Abstract
Improving glioblastoma multiforme (GBM) treatment with radio-chemotherapy remains a challenge. Topotecan is an attractive option as it exhibits growth inhibition of human glioma as well as brain penetration. The present study assessed the combination of radiotherapy (60 Gy/30 fractions/40 days) and topotecan (0.9 mg/m2/day on days 1–5 on weeks 1, 3 and 5) in 50 adults with histologically proven and untreated GBM. The incidence of non-hematological toxicities was low and grade 3–4 hematological toxicities were reported in 20 patients (mainly lymphopenia and neutropenia). Partial response and stabilization rates were 2% and 32%, respectively, with an overall time to progression of 12 weeks. One-year overall survival (OS) rate was 42%, with a median OS of 40 weeks. Topotecan in combination with radiotherapy was well tolerated. However, while response and stabilization concerned one-third of the patients, the study did not show increased benefits in terms of survival in patients with unresectable GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In adults, the treatment of glioblastoma, sometimes still labeled “multiforme” (GBM), is based on surgery followed by radiotherapy. However, adjuvant radiotherapy alone is associated with a poor prognosis and a median survival not exceeding 10 to 12 months [1], depending on key prognostic factors including age, performance status and type of surgery (total, partial or biopsy) [1–3]. Since the reports of benefits in patients with GBM treated by irradiation and temozolomide, the combination of radiotherapy and chemotherapy has been recognized as the standard treatment of the disease after resection [4]. The choice of appropriate drugs remains crucial, but is limited owing to the low activity and ability of most of cytotoxic drugs to penetrate the brain.
Topotecan, a topoisomerase 1 inhibitor, appears as an attractive candidate as it exhibits radiosensitization, cell growth inhibiting effects on human glioma cells in vitro [5–7] and in vivo [8, 9], and an exceptional brain penetration [10–12] without substantial local or systemic adverse effects. Administration of topotecan by continuous i.v. (c.i.v.) infusion has shown the best therapeutic index and a synergistic effect with RT [6, 7, 13]. Previous phase 2 studies with topotecan in newly diagnosed or recurrent malignant glioma have shown a modest activity, but the administration schedules were different, including various doses and infusion periods varying from 24 to 120 h [14–17]. In a phase 1 study with topotecan administered continuously for 5 days every 2 weeks in combination with concurrent RT, we demonstrated that the dose to be recommended was 0.9 mg/m2/day, and that CIV of topotecan with RT was feasible as an ambulatory treatment and that it could improve patients’ quality of life [18].
The present prospective multicenter phase 2 study was designed to assess the antitumor activity, measured by 12-month overall survival (OS), and the toxicity of c.i.v. topotecan in combination with RT in adults with unresectable GBM.
Patients and methods
The study has been performed in accordance with the ethical standards (declaration of Helsinki, 1964) and the protocol has been approved by Rennes ethic committee.
Inclusion criteria
Newly diagnosed patients aged 18–70 years with histologically proven GBM were eligible for the study if they had incomplete resection demonstrated by post-operative Magnetic Resonance Imaging (MRI). Other eligibility criteria included a life expectancy ≥12 weeks, a performance status ≤2 on the WHO scale, normal hematopoietic function (hemoglobin level ≥10 g/dl, neutrophil granulocyte count ≥1.5 × 109/l, platelet count ≥100 × 109/l), normal hepatic function (bilirubin level ≤1.5 × the upper limit of normal [ULN], transaminase level ≤2.5 × the ULN, alkaline phosphatase level ≤5 × the ULN) and normal renal function (serum creatinine level ≤1.5 × the ULN). Patients had to sign an informed consent form.
Exclusion criteria
Patients were ineligible if the tumor had a predominant histology of oligodendroglioma or if complete surgical resection and/or local administration of carmustine wafers had been performed. Patients with concurrent malignancy, psychiatric disorders or any medical condition that could interfere with treatment administration were also excluded. Pregnant or lactating women were ineligible.
