Abstract
This paper investigates the distribution of arbuscular mycorrhizal fungi (AMF) spores and AMF colonization in a field study in southeastern Brazil. Response to AMF and rhizobial inoculation was studied in monocultures of Plathymenia reticulata and mixed plantations with both Tabebuia heptaphylla and Eucalyptus camaldulensis in a sandy soil during two consecutive years. P. reticulata height and diameter and mycorrhizal colonization and AMF diversity were measured in dry and rainy periods. The inoculated treatment of E. camaldulensis, T. heptaphylla and P. reticulata mixed plants showed higher height and diameter growth of P. reticulata used as well as increased root colonization and AMF spore numbers. Spore populations were found to belong to five genera: Acaulospora, Entrophospora, Glomus, Gigaspora and Scutellospora, with Glomus dominating. Agroforestry practices including use of leguminous tree P. reticulata effectively maintained AMF spore numbers in soils and high AMF colonization levels compared with monocultures, proving an efficient system for productivity and sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the semi-arid region of the Minas Gerais state in Brazil, the dominant vegetation is woody Caatinga species which attain a height of 30 m and have intense leaf loss during the dry season. Caatinga, which means white forest, because only the stems of drought deciduous trees can be seen in the dry season, is a biome inserted under high levels of solar radiation, annual average temperature and evaporation. Also, low and irregular precipitation is limited to a very short period (Prado 2003).
After forest clearing, the “Carrasco” vegetation invades the area, and early-successional tree (attaining up to 5 m height) and small shrub species with thin stems occur (Rizzini 1997). The latter abounds and significantly retards or stops the natural succession in northwest (NW) of Minas Gerais.
The Jaíba Project, one of the most important irrigation programs in Brazil using the São Francisco River, was established in the NW of Minas Gerais, aiming at promoting the region’s agricultural and social development. Demand for wood in this area has become a continuous threat to the preserved area, one of the largest protected areas of woody Caatinga or Dry Deciduous Forest (Rizzini 1997).
With the objective of catering for wood demand in the Jaíba Project an agroforestry system was installed at the Jaíba region to study the growth of intercropped species, Eucalyptus plants and native species of woody Caatinga (Plathymenia reticulata and Tabebuia heptaphylla). Plathymenia reticulata is able to associate symbiotically with rhizobia and/or mycorrhizal fungi, and the use of legumes increases soil fertility, biomass production (DeBell et al. 1985; Parrota 1999) and nutrient cycling, especially the N and P cycling in litter (Binkley et al. 1992). Eucalyptus species, due to their fast growth, show a good adaptation to different soils and climatic conditions, and high timber value, being increasingly used for reforestation (DeBell et al. 1985; Khanna 1997; May and Attiwill 2003). Eucalyptus spp. have the capacity to form two types of mycorrhizas, arbuscular (AM) and ectomycorrhiza (ECM) (Zambolim and Barros 1982). Almost all studies report a temporal replacement, initially dominated by arbuscular mycorrhizal fungi (AMF) and then by ECM (Carrenho et al. 2008). Nevertheless, Adjoud-Sadadou and Halli-Hargas (2000) reported that the replacement of AM by ECM in Eucalyptus is not a general phenomenon in this genus under natural conditions in Algeria. Thus Eucalyptus growth responses to inoculation with AMF remain controversial.
Tree species, such as Leucaena leucocephala (Lam.) de Wit (Manjunath et al. 1984), Albizzia lebbeck (L.) Benth. (Faria et al. 1995), Centrolobium tomentosum Guill. ex Benth. (Marques et al. 2001) and Anadenanthera peregrina Speg. (Gross et al. 2004; Pagano et al. 2007, 2008), are legumes that had their growth and nodulation improved when inoculated with rhizobia and/or AMF, and show that rehabilitation of tropical land has proved more successful if plants are inoculated with AMF (Cuenca et al. 1998; Marques et al. 2001).
