Abstract
Scots pine (Pinus sylvestris L.) has higher early growth rates than Norway spruce (Picea abies (L.) H. Karst.). To help efforts to understand possible reasons for this difference and identify appropriate regeneration methods, we labeled seedlings with 15N in the nursery to probe nitrogen (N) uptake and retranslocation in Scots pine and Norway spruce seedlings at both harsh and fertile sites in southern and northern Sweden. For this, 15N dilution was measured during the first two years after planting. We also examined the potential N2-fixation capacity in fine roots after 5–7 growing seasons. Use of 15N-labeled seedlings enabled clear discernment of contributions of uptake of new N and retranslocation of old N to new foliage. Scots pine seedlings had higher proportions of N derived from uptake (Ndfu) than Norway spruce seedlings, and higher growth. Scots pine seedlings were less responsive to site preparation treatments compared to Norway spruce that has a greater need for appropriate silvicultural measures to grow well. After the second growing season, the contribution of N derived from retranslocation (Ndfr) to N in new foliage had diminished to 10–20% at all except the harshest site, where both species were more dependent on Ndfr. The potential N2-fixation capacity in fine roots of the two species differed, but in both cases the contribution of N2-fixation to N-acquisition was negligible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many terrestrial and marine ecosystems in the world are nitrogen (N) limited, due to low availability or low absolute quantities of N. Nitrogen limitation is also prevalent in most managed forest ecosystems (Tamm 1991; Vitousek et al. 1997), especially in boreal and temperate zones (Vitousek and Howarth 1991; Fenn et al. 1998; Bergh et al. 1999). Hence, tree biomass accumulation and productivity generally increase with increases in N availability. Nitrogen can be derived from internal storage sources by retranslocation, or from external sources by mineralization, N2-fixation, transfer of organic N via mycorrhiza to roots, N deposition or fertilization (Millard and Grelet 2010). On average, approximately 50% of N used by trees may be derived from N uptake and 50% from retranslocation, but there are large variations in quantified amounts due to effects of factors such as species, tree age, tree N status, quantification method, time of sampling, and environmental parameters (Millard and Grelet 2010; Villar-Salvador et al. 2015). For example, N uptake is reportedly higher and dependence on retranslocated N lower in environments with higher N availability (Millard and Grelet 2010).
N can also be derived from N2-fixation by free-living or endophytic diazotrophs (bacteria and archaea). Inter alia, cyanobacteria living symbiotically with feather mosses may provide important N inputs in boreal ecosystems (DeLuca et al. 2002; Stuiver et al. 2016), and N2 is fixed by symbionts in root nodules of some tree species, such as Frankia in Alnus spp. and Rhizobia in Acacia spp. (Binkley et al. 1994; Brockwell et al. 2005). Fixed N2 has been detected in aboveground tree residues (Brunner and Kimmins 2003), roots (Granhall and Lindberg 1978; Mäkipää et al. 2018), foliage and associated soil (Son 2001) of several tree species. However, only few studies have investigated N2-fixation in roots of coniferous trees growing in boreal conditions. Those studies have found low fixation rates in fine roots of Norway spruce and Scots pine in Sweden (Granhall and Lindberg 1978), and in woody roots of Norway spruce and silver birch (Betula pendula Roth.) in Finland (Mäkipää et al. 2018). In addition, several bacteria (endophytic diazotrophs) living in internal tissues of lodgepole pine (Pinus contorta var. latifolia Engelm. ex S. Watson) and hybrid white spruce (Picea glauca x engelmannii) have been identified, indicating potential for N2-fixation that may facilitate tree growth in nutrient-poor environments (Padda et al. 2018; Puri et al. 2018).
Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) H. Karst) are the most commonly planted tree species in Sweden, accounting for 96% of all planting (SFA 2023) and 80% of the total standing volume (SLU 2020). They have different growth patterns, as Scots pine is regarded as a pioneer species and has higher early growth rates than Norway spruce, being a late-successional species. This difference is often at least partly due to growth of Norway spruce seedlings being hampered, to varying degrees, by growth check (also called planting shock) caused by factors including low fertility, water stress and frost damage, either singly or in any combination (Grossnickle 2000). Hence, initial N uptake by planted seedlings, is crucial for high initial growth rates (Margolis and Brand 1990), and depends on nutrient uptake from the previous year (Malik and Timmer 1996; Nilsson and Örlander 1999; Grossnickle 2000; Nordborg et al. 2003). For example, Johansson et al. (2012) found that N fertilization substantially increased growth of planted Norway spruce seedlings in an optimization study. Other studies have found similar growth increases after fertilization in combination with site preparation, of both Norway spruce seedlings (Nordborg and Nilsson 2003; Nordborg et al. 2003), and several North American conifers (Munson et al. 1993; Thiffault and Jobidon 2006; Thiffault et al. 2017). A common method to promote survival and growth of planted seedlings in Sweden is mechanical site preparation, as 89% of all regenerations are scarified (SFA 2022). The most common methods used are mounding or disc trenching (Nilsson et al. 2010). Site preparation improves the conditions for the seedlings as it for example reduces competing ground vegetation (Thiffault et al. 2005; Johansson et al. 2013), increases nutrient mineralization (Örlander et al. 1990; Lebel et al. 2008) and reduces competition for nutrients (Thiffault et al. 2004).
Useful tools for exploring plants’ interactions with their environments, such as N uptake and utilization by trees, include stable isotopes (Dawson et al. 2002; Fry 2006). They can be used at naturally occurring levels (‘natural abundance’) or levels beyond natural ranges (‘enrichment’) using labeled substances (Dawson et al. 2002). Enrichment methods enable tracking of elements’ fates, with minimal changes to natural behavior. The abundance of an isotope (e.g. 15N) relative to other isotopes of the same element in a labeled substance exceeds (and is compared to) the natural or background abundance (Dawson et al. 2002). The average terrestrial abundance of 15N is 0.3663%, and 14N accounts for the other 99.6337% of N (Fry 2006). Thus, in a typical experimental approach, substances with higher abundance of 15N are added to soil in some plots, which are compared to unlabeled plots (control). Typically this is done the year before sampling to enable tracking of the retranslocation of N from that year, or labeled substances are added and samples are collected in the same year to quantify N uptake and retranslocation from all previous years (Millard and Grelet 2010).