Study design
Treatment had to be started within 42 days following surgery. The treatment procedure shown in Table 1 was performed as previously described [18]. Briefly, after establishment of an implantable port, topotecan (Hycamtin®, GlaxoSmithKline, Marly-Le-Roi, France) was administered as a c.i.v. infusion by a programmable pump, from day 1 to day 5 (120 h) on weeks 1, 3, and 5 during RT, at 0.9 mg/m2/day. On day 1 of each chemotherapy cycle, infusion was set up at least 1 h before the RT session. Beginning a cycle of chemotherapy with topotecan on each subsequent day 1 required a hemoglobin level ≥10 g/dl (posttransfusion rates were allowed), a neutrophil granulocyte count ≥0.5 × 109/l, and a platelet count ≥75 × 109/l. In cases of neutropenia with an absolute neutrophil count <0.5 × 109/l, treatment with granulocyte-colony-stimulating factor was allowed, while not recommended. Red-cell transfusions were recommended in instances of anemia (hemoglobin level <10 g/dl) and platelet transfusions in cases of thrombocytopenia (platelet counts <30 × 109/l). Chemotherapy was postponed until these levels were reached but no cycle had to be delivered after the end of the RT. Dose reductions at 0.7 mg/m²/day were used in case of grade 3 toxicities except for alopecia and nausea.
RT was delivered with high energy photons (6 MV and/or 18 MV) and a multileaf collimator. The use of thermoformed masks coupled with supine position of the patients ensured reproducibility. A post-contrast CT scan was then performed (contiguous 3 mm-thick slices along the whole brain). All images were transferred to a Dosigray 3D planning system and an identical technique was used for all patients. The Gross Tumor Volume (GTV) comprised the void of the operative cavity and the tumor remnant, and the Clinical Target Volume was located 25 mm around the GTV. Patients received a 2 Gy daily dose 5 days/week for 30 fractions. The planned total dose was 60 Gy delivered over 6 weeks.
Toxicity assessment
Toxicities were graded according to the National Cancer Institute’s Common Toxicity Criteria Adverse Event (NCI-CTCAE) scale (version 2.0). Before being included in the study, patients underwent complete clinical examination, blood cell and platelet counts, serum biochemistry, electrocardiogram, and chest X-ray. During the treatment, blood cell and platelet counts were measured weekly. In addition, at the beginning of each cycle of chemotherapy, patients underwent clinical examinations, assessment of WHO performance status, and biochemistry. At week 6 and every third subsequent month posttreatment, the same parameters (with the exception of biochemistry) were evaluated until tumor progression.
Efficacy assessment
A MRI of the brain with and without gadolinium enhancement was performed for each patient within 4 days postoperatively. Before being included in the study, each patient underwent neurological examination including mini-mental state examination (MMSE). The dose of corticosteroids needed was registered as well as that of anticonvulsants. At week 6 and every subsequent month post-treatment, neurological examinations of the brain with MMSE and MRI were evaluated until tumor progression.
Objective response was assessed 6 weeks after the completion of the treatment, based on clinical signs and on MRI, and every 3 months thereafter. OS was assessed from the date of surgery until the date of death, and survival without disease progression was assessed from the date of initial surgery until the date of observed recurrence. Median OS value, 12-month OS and survival curves were calculated using the Kaplan-Meier method.
Statistical methodology
OS has been chosen as a parameter of efficacy as it is supposed to give a rough evaluation independent of the follow-up procedures. However, it may vary with initial prognostic factors [2]. Most publications have shown that incomplete removal is one of the key prognostic factor with a 12-month OS ranging from 15% [3] to 25% [19] after biopsy, from 20% [20] to 45% [21] after partial removal. In 2001, when the study was started, the 12-month OS after RT was expected about 25%. Our trial was powered so that this innovative treatment should increase OS to 45% to conclude to an efficacy ranging between 31 and 60% (CI 95%) 12-month OS.
Results
Fifty-one patients were enrolled into this phase 2 trial between October 2001 and April 2004 in 7 participating centers. One patient did not begin the combined treatment due to early progression and was excluded of the final analysis. Finally, 50 patients were evaluable for toxicity and efficacy with a median follow-up of 106 weeks. Patient characteristics are summarized in Table 2. Twenty-eight patients (56%) had stereotactic biopsy and 22 (44%) had partial resection. The median time between surgery and the first dose of irradiation was 35 ± 7.5 days. Only 6 out of 50 patients did not receive prophylactic enzyme-inducing anti-epileptic drugs (EIAEDs).