Mycorrhizas improve plant nutrient cycling (Smith and Read 1997), soil structure (Miller and Jastrow 1992; Wright and Upadhyaya 1998) and biological soil quality (Cardoso and Kuyper 2006), not only regulating the functional populations in the rhizosphere (Matsumoto et al. 2005), but also decomposing the litter (Aristizábal et al. 2004; Scotti and Correa 2004). Therefore, transfer of nitrogen and phosphorus of decomposed leaves to host plants is improved (Hodge et al. 2001). The traditional taxonomy of AMF is based on spore morphology; however, in some cases difficulties have been found in AMF studies regarding morphological identification (Kirk et al. 2004). Plants growing under stress conditions show higher mycorrhizal dependence, especially because AM symbiosis improves water and nutrient supply, increasing their survival (Augé et al. 1987; Subramanian et al. 1997), particularly in semi-arid and arid environments (Varma 1995; Nouaim and Chaussod 1996). Mixed species plantations usually allow a larger diversity and/or abundance of AMF than monocultures (Cardoso et al. 2003; Cardoso and Kuyper 2006; Muleta et al. 2008). There are few reports showing AMF occurrence, distribution and diversity in the Caatinga region, most of them in cultivated soils (Maia and Trufem 1990; Yano-Melo et al. 1997, 2003). However, Albuquerque (2008) evaluated three natural areas in the Caatinga biome and verified that diversity varied according to environmental conditions, mainly plant cover and soil properties.
The present paper aims to: (a) examine the intercropping effect among Eucalyptus camaldulensis and T. heptaphylla over the growth of native species P. reticulata and over soil biology and fertility, (b) estimate root colonization and fungal diversity (density and richness) of AMF spores during the rainy and dry periods, and (c) evaluate the response to inoculation with Rhizobium and AMF on native P. reticulata species plant growth, in pure and mixed stand, as a productive model for revegetation of local degraded areas. The results provide the first indication of the diversity and species composition of AM fungi in the dry forest of Minas Gerais, Brazil.
Materials and methods
Study areas
The experimental study area was at the Jaíba Project of Irrigation (15°09′03″S 43°49′26″W), between São Francisco River and Verde Grande River, in the north region of Minas Gerais, Brazil. The climate is semi-arid BSh according to Köppen’s classification (Silva et al. 2006). Annual precipitation is 1 mm (July) to 217 mm (December), mean temperature varying between 14.8°C (July) and 34.0 (October), and the annual mean of potential evaporation being 4.88 mm day−1 (Codevasf 2004). Annual mean precipitations (871 mm) are concentrated in the November–March months (Rodrigues et al. 2001). Rainfall at this site was recorded throughout the study (2003–2004; Fig. 1).
Soil sampling and analysis
Rhizospheric soil samples were collected from the top 20 cm. Three samples/block for each plant species and for each treatment were collected, totalizing 54 samples. The samples were analyzed for chemical and physical properties. The soil analysis was performed by the Brazilian company of agricultural research (Embrapa 1979). Soil pH (H2O), cation exchange capacity (CEC) and base saturation (BS) were determined. The texture of the soil is sandy loam (Embrapa 1999), its physical and chemical characteristics being shown in Table 1.
Field experimental design
The experimental site (<1 ha) was cleared of “Carrasco” vegetation and seedlings were transplanted after the rainy season (February) in 2003. Plathymenia reticulata Benth (Leguminosae), E. camaldulensis Dehnh (Myrtaceae) and T. heptaphylla (Vell.) Tol. (Bignoniaceae) were cultivated in mixture and in monocultures.
The experimental design consisted of three replicate blocks (each 24 × 12 m for single plantations, and 24 × 18 m for mixed ones). A randomized block design with six treatments was used. Within the mixed plot, the seedlings were planted in columns of three species each, 48 plants per plot randomized by species within the plot with a 3-m spacing between individual seedlings.