The aim of the study reported here was to elucidate more clearly the relative importance of N uptake and N retranslocation for Scots pine and Norway spruce seedlings during the first two years after planting, possible associated growth responses, and the potential role of N2-fixation. For this purpose, we used seedlings fertilized with 15N-enriched fertilizer cultivated throughout their development in the nursery from seed to seedling ready for deployment. This strategy enabled assessment of the proportions of N in current-year needles derived from uptake and retranslocation after field planting (hereafter Ndfu and Ndfr, for convenience). The acquired data were related to N concentrations and leading shoot growth in the following year. The potential N2-fixation capacity in fine roots of Norway spruce and Scots pine seedlings was also tested. For this, we used the acetylene reduction assay (Hardy et al. 1968), which exploits the ability of nitrogenases responsible for N-fixation to also reduce acetylene gas to ethylene. The study was established at four sites with widely differing fertility and geographic conditions in Sweden. The following hypotheses were tested:
-
1.
Scots pine seedlings have higher N uptake, while Norway spruce seedlings are more dependent on N retranslocation.
-
2.
The higher N uptake of Scots pine than Norway spruce seedlings is due to differences in N2-fixation rates in their fine roots.
Material and methods
Experimental design
The hypotheses were tested in field trials replicated at four forest sites. A more detailed description of the experiment has been presented by Nilsson et al. (2019). The sites were selected based on site indices of the previous crop trees, to represent one nutrient-poor and one fertile site in both southern and northern Sweden (Table 1), designated NorthPoor, NorthFertile, SouthPoor and SouthFertile, respectively. The previous crop trees were harvested during the winter before planting, except at the SouthPoor site, where they were harvested a year earlier.
Site preparation treatments
A split plot design with four blocks was applied at all sites. Each block covered 13 × 41 m, divided into three main plots (13 × 11 m), with a 4 m undisturbed buffer zone between them. Each main plot was assigned to one of three site preparation treatments, which was applied to its entire area (not spot-wise). In the main plot, the inner ca. 7 × 8 m was used for planting, creating a second 2–2.5 m buffer zone to the edge of the treated main plot. The size of main plots and inner areas varied slightly between the two regions. The treatments were: no site preparation (control); removed humus (RH, i.e., removal of the organic layer in the entire main plot leaving a top layer of mineral soil); and deep soil cultivation (DSC, i.e., inverting the entire soil profile to a depth of 60 cm, burying the topsoil under 10–20 cm of mineral soil). Each of the site preparation treatments was applied in the spring or early summer using an excavator operated from outside the plots to avoid soil compaction. The two sites in southern Sweden were fenced to prevent browsing damage, but this was assumed not necessary at the northern sites.
Nursery preparation
The planting material consisted of seedlings of provenances considered to be well adapted to the respective sites (Table 1), provided by the Forestry Research Institute of Sweden (Skogforsk). All seedlings were sown in containers filled with unfertilized peat. The sizes of the containers were 50 and 90 cm3 in northern and southern Sweden, respectively, following standard nursery protocols in each area. The seedlings were grown in a greenhouse following a conventional growing scheme and stored in a freezer (at -4 to -2 °C) during the winter (Table 2).
Labeling was designed to label the N pools of the seedlings uniformly and homogeneously before their planting in forest. By doing this type of labeling the 15N signature of seedling internal N will be different from signature of unlabeled N from the forest soil. Consequently, any N uptake from the soil will dilute the 15N signature of the internal N pool of the seedling. Hence, to enable tracing of the 15N concentration in new needles, concentrated 15N powder as ammonium nitrate (15NH415NO3) (Cambridge Isotope Laboratories Inc. Ammonium nitrate 15N2 98% +), was added in the nursery to the growth media by mixing with the standard fertilizer (NPK 9–1-6) to obtain a labeling of approximately 1 atom% of total N compounds. Fertigation was repeated twice per week per seedling with 5 mg N, from seeding to outplanting.
To determine the baseline concentration of 15N and check that its concentration was similar in all seedlings at planting, needles from six seedlings from each batch and species were analyzed, 24 seedlings in total. The samples were dried for 24 h in 65˚C and ground with a ball mill (Retsch MM 400), then their 15N concentration was determined using an isotope ratio mass spectrometer (Thermo Fisher Scientific. Delta V) coupled to an element analyzer (Thermo Fisher Scientific. Flash Elemental Analyzer), at SLU – the Swedish University of Agricultural Sciences in Umeå, Sweden.
Planting
Scots pine and Norway spruce seedlings enriched to a similar 15N level were randomly interplanted in the main plots at all sites with 1 m spacing in 2011 and 2012 at the northern and southern sites, respectively. In each plot, 20 or 29 seedlings of each species were planted during spring or early summer at the northern and southern sites, respectively. All seedlings (2352 in total) were planted at a conventional planting depth, with the entire root system from the container a few cm under the soil surface. Each seedling was treated with insecticide (Bayer AB—Bayer CropScience. Merit Forest WG, active substance Imidacloprid) to protect it from pine weevil (Hylobius abietis) at planting, then re-treated in the first spring at the northern sites, and both the first spring and second autumn at the southern sites.
Field measurements and sampling
Immediately after planting, initial dimensions (height from the ground and diameter at ground level) of every seedling were measured. Thereafter, these dimensions (and leading shoot length) were annually re-measured after the growing season during the first 5–6 years. For more details regarding seedling damage, mortality, vegetation cover and soil temperature, see Nilsson et al. (2019). Three seedlings of each species were excavated from every main plot after the first and second growing seasons, in total 192 seedlings. Seedlings in each plot were assigned to three height-based classes, one seedling of each class was excavated, and pooled by site preparation treatment to analyze 15N contents of the new needles. This sampling was stopped after two growing seasons as the 15N level approached the natural level of 0.3663 atom% at most sites.