Treatment was early stopped in 13 cases, due to disease progression (n = 10), grade 4 hematologic toxicity (n = 2) and one unknown reason. As shown in Table 3, toxicity was mainly hematologic. Among the seven serious adverse events (AE) considered to be due to the treatment, six were grade 4 NCI-CTCAE (diarrhea = 2, febrile neutropenia = 2, catheter-related infection = 1, anemia = 1) and one was a grade 3 NCI-CTCAE somnolence. Nine red-cell infusions and three platelet infusions were necessary during radiochemotherapy. No late toxicity, particularly neurologic toxicity, was observed.
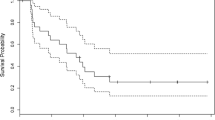
The objective response rate was low with only one partial response and 16 stable diseases for a clinical benefit of 34%. Median time to progression was 12 weeks. Median OS was 36 weeks after stereotactic biopsy and 49 weeks after partial resection for a median OS of 40 weeks (CI 95%: 31–49 weeks) for the whole population (Figs. 1 and 2). One-year and 2-year OS were 42% (CI 95%: 28–56%) and 10%.
The percentage of patients with a good performance status (WHO 0 or 1) decreased during treatment period from 82% at the inclusion to 61% at the end of the treatment. Median dosage of equivalent prednisolone increased from 48 mg/day to 60 mg/day and the MMSE did not change significantly (26–28). No late neurologic toxicity was reported. However, the poor availability of data made the statistical assessment of these parameters limited during the months following the end of treatment. No analysis has been carried out according to whether or not patients were taking EIAEDs.
After progression, 28 patients (56%) were treated with surgery (n = 2) and/or 2nd and/or 3rd line of chemotherapy with temozolomide (n = 19), nitrosourea (n = 11) and/or other agents (n = 2). Median OS was similar in the 28 patients receiving 2nd line chemotherapy with or without temozolomide (57 weeks for both).
Discussion
Because of its known effect on human glioma cell growth inhibition, coupled with an exceptional capacity to penetrate brain, topotecan has been assessed in the treatment of glioma through various clinical trials including different administration and combination schedules.
Early phase 2 trials using topotecan intravenously as second-line chemotherapy in patients with relapsed high-grade glioma have not revealed a significant anti-tumoral activity [15–17]. However, c.i.v. infusion of topotecan has been shown to delay disease progression [6, 13, 16].
In the present study, the efficacy of the combination of topotecan with RT was relevant when stabilization was considered in addition to the modest response rate, with a control of the disease observed for one-third of the patients, the median OS being of 40 weeks. Based on recent studies of concomitant treatment of brain tumors with radiotherapy and temozolomide, one can postulate that response rates may have been underestimated by pseudoprogression. This phenomenon, not planned to be investigated at the time of our study, is expected to occur in up to 20% of the patients treated with concomitant radiochemotherapy [22, 23]. However, pseudoprogression was not shown to influence survival of patients with GBM [23], which was chosen as the main efficacy endpoint of our study. Furthermore, one cannot exclude that efficacy could have been underestimated due to interactions between EIAEDs and topotecan. However, a sub-group analysis would not have been powerful because of the low size of the group of patients who did not receive prophylactic EIAEDs (6/50), and then had not been planned.. In terms of grades 3–4 toxicities, rates of both neutropenia and thrombocytopenia, known as the dose-limiting toxicities of topotecan when used at standard doses, were <40%, and the rate of non hematological toxicities, as expected, was low. Thus, the impact of these results has to be discussed in the context of previous data with a combination of RT and topotecan and other cytotoxic drugs.
Initial trials with the standard dose of topotecan in combination with RT have shown classical topotecan-related hematological toxicities. In the Radiotherapy Oncology Group (RTOG) phase 1, 47 GBM patients received a cranial RT (60 Gy/30 fractions) and three cycles of topotecan administered during RT at 21-day intervals (daily 30-min intravenous infusions for 5 days with dose increments from 0.5 mg/m²/day to 1.5 mg/m²/day) [24]. Among the 17 patients who accrued to 1.5 mg/m²/day, six experienced grade 4 neutropenia, and four grade 3 neutropenia. Median OS was 9.7 months for all patients. In the phase 2 study designed with a similar schedule (1.5 mg/m²/day) and including 87 patients enrolled after biopsy (29%), partial resection (48%) or macroscopically complete resection (21%), grade 4 acute toxicity (primarily hematologic) was reported in 54% of patients, while four patients experienced grade 3 late central nervous system toxicity [25]. The median OS was 9.3 months and the 1-year OS rate was 32%. After comparison of the study population with matched patients from the RTOG database, this schedule did not appear to produce benefits in terms of survival over previously tested therapies, so that no further development was advised. These data with topotecan administered as short-term infusions are similar to those reported in the present study with c.i.v. infusion in terms of both toxicity and overall survival.
