be explained by the fact that time from diagnosis to re-irradiation was much longer for patients with WHO grade III tumors and the pretreatment was much heavier than for patients with WHO grade IV tumors. This phenomenon has already been observed in our previous study on re-irradiation with or without bevacizumab [18]. Another reason and potential bias is the fact that some of the grade III tumors could have been glioblastoma multiforme (GBM) at recurrence, as they were only diagnosed by RANO criteria.
In the [18F]FET–PET evaluation, none of the conventional values (SUVmax/BG, SUVmean/BG, and BTV) correlated with PFS or survival after re-irradiation. Although SUVmax/BG and BTV decreased significantly after re-irradiation, neither pre- and posttherapeutic parameters nor the pre-/posttherapeutic ratio showed significant influence on PFS or survival in this cohort. Due to the retrospective study design, posttherapeutic PET scans were not performed systematically, resulting in varying posttherapeutic scanning time (interquartile range 1–3 months after re-irradiation). Previous studies investigating assessment of treatment response by [18F]FET–PET suggested an early timeframe (7–10 days) after completion of radiochemotherapy as optimal for evaluation [16, 19], so that the later scans might have confounded the prognostic influence of posttherapeutic PET.
The main finding of the present study is that only kinetic analysis at the time of re-irradiation revealed an independent prognostic result for survival (p = 0.001) and PFS (marginally significant): patients with increasing TACs prior to re-irradiation had significantly longer survival compared with those with decreasing TACs. Hence, one could assume that recurrent tumor lesions with mainly increasing TACs might be less aggressive, potentially determining the clinical course and influencing the treatment response.
Interestingly, our data revealed that kinetic analysis lost its prognostic value after re-irradiation. The most probable explanation might be the utilization of antiangiogenic medication (bevacizumab) in the majority of patients, which could have substantially influenced the tracer washout, e.g., by regionally decreased blood flow [20], and therefore might confound the reliability of kinetic analyses after antiangiogenic therapy. Further studies on kinetic changes after antiangiogenic therapy are warranted to better interpret kinetic findings after bevacizumab treatment. The true benefit of kinetics in clinical practice might consist of identifying patient groups who would profit most from re-irradiation.
Conclusions
In patients with recurrent malignant glioma considered for re-irradiation, conventional [18F]FET–PET analysis does not provide any prognostic value for PFS or posttreatment survival. Kinetic analysis, however, reveals a significantly better outcome for patients with initially increasing time–activity curves (pretherapeutic but not posttherapeutic).
Identification of patients with better prognosis by dynamic [18F]FET–PET acquisition is important, as they might profit most from re-irradiation therapy.
References
Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, Bozzao A, Lanzetta G, Scarpino S, Arcella A, Enrici RM (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97:377–381
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G (2007) Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol 81:287–294
Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth FW, Tonn JC, Belka C (2011) Therapeutic options for recurrent malignant glioma. Radiother Oncol 98:1–14
Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26:4189–4199
Metellus P, Colin C, Taieb D, Guedj E, Nanni-Metellus I, de Paula AM, Colavolpe C, Fuentes S, Dufour H, Barrie M, Chinot O, Ouafik L, Figarella-Branger D (2011) IDH mutation status impact on in vivo hypoxia biomarkers expression: new insights from a clinical, nuclear imaging and immunohistochemical study in 33 glioma patients. J Neurooncol 105:591–600
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, Christian B, Ruppert J, Bernstein T, Kraus G, Mantil JC (2003) Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol 64:227–237
Charnley N, West CM, Barnett CM, Brock C, Bydder GM, Glaser M, Newlands ES, Swindell R, Matthews J, Price P (2006) Early change in glucose metabolic rate measured using FDG-PET in patients with high-grade glioma predicts response to temozolomide but not temozolomide plus radiotherapy. Int J Radiat Oncol Biol Phys 66:331–338
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, Schnell O, Suchorska B, Kreth FW, Tonn JC, Bartenstein P, la Fougere C, Belka C (2011) FET–PET for malignant glioma treatment planning. Radiother Oncol 99:44–48
Piroth MD, Pinkawa M, Holy R, Stoffels G, Demirel C, Attieh C, Kaiser HJ, Langen KJ, Eble MJ (2009) Integrated-boost IMRT or 3-D-CRT using FET–PET based auto-contoured target volume delineation for glioblastoma multiforme–a dosimetric comparison. Radiat Oncol 4:57
Wyss M, Hofer S, Bruehlmeier M, Hefti M, Uhlmann C, Bartschi E, Buettner UW, Roelcke U (2009) Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol 95:87–93
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K (2004) Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol 13:806–819
Piroth MD, Holy R, Pinkawa M, Stoffels G, Kaiser HJ, Galldiks N, Herzog H, Coenen HH, Eble MJ, Langen KJ (2011) Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol 99:218–224
Piroth MD, Pinkawa M, Holy R, Klotz J, Nussen S, Stoffels G, Coenen HH, Kaiser HJ, Langen KJ, Eble MJ (2011) Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys 80:176–184
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ, Jansen P, Coenen HH, Steiger HJ, Langen KJ (2007) Prognostic value of O-(2–18F-fluoroethyl)-l-tyrosine PET and MRI in low-grade glioma. J Nucl Med 48:519–527
Niyazi M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, Geisler J, la Fougère C, Ertl L, Linn J, Siefert A, Belka C (2012) Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 82:67–76
Galldiks N, Langen KJ, Holy R, Pinkawa M, Stoffels G, Nolte KW, Kaiser HJ, Filss CP, Fink GR, Coenen HH, Eble MJ, Piroth MD (2012) Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-l-tyrosine PET in comparison to MRI. J Nucl Med 53:1048–1057
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6:553–563
Conflict of interest
The authors declare that conflicts of interest do not exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maximilian Niyazi and Nathalie Jansen contributed equally
Rights and permissions
About this article
Cite this article
Niyazi, M., Jansen, N., Ganswindt, U. et al. Re-irradiation in recurrent malignant glioma: prognostic value of [18F]FET–PET. J Neurooncol 110, 389–395 (2012). https://doi.org/10.1007/s11060-012-0980-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0980-7