To analyze potential N2-fixation capacity, three of the tallest seedlings of each species were selected in every plot during 2017 (after 5–7 growing seasons) and one of them was randomly sampled during the two fine root sampling occasions. Fine roots (< 2 mm diameter) were sampled twice (in late spring and early autumn) to capture seasonal differences. Fine roots were manually collected by following the roots of the seedling and sampled with spade and scissors, put in a plastic bag and stored in a cooler during the fieldwork. Samples were then stored for less than a week in darkness at 4 ˚C until the start of the laboratory analyses. A total of 192 samples (one per block per species per sampling occasion), or 12 replications per site per species and sampling occasion were used in the analyses.
15N concentration in new needles
We calculated proportions of N in needles of new shoots derived from N acquisition in the field and N retranslocation (‘N derived from uptake’, or Ndfu, and ‘N derived from retranslocation’, or Ndfr, respectively) after the two first growing seasons. Monitoring changes in 15N concentrations could be considered an adaptation of typical isotope dilution methods (Barraclough 1995; Dawson et al. 2002; Murphy et al. 2003; Millard and Grelet 2010), where 15N-labeled substances are added to the soil in some plots, but not controls, in the sampling year or the preceding year to quantify retranslocation from all previous years or that specific year. In contrast, we enriched our seedlings to high levels of 15N and followed the 15N dynamics in new needles to enable investigation of possible connections to the seedlings’ growth and relative levels of newly acquired and stored N in new needles, and conversely their dependence on retranslocated labeled N from enriched fertilizer applied in the nursery.
Ndfr and Ndfu were calculated using the following formula:
Where 15Nnursery = Atom% 15N in the seedling batch analyzed before planting in the field; 15Nsoil = Atom% 15N in N from the soil, approximated to 0.3663; 15Nneedles = Atom% 15N in the needles of new shoots at a given time.
The equation follows the approach of Deléens et al. (1994).
Potential N2-fixation capacity
To analyze potential N2-fixation capacity in fine roots associated with both species, fine roots were harvested from the collected field material, and carefully cleaned with distilled water to remove contaminating soil particles. The roots were then placed in 22 ml glass vials, and nitrogenase activity was estimated using a 24-h acetylene reduction assay, essentially following the protocol previously used to estimate the activity in feather mosses (DeLuca et al. 2002; Stuiver et al. 2016). After removing 10% of the air in the vials and replacing it, by injection with acetylene, the vials with fine roots from the northern and southern trials were stored in a dark climate chamber for 24 h at 10 and 13 °C, respectively. The incubation temperatures were based on average temperatures recorded in the organic layer in the two regions. The ethylene concentration in each sample was then determined using a gas chromatograph (Perkin Elmer Inc. Clarus 500 GC). Finally, the fine root samples were dried at 70 °C for 48 h and weighed, their potential N2-fixation capacities were calculated, in ng N2 g−1 fine root day−1, from the amount of ethylene produced (determined using the universal gas law) and a conversion factor based on the numbers of electrons required for N2 fixation (8) and acetylene reduction (2.5), giving a theoretical ratio of 8/2.5 = 3.2 (Bellenger et al. 2014). The factor used in most studies is between 3 and 4, as factors typically derived from field samples have been found to be around 4 (Hardy et al. 1973). It should be noted that Roskoski (1981) found that conversion factors could range from 0.1 to 20, but this was with samples that had been incubated for 5 or 9 days with 15N2 and then analyzed for C2H2 reduction. We used the conversion factor 3, which is commonly applied in similar studies, as described in several reviews (reviews by Son 2001; Brunner and Kimmins 2003; Bellenger et al. 2014). The weight of dried samples used in the incubations varied from 0.04 to 0.75 g, with a mean of 0.26 g.
Statistical analyses
Ndfu values and potential N2-fixation capacities of the fine roots of seedlings sampled at each site, species and site preparation treatment were calculated and used in Analysis of Variance (ANOVA) of species and site preperation effects. Mixed models of effects across and within sites were constructed using the R statistical packages lmerTest (Kuznetsova et al. 2017) and lme4 (Bates et al. 2015), with sites and blocks as random effects, and site preparation and species as fixed effects.
The following split-plot model was used to assess the effects across sites:
Here yijk is the response variable (Ndfu or potential N2-fixation) of the kth replicate of site preparation treatment (C, RH or DSC) i, and species j, µ = overall mean, sk = site, random effect, (k = 1,…,4), bkl = block within site, random effect, (l = 1,…,4), αi = fixed effect of the ith site preparation treatment (i = 1,…,3), δikl = site preparation treatment experimental error, βj = fixed effect of the jth split-plot species (j = 1,2), (αβ)ij = interaction between site preparation and species, εijk = split-plot experimental error (species experimental error).
In addition, the following split-plot model was used to assess effects within sites, which was to for example analyze Ndfu at the NorthPoor site after the second growing season for, as15N levels were approaching natural abundance (0.3663 atom% 15N), corresponding to 100% Ndfu, in shoots of both species at the three other sites, using the method described above, except for exclusion of site from the model:
where yijk is the response variable (Ndfu) of the kth replicate of site preparation treatment (C, RH or DSC) i, and species j. The other terms are the same as above.
When significant differences were detected, they were identified by Tukey’s post hoc test. The NorthPoor site was excluded from the analysis across all sites of Ndfu after the second growing season, as seedlings there had considerably lower Ndfu than at the other three sites. The explanatory variables were log-transformed when necessary to meet homogeneity of variance requirements for ANOVA. In all analyses, differences were deemed significant if p < 0.05.