In a phase I study of twice-daily accelerated RT (57.75 Gy/33 fractions/3.5 weeks) combined with 21-day c.i.v. topotecan administered concurrently from 0.3 to 0.7 mg/m²/day in 20 patients with GBM [26], toxicities were moderate, which included two grade 4 neutropenia and one grade 4 thrombocytopenia, so that the recommended dose was 0.6 mg/m²/day. Fourty-two patients were then included in a phase 2 study with this schedule of topotecan [27]. Grade 3–4 hematological toxicities were low (34% leucopenia and 12% thrombocytopenia), and median OS (10 months) as well as one-year OS rate (38%) were similar to what had been observed in previous studies cited and to the data reported in the present one. However, in a study including 60 patients treated with RT (60 Gy/30 fractions) and an absolute dose of 0.5 mg of intravenous topotecan one hour prior to RT [28], hematological toxicity was low, quality of life remained preserved and outpatient treatment was possible. The median survival time of 15 months observed in this study was particularly long, and led to the conclusion that a randomized double-blind placebo controlled parallel design-based clinical trial had to be performed.
The question that emerged is whether or not the efficacy of topotecan could be increased in patients treated for gliomas. While concurrent treatment with anticonvulsants and dexamethasone has been shown to enhance the clearance of drugs such as 9-amino camptothecin [29] and irinotecan [30], much less data are available concerning topotecan. Because topotecan is metabolised by cytochrome P450 enzyme system, minimizing drug interactions by avoiding the concomitant use of these therapies that are also metabolized through this system is expected to enhance outcomes [31].
The strategy of topotecan combination with other cytotoxic drugs did not result in encouraging results for the treatment of high grade gliomas. For instance, paclitaxel and topotecan exhibited modest activity in adults with recurrent or refractory GBM [32]. However, topotecan appeared to be quite unaffected by the most common multidrug resistance mechanisms so that it may be expected to potentiate cytotoxicity of alkylators like thiotepa or carboplatin for treating brain tumors in children and young adults [33].
In terms of radiochemotherapy, other camptothecin analogs have been tested as radiosensitizers, among which irinotecan may be more active than topotecan. The positive results (70% of clinical benefit) of the study by Friedman et al. including 60 patients with malignant glioma treated with irinotecan [30] were not confirmed by others [34]. So far, irinotecan has not been evaluated in combination with RT in the treatment of GBM.
Studies with temozolomide were the first to demonstrate the efficacy of radiochemotherapy followed by adjuvant chemotherapy [4, 35]. In the initial phase 2 study performed in 64 patients, median OS was 16 months and one- and 2-year survival rates were 58% and 31%, respectively [35]. Those results were confirmed in a phase 3 study including 573 patients with GBM receiving RT alone (median OS = 12.1 months; 2-year survival rate = 10.4%) or RT plus daily temozolomide followed by six cycles of adjuvant temozolomide (median OS = 14.6 months; 2-year OS rate = 26.5%) [4], and later observed in an other randomized phase 2 trial [36]. Even though 84% of patients included in the phase 3 study with a continuous administration of temozolomide had undergone debulking surgery [4], 2-year survival appeared clearly superior than that assessed in our study (26.5% versus 10%, respectively), the results of which remaining equivalent to those reported in other studies with topotecan (Table 4.) Sub-group assessment of patients belonging to similar prognostic groups, such as patients who only received a partial resection, still showed a difference in median OS (15.8 versus 11.3 months). Moreover, tolerance appeared to be better with temozolomide, as suggested by the rates of grade 3–4 hematological toxicity (16% versus 29–59% in radiochemotherapy studies using topotecan) (Table 4). Only the study with topotecan at an absolute dose of 0.5 mg reported lower rates of toxicity with topotecan [28].