A general linear regression model with dummy variables for species and interaction between dummy variables and independent numeric variables, with the following formula, was constructed for analyzing Ndfu or N concentration, and leading shoot growth the following year:
where X1 = independent variable (Ndfu or N concentration), X2 = species dummy variable, 1 if Scots pine and 0 if Norway spruce, β0 and β1 and β2 and β3 = coefficients to be estimated, εi = error term.
Results
N uptake
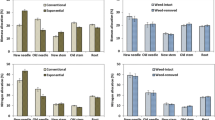
Significant differences in Ndfu were found between species across site preparation treatments and sites after the first and second growing seasons (Table 3). After the first growing season, Ndfu was significantly higher in plots with no site preparation (control) than in DSC treatment plots (p = 0.0376) across sites and species. Differences in Ndfu among sites reduced the possibility of robust ANOVA after the second growing season, as seedlings at the NorthPoor site, especially Norway spruce seedlings, had lower Ndfu and higher Ndfr (and thus higher dependence on N retranslocation) than those at other sites (Fig. 1 and 5). Therefore, data pertaining to Ndfu at the NorthPoor site and the other three sites after the second growing season were analyzed separately for the overall across sites analysis. After the second growing season, Ndfu was significantly lower in RH plots than in control and DSC plots (p = 0.0163 and 0.0387, respectively) across the other three sites. At the NorthPoor site, Ndfu was significantly lower under the DSC treatment than under the control and RH treatments (p = 0.0013 and 0.0036, respectively), and seedlings of both species did almost have no Ndfu in their new needles after the first growing season. Significant differences in Ndfu between species were found within sites among treatments after the first and second growing seasons, with lower Ndfu both at the NorthPoor site and lower for Norway spruce in most site preparation treatments at all sites, especially in the RH treatment after the first growing season except at the SouthFertile site (Fig. 1). Furthermore, significant differences in Ndfu between species across site preparation treatments were also found within sites (Fig. 5). At all sites except the NorthPoor site 15N levels were approaching natural abundance (0.3663 atom% 15N), corresponding to 100% Ndfu, in shoots of both species after the second growing season (Figs. 1 and 5).
Ndfu (in percentage of N in needles of new shoots derived from uptake) during the first two growing seasons at each site and under each site preparation treatment: Control (no site preparation), Removed Humus (RH) and Deep Soil Cultivation (DSC). The asterisks indicate significant (p < 0.05) differences between species. 192 seedlings were sampled and each data point is represented by 12 seedlings. Standard error at NorthPoor year 1 is 4.632, and year 2 5.899. Standard error at NorthFertile year 1 is 10.943, and year 2 2.6439. Standard error at SouthPoor year 1 is 7.5027, and year 2 3.996. Standard error at SouthFertile year 1 is 6.666, and year 2 2.135. Ndfu at each site across treatments are shown in Fig. 5 in the Appendix
The results also show that there was a significant difference between species for Ndfu after the first growing season and growth of the leading shoot in the following year across all sites (p < 0.0001). As there was a significant interaction between Ndfu and species (p = 0.0062), the slope of Scots pine had a higher intersection and was steeper than that of Norway spruce, meaning that an increase of N uptake leads to more leading shoot growth the following year for Scots pine. Increases in Ndfu were associated with increases in leading shoot growth of Scots pine seedlings at all sites and Norway spruce seedlings in the south. This was much weaker for Norway spruce in the north, especially at the NorthFertile site (Fig. 2a). Significant difference between species for Ndfu and leading shoot growth in the following year was also found after the second growing season at all sites except NorthPoor (p < 0.0017) (Fig. 2b) and at the NorthPoor site alone (p < 0.0251) (Fig. 2c). No significant between-species difference in this respect was found at the NorthPoor site after the second growing season. However, there was a nearly significant interaction between Ndfu and species (p = 0.0530), indicating that increases in leading shoot growth in the following year associated with increases in Ndfu tended to be higher in Scots pine seedlings (Fig. 2c).
a–c Ndfu (in percentage of N in needles of new shoots derived from uptake) versus leading shoot growth in the following year of Scots pine (open symbols, dashed line) and Norway spruce (solid symbols, full line) seedlings, after: a the first growing season at all sites; b the second growing season at all except the NorthPoor site; c the second growing season at the NorthPoor site
N concentration after the first growing season and growth of the leading shoot in the following year were also significantly different between species (p < 0.0001), and increases in N concentration were associated with larger growth increases in Scots pine than in Norway spruce seedlings as Scots pine had both higher intersection and steeper slope (p = 0.0287) (Fig. 3a). In further similarity to the relationship between growth of leading shoots of Norway spruce and Ndfu (Fig. 2a), leading shoot growth was very low even with high N concentrations in the north. As 15N levels were approaching natural abundance at all sites except NorthPoor after the second growing season, a significant difference between species for N concentration and leading shoot growth was found at the NorthPoor site (p = 0.0347) (Fig. 3c), but not at the other three sites combined (Fig. 3b).
a–c N concentration in needles of new shoots versus leading shoot growth in the following year of Scots pine (open symbols, dashed line) and Norway spruce (solid symbols, full line) seedlings after: a the first growing season at all sites; b the second growing season at all except the NorthPoor site; c the second growing season at the NorthPoor site
Potential N2-fixation capacity
Potential N2-fixation capacity, estimated by the acetylene reduction assay, varied between species and sites, ranging from 5.7 to 48 ng N2 g fine root−1 day−1 (Fig. 4). The Scots pine fine roots had significantly higher potential N2-fixation capacity than the Norway spruce across (p < 0.0001) and within all sites, at both the spring and autumn sampling occasions (Fig. 4), but no treatment or interaction effects between treatment and species were found.
Potential N2-fixation (ng N2 g fine root−1 day−1; right y-axis) rates in fine roots of Scots pine and Norway spruce saplings at all sites at the first and second sampling occasions and their ethylene production (nmol C2H4 g fine root−1 day−1; left y-axis) in assays. Each bar shows the mean of 12 samples and error bars represent standard errors
Discussion
N uptake
The presented results corroborate our first hypothesis, that Scots pine seedlings have higher N uptake, as they had higher Ndfu and correspondingly lower Ndfr values than Norway spruce seedlings at all sites after the first and second growing seasons. As 15N was measured annually in new needles, high Ndfu values indicate that seedlings had mostly utilized non-enriched N from the soil, while seedlings with lower Ndfu values were more dependent on the enriched N they obtained in the nursery under sufficient fertilization regime, and their N uptake rates were lower. Ndfu had reached almost 100% after two growing seasons, indicating that it accounted for almost all N in new needles, and Ndfr made minor contributions, at three of the sites, while there was still strong dependence on N retranslocation at the NorthPoor site.
Overall, Norway spruce was more sensitive to the site preparation treatments than Scots pine, as previously found (Nilsson et al. 2019). Reasons for this difference are still unknown, even though Norway spruce is more affected by resource availability as it has shown better growth response in fertilization experiments (Hedwall et al. 2014). However, the lower Ndfu in Norway spruce and higher Ndfu in Scots pine seedlings is consistent with Scots pine being a pioneer species with high early growth rates, Norway spruce having more sustained growth patterns, and frequent occurrence of growth check (Grossnickle 2000). It is hard to make general recommendations of site preparation techniques and species based on our results as the time frame was relatively short. However, Norway spruce should not be planted in planting spots with removed humus and a long distance to the nutrient rich organic material, which can be created by both disc trenching and mounding, as the seedlings seemed to struggle with N uptake, compared to Scots pine that was less affected in such planting spots. It could also be tempting to disregard planting spots as were created in the DSC site preparation treatment, especially in the north, as the Ndfu was low, but growth in such environment has been shown to perform well with time (Nilsson et al. 2019). Furthermore, it has been shown that seedlings planted without site preparation have a higher risk of both damage and mortality caused by pine weevil (Wallertz et al. 2018). Hence, choosing the right planting spot where the roots can reach the organic material relatively fast should be prioritized.
Retranslocation of nutrients and carbohydrates from older needles and other parts is crucial for tree growth (Millard and Grelet 2010; Uscola et al. 2015; Villar-Salvador et al. 2015). Results of this study show that increases in Ndfu after the first and second growing season led to increased leading shoot growth in the following year, in accordance with previous studies (Nilsson and Örlander 1999; Grossnickle 2000; Nordborg et al. 2003). Interestingly, increases in Ndfu for Norway spruce in the north did not result in longer leading shoots in the following year, indicating that the limiting factor was something other than N, e.g., low soil temperature, soil water availability (Margolis and Brand 1990), or nutrient levels (Grossnickle 2000). This is consistent with observations of higher dependency on retranslocation in low-N environments (such as the NorthPoor site) in many tree species (Millard and Grelet 2010), including Scots pine (Proe et al. 2000). The observation that Ndfu was approaching 100% after the second growing season shows that the labeled N supplied in the nursery was no longer crucial for the seedlings. Hence, increases in their growth were heavily dependent on N uptake, indicating that retranslocation of initial N was much less important than N uptake. However, seedlings at the NorthPoor site still seemed to rely on N retranslocation. From another perspective, the results also suggest that some of the N in new needles was still derived from retranslocation of N supplied in the nursery, even after the second growing season, and dependence on this N was higher in Norway spruce seedlings. High dependence on retranslocated N, especially in low N environments, is the main reason for nutrient loading in the nursery, which was not done in this study. Nutrient loading provides seedlings with additional nutrient reserves that can be utilized in the following growing season, thereby enhancing growth of seedlings of various species (Malik and Timmer 1996; Salifu and Timmer 2003a, 2003b; Salifu and Jacobs 2006; Salifu et al. 2009), including Norway spruce, but the effect is short-lived in the field and fades away after the first or second growing seasons (Grossnickle 2000; Heiskanen et al. 2009).
N concentration after the first growing season and leading shoot growth the following year showed many similarities to leading shoot growths’ relationship with Ndfu, in accordance with previous findings (Malik and Timmer 1996; Villar-Salvador et al. 2015). However, after the second growing season, this only persisted at the NorthPoor site. That N concentration after the second growing season and leading shoot growth the following year did not show any clear pattern at the three sites except NorthPoor is in line with previous studies showing that N concentration and growth rate are not necessarily related (Salifu and Timmer 2003b).
Implications of differences between the 15N dilution approach applied in this study and typical approaches (also called pulse-chase technique) also warrant consideration. In our approach, growing seedlings from seeds with labeled N to the stage at which they were planted, ensures that all organs and tissues display the same 15N signal. It means that any change in this signal can be attributed to uptake of N from the soil. The typical approach described by Millard and Grelet (2010) where plants are supplied 15N-labeled N sources either before or the same year in which N uptake and retranslocation are determined may result in heterogeneously labeled seedlings, as distributions of labeled N may vary between different plant tissues and organs, thereby complicating interpretation of 15N patterns in different plant tissues.
N2-fixation capacity
The second hypothesis, that Scots pines’ higher N uptake may be due to N2-fixation in fine roots, was rejected, as N2-fixation rates were very low in comparison to rates of N acquisition from the soil or retranslocation. A conservative sensitivity analysis based on seedling weight, and assumptions that the entire seedling biomass had the same maximum potential N2-fixation capacity and 1–2% N content (Nilsson et al. 1996; Bergquist and Örlander 1998), indicated that N2-fixation accounted for at most ca. 0.1–0.2% of the seedlings’ total N uptake. Thus, although potential N2-fixation rates were higher in Scots pine seedlings than Norway spruce seedlings they were far too low to explain the between-species difference in N uptake. Thus, the contribution of N from N2-fixation was neglected for the Ndfu and Ndfr values.