Concerning the expected efficacy of temozolomide, one must distinguish patients with GBM containing a methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter that experienced benefits with temozolomide, and those who did not have a methylated MGMT promoter [37]. Thus, a molecule that inhibits MGMT might be of interest, despite not encouraging initial results [38]. A future approach might be to combine oral temozolomide with in situ BCNU polymers [39] or other radiosensitizing molecules such as topotecan. In this respect, the results of Balzarotti et al. have suggested a synergistic effect of temozolomide and topotecan in association against human glioma cell lines [40], but the risk of an enhance myelotoxicity could be an issue.
In conclusion, this study shows that topotecan combined with concurrent RT is well tolerated and allows reducing or stabilizing the disease in one-third of patients with incomplete resected GBM. However, the efficacy of the combination in terms of survival was not better than that previously reported with other administration schedules, which does not justify the investigation of this regimen in a phase 3 trial. Current avenues of research should better be focused on new systemic agents, mainly including targeted therapies.
References
Surawicz TS, Davis F, Freels S et al (1998) Brain tumor survival: results from the National Cancer Data Base. J Neurooncol 40:151–160. doi:10.1023/A:1006091608586
Curran JC, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710. doi:10.1093/jnci/85.9.704
Kreth FW, Berlis A, Spiropoulou V et al (1999) The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer 86:2117–2123. doi :10.1002/(SICI)1097-0142(19991115)86:10<2117::AID-CNCR33>3.0.CO;2-8
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Mattern MR, Hofmann GA, McCabe FL, Johnson RK (1991) Synergistic cell killing by ionizing radiation and topoisomerase I inhibitor topotecan (SK&F 104864). Cancer Res 51:5813–5816
Lamond JP, Mehta MP, Boothman DA (1996) The potential of topoisomerase I inhibitors in the treatment of CNS malignancies: report of a synergistic effect between topotecan and radiation. J Neurooncol 30:1–6. doi:10.1007/BF00177437
Pinel S, Chastagner P, Merlin JL et al (2006) Topotecan can compensate for protracted radiation treatment time effects in high grade glioma xenografts. J Neurooncol 76:31–38. doi:10.1007/s11060-005-3666-6
Friedman HS, Houghton PJ, Schold SC et al (1994) Activity of 9-dimethylaminomethyl-10-hydroxycamptothecin against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol 34:171–174
Chastagner P, Kozin SV, Taghian A (2001) Topotecan selectively enhances the radioresponse of human small-cell lung carcinoma and glioblastoma multiforme xenografts in nude mice. Int J Radiat Oncol Biol Phys 50:777–782. doi:10.1016/S0360-3016(01)01501-2
Baker SD, Heideman RL, Crom WR et al (1996) Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemother Pharmacol 37:195–202. doi:10.1007/BF00688317
Straathof CS, van den Bent MJ, Loos WJ et al (1999) The accumulation of topotecan in 9L glioma and in brain parenchyma with and without dexamethasone administration. J Neurooncol 42:117–122. doi:10.1023/A:1006166716683
Stewart CF, Iacono LC, Chintagumpala M et al (2004) Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol 22:3357–3365. doi:10.1200/JCO.2004.10.103
Blaney SM, Balis FM, Cole DE et al (1993) Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res 53:1032–1036
Blaney SM, Phillips PC, Packer RJ et al (1996) Phase II evaluation of topotecan for pediatric central nervous system tumors. Cancer 78:527–531. doi :10.1002/(SICI)1097-0142(19960801)78:3<527::AID-CNCR21>3.0.CO;2-#
Macdonald D, Cairncross G, Stewart D et al (1996) Phase II study of topotecan in patients with recurrent malignant glioma. National Clinical Institute of Canada Clinical Trials Group. Ann Oncol 7:205–207
Friedman HS, Kerby T, Fields S et al (1999) Topotecan treatment of adults with primary malignant glioma. The Brain Tumor Center at Duke. Cancer 85:1160–1165. doi :10.1002/(SICI)1097-0142(19990301)85:5<1160::AID-CNCR21>3.0.CO;2-F
Burch PA, Bernath AM, Cascino TL et al (2000) A North Central Cancer Treatment Group phase II trial of topotecan in relapsed gliomas. Invest New Drugs 18:275–280. doi:10.1023/A:1006438109266