The N2-fixation capacity of Norway spruce we derived was within the range reported by Mäkipää et al. (2018) in Finland, despite their focus on woody root material (> 5 mm in diameter). Moreover, we determined the maximum potential N2-fixation capacity of the fine roots. As no controls with fine root samples without acetylene were included in our assays it should be noted that the roots may have emitted ethylene due to stress or some other response, as organs of many plants, including pine roots, may do (Stumpff and Johnson 1987). However, in a pilot study Mäkipää et al. (2018) detected no ethylene production without acetylene addition in Norway spruce or silver birch roots subjected to similar conditions. Moreover, any corrections to account for naturally emitted ethylene in our study would have decreased the derived N2-fixation capacities. Extrapolation of ethylene production-based estimates of N2-fixation rates in roots of middle-aged and mature Norway spruce trees by Mäkipää et al. (2018) resulted in values of just 0.06 and 0.15 kg ha−1 year−1, tiny fractions of the total stand-level uptake of N by mature trees, which reportedly amounts to 15–50 kg ha−1 year−1 in northern Sweden and Finland (Korhonen et al. 2013; Sponseller et al. 2016).
Conclusions
There are four main findings of this study. First, use of 15N-labeled seedlings enabled disentanglement of the role of uptake of new N and retranslocation of old N into new foliage. Second, increases in both the Ndfu and N concentration in foliage after the first growing season led to increased leading shoot growth in the second growing season. Third, temporal patterns of changes in N uptake and retranslocation in Norway spruce and Scots pine seedlings were similar. Fourth, there were low rates of potential N2-fixation capacity, and despite differences in capacities between Scots pine and Norway spruce, the contribution of N2-fixation to N acquisition was negligible in both species.
These patterns suggest that N uptake following planting is crucial for seedling growth, and retranslocated N alone is insufficient for early seedling growth. Scots pine seedlings had clearly higher Ndfu values, and thus apparently higher N uptake rates, indicating that they are less responsive to site preparation treatments and can acquire more N than Norway spruce seedlings during the first years after planting, and grow more rapidly after the first year with increasing Ndfu. Conversely, Norway spruce has greater need for appropriate silvicultural measures to grow well. Further investigation of limiting factors for the growth of Norway spruce seedlings in the north is warranted (e.g., through optimization studies), as their leading shoot growth was low after the second growing season despite high Ndfu values. The complexity of N2-fixation in roots, including variations associated with differences in root diameter and age, also warrants further attention in future research.
Data availability
The data this study is based upon are available from the corresponding author on reasonable request.
References
Anon (1981) FAO–UNESCO Soil map of the world (Vol. 5 Europe). Rome: United Nations Educational, Scientific and Cultural Organization.
Barraclough D (1995) 15N isotope dilution techniques to study soil nitrogen transformations and plant uptake. Fertilizer Research 42(1–3):185–192. https://doi.org/10.1007/bf00750513
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AML (2014) Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol Biochem 69:413–420. https://doi.org/10.1016/j.soilbio.2013.11.015
Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For Ecol Manage 119(1–3):51–62. https://doi.org/10.1016/s0378-1127(98)00509-x
Bergquist J, Örlander G (1998) Browsing damage by roe deer on Norway spruce seedlings planted on clearcuts of different ages - 1. Effect of slash removal, vegetation development, and roe deer density. For Ecol Manag 105(1–3):283–293. https://doi.org/10.1016/s0378-1127(97)00297-1
Binkley D, Cromack K, Baker D (1994) Nittrogen fixation by red alder: Biology, rates, and controls. In: Hibbs DE, DeBell DS, Tarrant RF (eds) The biology and management of red alder. Oregon State University Press, Corvallis, pp 57–72
Brockwell J, Searle SD, Jeavons AC. (2005). Nitrogen fixation in Acacias: An untapped resource for sustainable plantations, farm forestry and land reclamation. Australian Centre for International Agricultural Research (ACIAR) Monograph No. 115
Brunner A, Kimmins JP (2003) Nitrogen fixation in coarse woody debris of Thuja plicata and Tsuga heterophylla forests on northern Vancouver Island. Can J for Res-Revue Canadienne De Recherche Forestiere 33(9):1670–1682. https://doi.org/10.1139/x03-085
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451
Deléens E, Cliquet J, Prioul J (1994) Use of 13C and 15N plant label near natural abundance for monitoring carbon and nitrogen partitioning. Funct Plant Biol 21(2):133–146
DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419(6910):917–920. https://doi.org/10.1038/nature01051
Fenn ME, Poth MA, Aber JD, Baron JS, Bormann BT, Johnson DW, Lemly AD, McNulty SG, Ryan DF, Stottlemyer R (1998) Nitrogen excess in North American ecosystems: predisposing factors, ecosystem responses, and management strategies. Ecol Appl 8(3):706–733. https://doi.org/10.2307/2641261
Fry B (2006) Stable isotope ecology. Springer, New York
Granhall U, Lindberg T (1978) Nitrogen fixation in some coniferous forest ecosystems. Ecological Bulletins (Stockholm)(26), 178–192.
Grossnickle SC (2000) Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Research Press, Ottawa
Hägglund B, Lundmark, JE (1987) Handledning i bonitering med skogshögskolans boniteringssystem. Del 1 Definitioner och anvisningar (3 ed.). Jönköping: Skogsstyrelsen.
Hägglund B, Lundmark JE (2007) Handledning i bonitering med skogshögskolans boniteringssystem. Del 2 Diagram och tabeller (5 ed.). Jönköping: Skogsstyrelsen.
Hardy RWF, Burns RC, Holsten RD (1973) Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5(1):47–81. https://doi.org/10.1016/0038-0717(73)90093-X
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) Acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43(8):1185–2000. https://doi.org/10.1104/pp.43.8.1185
Hedwall P-O, Gong P, Ingerslev M, Bergh J (2014) Fertilization in northern forests: biological, economic and environmental constraints and possibilities. Scand J for Res 29(4):301–311. https://doi.org/10.1080/02827581.2014.926096
Heiskanen J, Lahti M, Luoranen J, Rikala R (2009) Nutrient loading has a transitory effect on the nitrogen status and growth of outplanted Norway spruce seedlings. Silva Fennica 43(2):249–260. https://doi.org/10.14214/sf.210
Johansson K, Langvall O, Bergh J (2012) Optimization of environmental factors affecting initial growth of Norway spruce seedlings. Silva Fennica 46(1):27–38. https://doi.org/10.14214/sf.64
Johansson K, Ring E, Högbom L (2013) Effects of pre-harvest fertilization and subsequent soil scarification on the growth of planted Pinus sylvestris seedlings and ground vegetation after clear-felling. Silva Fennica 47(4):1–18
Korhonen JFJ, Pihlatie M, Pumpanen J, Aaltonen H, Hari P, Levula J, Kieloaho AJ, Nikinmaa E, Vesala T, Ilvesniemi H (2013) Nitrogen balance of a boreal Scots pine forest. Biogeosciences 10(2):1083–1095. https://doi.org/10.5194/bg-10-1083-2013
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82(13):1–26
Lebel P, Thiffault N, Bradley RL (2008) Kalmia removal increases nutrient supply and growth of black spruce seedlings: an effect fertilizer cannot emulate. For Ecol Manag 256(10):1780–1784. https://doi.org/10.1016/j.foreco.2008.02.050
Malik V, Timmer VR (1996) Growth, nutrient dynamics, and interspecific competition of nutrient-loaded black spruce seedlings on a boreal mixedwood site. Can J for Res-Revue Canadienne De Recherche Forestiere 26(9):1651–1659. https://doi.org/10.1139/x26-186
Margolis HA, Brand DG (1990) An ecophysiological basis for understanding plantation establishment. Can J for Res-Revue Canadienne De Recherche Forestiere 20(4):375–390. https://doi.org/10.1139/x90-056
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30(9):1083–1095. https://doi.org/10.1093/treephys/tpq042
Munson AD, Margolis HA, Brand DG (1993) Intensive silvicultural treatment: impacts on soil fertility and planted conifer response. Soil Sci Soc Am J 57(1):246–255. https://doi.org/10.2136/sssaj1993.03615995005700010043x
Murphy DV, Recous S, Stockdale EA, Fillery IRP, Jensen LS, Hatch DJ, Goulding KWT (2003) Gross nitrogen fluxes in soil: theory, measurement and application of 15N pool dilution techniques. In: Sparks DL (ed) Advances in agronomy, vol 79. Elsevier Academic Press Inc, San Diego, pp 69–118
Mäkipää R, Huhtiniemi S, Kaseva J, Smolander A (2018) Asymbiotic nitrogen fixation on woody roots of Norway spruce and silver birch. Can J for Res 48(2):172–179. https://doi.org/10.1139/cjfr-2017-0270
Morén AS, Perttu K (1994) Regional temperature and radiation indices and their adjustment to horizontal and inclined forest land. Studia Forestalia Suecica 194:19
Nilsson O, Hjelm K, Nilsson U (2019) Early growth of planted Norway spruce and Scots pine after site preparation in Sweden. Scand J for Res 34(8):678–688. https://doi.org/10.1080/02827581.2019.1659398
Nilsson U, Gemmel P, Hällgren J-E (1996) Competing vegetation effects on initial growth of planted Picea abies. NZ J Forest Sci 26(1/2):84–98
Nilsson U, Luoranen J, Kolström T, Örlander G, Puttonen P (2010) Reforestation with planting in northern Europe. Scand J for Res 25(4):283–294. https://doi.org/10.1080/02827581.2010.498384
Nilsson U, Örlander G (1999) Vegetation management on grass-dominated clearcuts planted with Norway spruce in southern Sweden. Can J for Res-Revue Canadienne De Recherche Forestiere 29(7):1015–1026. https://doi.org/10.1139/cjfr-29-7-1015
Nordborg F, Nilsson U (2003) Growth, damage and net nitrogen uptake in Picea abies (L.) Karst. seedlings, effects of site preparation and fertilisation. Annals for Sci 60(7):657–666. https://doi.org/10.1051/forest:2003058
Nordborg F, Nilsson U, Örlander G (2003) Effects of different soil treatments on growth and net nitrogen uptake of newly planted Picea abies (L.) Karst. seedlings. For Ecol Manag 180(1–3):571–582. https://doi.org/10.1016/s0378-1127(02)00650-3
Örlander G, Gemmel P, Hunt J. (1990). Site preparation: a Swedish overview. In: FRDA Report, BC Ministry of Forests, Victoria, Canada
Padda KP, Puri A, Chanway CP (2018) Isolation and identification of endophytic diazotrophs from lodgepole pine trees growing at unreclaimed gravel mining pits in central interior British Columbia, Canada. Can J for Res 48(12):1601–1606. https://doi.org/10.1139/cjfr-2018-0347
Perkin Elmer Inc. Clarus 500 GC. Waltham, Massachusetts, USA
Proe MF, Midwood AJ, Craig J (2000) Use of stable isotopes to quantify nitrogen, potassium and magnesium dynamics in young Scots pine (Pinus sylvestris). New Phytol 146(3):461–469. https://doi.org/10.1046/j.1469-8137.2000.00658.x
Puri A, Padda KP, Chanway CP (2018) Evidence of endophytic diazotrophic bacteria in lodgepole pine and hybrid white spruce trees growing in soils with different nutrient statuses in the West Chilcotin region of British Columbia, Canada. For Ecol Manage 430:558–565. https://doi.org/10.1016/j.foreco.2018.08.049
Roskoski JP (1981) Comparative C2H2 reduction and 15N2 fixation in deciduous wood litter. Soil Biol Biochem 13(1):83–85. https://doi.org/10.1016/0038-0717(81)90109-7