Lesimple T, Ben Hassel M, Gédouin D et al (2003) Phase I study of topotecan in combination with concurrent radiotherapy in adults with glioblastoma. J Neurooncol 65:141–148. doi:10.1023/B:NEON.0000003647.66788.3b
Simpson JR, Horton J, Scott C et al (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. In J Radiat Oncol Biol Phys 26:239–244
Obwegeser A, Ortler M, Seiwald M et al (1995) Therapy of glioblastoma multiforme: a cumulative experience of 10 years. Acta Neurochir (Wien) 137:29–33. doi:10.1007/BF02188776
Albert FK, Forsting M, Sartor K et al (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45–60. doi:10.1097/00006123-199401000-00008
Brandes AA, Tosoni A, Spagnolli F et al (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro-oncol 10:361–367. doi:10.1215/15228517-2008-008
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410. doi:10.1002/cncr.23562
Fisher BJ, Scott C, Macdonald DR et al (2001) Phase I study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group Trial 9507. J Clin Oncol 19:1111–1117
Fisher B, Won M, Macdonald D, Johnson DW, Roa W (2002) Phase II study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group 9513. Int J Radiat Oncol Biol Phys 53:980–986. doi:10.1016/S0360-3016(02)02817-1
Grabenbauer GG, Anders K, Fietkau RJ et al (2002) Prolonged infusional topotecan and accelerated hyperfractionated 3d-conformal radiation in patients with newly diagnosed glioblastoma-a phase I study. J Neurooncol 60:269–275. doi:10.1023/A:1021100413142
Klautke G, Schütze M, Bombor I et al (2006) Concurrent chemoradiotherapy and adjuvant chemotherapy with topotecan for patients with glioblastoma multiforme. J Neurooncol 77:199–205. doi:10.1007/s11060-005-9028-6
Gross MW, Altscher R, Brandtner M et al (2005) Open-label simultaneous radio-chemotherapy of glioblastoma multiforme with topotecan in adults. Clin Neurol Neurosurg 107:207–213. doi:10.1016/j.clineuro.2004.07.016
Grossman SA, Hochberg F, Fisher J et al (1998) Increased 9-aminocampothecin dose requirements in patients on anticonvulsants. The new approaches to brain tumor therapy. Cancer Chemother Pharmacol 42:118–126. doi:10.1007/s002800050794
Friedman HS, Petros WP, Friedman AH et al (1999) Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17:1516–1525
Motl S, Zhuang Y, Waters CM, Stewart CF (2006) Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet 45:871–903. doi:10.2165/00003088-200645090-00002
Pipas JM, Meyer LP, Rhodes CH et al (2005) A phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent or refractory glioblastoma muliforme or anaplastic astrocytoma. J Neurooncol 71:301–305. doi:10.1007/s11060-004-2026-2
Kushner BH, Cheung NK, Kramer K et al (2001) Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transplant 28:551–556. doi:10.1038/sj.bmt.1703213
Raymond E, Fabbro M, Boige V et al (2003) Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy-naive patients with glioblastoma. Ann Oncol 14:603–614. doi:10.1093/annonc/mdg159
Stupp R, Dietrich PY, Ostermann-Kraljevic S et al (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20:1375–1382. doi:10.1200/JCO.20.5.1375
Athanassiou H, Synodinou M, Maragoudakis E et al (2005) Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol 23:2372–2377. doi:10.1200/JCO.2005.00.331
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Quinn JA, Pluda J, Dolan ME et al (2002) Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol 20:2277–2283. doi:10.1200/JCO.2002.09.084
Westphal M, Hilt DC, Bortey E et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol 5:79–88. doi:10.1215/15228517-5-2-79
Balzarotti M, Ciusani E, Calatozzolo C et al (2004) Effect of association of temozolomide with other chemotherapic agents on cell growth inhibition in glioma cell lines. Oncol Res 14:325–330
Acknowledgments
The authors are grateful to GlaxoSmithKline, Marly-Le-Roi, France, for providing the Topotecan and for logistic assistance, and to Biotrial, Rennes, France, for their contribution to the realization of the study. The authors wish to thank Hervé Bismut who provided editorial assistance in the preparation of the manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lesimple, T., Riffaud, L., Frappaz, D. et al. Topotecan in combination with radiotherapy in unresectable glioblastoma: a phase 2 study. J Neurooncol 93, 253–260 (2009). https://doi.org/10.1007/s11060-008-9774-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9774-3