Salifu KF, Islam MA, Jacobs DF (2009) Retranslocation, plant, and soil recovery of Nitrogen-15 aplied to breroot back wlnut seedlings. Commun Soil Sci Plant Anal 40(9–10):1408–1417. https://doi.org/10.1080/00103620902818062
Salifu KF, Jacobs DF (2006) Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Ann for Sci 63(3):231–237. https://doi.org/10.1051/forest:2006001
Salifu KF, Timmer VR (2003a) Nitrogen retranslocation response of young Picea mariana to nitrogen-15 supply. Soil Sci Soc Am J 67(1):309–317
Salifu KF, Timmer VR (2003b) Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Can J for Res-Revue Canadienne De Recherche Forestiere 33(7):1287–1294. https://doi.org/10.1139/x03-057
SFA (2022) Swedish Forest Agency, Regeneration Quality. Retrieved from https://www.skogsstyrelsen.se/globalassets/statistik/statistikfaktablad/jo0311-statistikfaktablad-atervaxternas-kvalitet-2022.pdf. Accessed 2023-02-21
SFA (2023) Swedish Forest Agency, Forest seedlings. Retrieved from https://pxweb.skogsstyrelsen.se/pxweb/sv/Skogsstyrelsens%20statistikdatabas/Skogsstyrelsens%20statistikdatabas__Skogsplantor/JO0313_1.px/?rxid=03eb67a3-87d7-486d-acce-92fc8082735d. Accessed 2023-10-31
SLU (2020) Swedish University of Agriultural Sciences, Forest statistics 2020. Retrieved from https://www.slu.se/globalassets/ew/org/centrb/rt/dokument/skogsdata/skogsdata_2020_webb.pdf. Accessed 2020-10-28
Son Y (2001) Non-symbiotic nitrogen fixation in forest ecosystems. Ecol Res 16(2):183–196. https://doi.org/10.1046/j.1440-1703.2001.00385.x
Sponseller RA, Gundale MJ, Futter M, Ring E, Nordin A, Näsholm T, Laudon H (2016) Nitrogen dynamics in managed boreal forests: recent advances and future research directions. Ambio 45:S175–S187. https://doi.org/10.1007/s13280-015-0755-4
Stuiver BM, Gundale MJ, Wardle DA, Nilsson MC (2016) Nitrogen fixation rates associated with the feather mosses Pleurozium schreberi and Hylocomium splendens during forest stand development following clear-cutting. For Ecol Manage 375:309–309. https://doi.org/10.1016/j.foreco.2016.05.038
Stumpff NJ, Johnson JD (1987) Ethylene production by loblolly pine seedlings associated with water stress. Physiol Plant 69(1):167–172. https://doi.org/10.1111/j.1399-3054.1987.tb01962.x
Tamm CO (1991) Nitrogen in terrestrial ecosystems. Questions of productivity, vegetational changes, and ecosystem stability. Springer, Berlin Heidelberg
Thiffault N, Jobidon R (2006) How to shift unproductive Kalmia angustifolia–Rhododendron groenlandicum heath to productive conifer plantation. Can J Forest Res-Revue Canadienne De Recherche Forestiere 36(10):2364–2376. https://doi.org/10.1139/x06-090
Thiffault N, Titus BD, English B (2017) Twenty-five years post-treatment conifer responses to silviculture on a Kalmia-dominated site in eastern Canada. For Chron 93(2):161–170. https://doi.org/10.5558/tfc2017-022
Thiffault N, Titus BD, Munson AD (2004) Black spruce seedlings in a Kalmia-Vaccinium association: microsite manipulation to explore interactions in the field. Can J Forest Res-Revue Canadienne De Recherche Forestiere 34(8):1657–1668. https://doi.org/10.1139/x04-046
Thiffault N, Titus BD, Munson AD (2005) Silvicultural options to promote seedling establishment on Kalmia-Vaccinium-dominated sites. Scand J for Res 20(2):110–121. https://doi.org/10.1080/02827580510008356
Uscola M, Villar-Salvador P, Gross P, Maillard P (2015) Fast growth involves high dependence on stored resources in seedlings of Mediterranean evergreen trees. Annals Botany 115(6):1001–1013
Villar-Salvador P, Uscola M, Jacobs DF (2015) The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New for 46(5):813–839. https://doi.org/10.1007/s11056-015-9499-z
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7(3):737–750. https://doi.org/10.2307/2269431
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13(2):87–115
Wallertz K, Björklund N, Hjelm K, Petersson M, Sundblad L-G (2018) Comparison of different site preparation techniques: quality of planting spots, seedling growth and pine weevil damage. New for. https://doi.org/10.1007/s11056-018-9634-8
Acknowledgements
The authors thank the landowners who provided suitable sites for this study. We are also grateful to the staff and research technicians of the Forestry Research Institute of Sweden (Skogforsk) in Ekebo and Sävar, the unit for field-based research at Asa Experimental Forest and Svartberget field station (SLU), Department of Forest Ecology and Management (SLU) in Umeå, and Southern Swedish Forest Research Centre (SLU) in Alnarp for their assistance in acquiring measurements or samples, help in laboratory work, or other contribution to the study. We also thank Olle Sjölin for initiating the study, and the Trees for the Future (T4F) program for financial support.
Funding
This work was supported by the research program Trees for the Future [T4F].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 5.
Ndfu (in percentage of N in needles of new shoots derived from uptake) at each site across treatments. The asterisks indicate significant differences between species. 192 seedlings were sampled and each data point is represented by 36 seedlings. Standard error at NorthPoor year 1 is 4.632, and year 2 5.899. Standard error at NorthFertile year 1 is 10.943, and year 2 2.6439. Standard error at SouthPoor year 1 is 7.5027, and year 2 3.996. Standard error at SouthFertile year 1 is 6.666, and year 2 2.135
.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nilsson, O., Nilsson, U., Näsholm, T. et al. Nitrogen uptake, retranslocation and potential N2-fixation in Scots pine and Norway spruce seedlings. New Forests 55, 1247–1266 (2024). https://doi.org/10.1007/s11056-024-10032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-024-10032-2