The treatments were: 1—monoculture of Plathymenia reticulata with complete fertilization; 2—monoculture of P. reticulata with 80% fertilization + inoculation with Rhizobium and AMF; 3—monoculture of E. camaldulensis with complete fertilization; 4—monoculture of E. camaldulensis with 80% fertilization + inoculation with AMF; 5—mixed plantation of E. camaldulensis, P. reticulata and T. heptaphylla with complete fertilization; 6—mixed plantation of E. camaldulensis, P. reticulata and T. heptaphylla with 80% fertilization + inoculation with Rhizobium and AMF. Complete fertilization consisted in triple superphosphate (500 kg ha−1), KCl (382 Kg ha−1), MgSO47H2O (50 kg ha−1), ZnSO47H2O (46.8 kg ha−1), Mo7O24H2O (1.76 kg ha−1), urea (222 kg ha−1) following Somasegaran and Hoben (1985) and was applied at the beginning of the plantation.
Inoculation
The leguminous P. reticulata was inoculated with Rhizobium and AMF. The Rhizobium strain BHCB-AGRh2, which has slow growth, was previously isolated from nodules of P. reticulata, collected at the forest reserve, and pre-screened for its effectiveness under greenhouse, nursery and field conditions (Scotti and Correa 2004). The bacterial inocula were provided at 1 ml per pot (107 cfu ml−1) according to Somasegaran and Hoben (1985).
Arbuscular mycorrhizal inoculation was accomplished by placing into each pot 1 ml of suspension composed by 150 spores ml−1 in a total of three species: Gigaspora margarita Becker and Hall, Scutellospora heterogama (Nicol. and Gerdemann) Walker and Sanders, and Glomus brohultii Sieverding and Herrera isolated from pot cultures with Brachiaria decumbens Stapf. The AMF strains used were from the Biological Science Institute, Belo Horizonte (BHCB) culture collection. The plant species T. heptaphylla was not inoculated and E. camaldulensis was inoculated with AMF.
Analysis of plant growth under field conditions
Plant height and diameter of P. reticulata were measured in field over 2 years. The sampling took place within the dry period (May–October 2003), the rainy period (November 2003–March 2004), and the dry period (May–October 2004). Growth data in different treatments were compared by Tukey’s test (P < 0.05) following ANOVA.
Root colonization
Roots of P. reticulata, T. heptaphylla and E. camaldulensis were collected at experimental site by excavating from the trunk to the lateral root system of each tree. Root samples were harvested around each species tree from 10 to 40 cm depth, from three trees at each block. Roots were fixed in FAA solution (5 ml of formaldehyde, 5 ml of acetic acid and 90 ml of ethyl alcohol) until samples could be processed. Roots were stained and assessed for mycorrhizal infection as follows. Roots were cleared and stained with Trypan Blue (Phillips and Hayman 1970). Roots were cut into 1 cm segments and 31 cm root fragments were examined per sample for their AM status under a compound microscope (100×). If at least one root segment was found to contain fungal mycelia, arbuscules or vesicles, then the sample was considered as an AM plant, recorded as “+”. Plants were recorded as non-mycorrhizal (“−”) when neither arbuscules/vesicles nor fungi form mycelia were detected in their root cortical cells. Quantification of mycorrhiza colonization was done according to McGonigle et al. (1990), and results were expressed as percentage of colonized segments. Eucalyptus root samples were checked for natural ectomycorrhizal colonization and quantified by a line intercept method (Brundrett et al. 1996). Fine roots with a fungal mantle and Hartig net were scored as ectomycorrhizal colonization. Dual mycorrhizae (AM and ECM) were observed and individually recorded for calculation. Percent colonization was arcsin (x/100)1/2 transformed. The data were subjected to one-way ANOVA using the MINITAB software version 13.2 and means were compared by Tukey’s test (P < 0.05).
AM fungal communities distribution
Three soil sub-samples were harvested around each tree species and mixed together, because spores can be aggregate distributed (Picone 2000). Composite samples were collected from three trees at each block in August 2003 (dry period) and March (rainy period) 2004 and October (dry period) 2004. Plathymenia reticulata and E. camaldulensis rhizospheric soils were collected at 10 cm depth for analysis of AMF spores. AMF spores were recovered from 100-g triplicate sub-samples of soil of each treatment in the field, separated from the soil by wet sieving and decanting (Gerdemann and Nicolson 1963) and sucrose centrifugation (Walker et al. 1982); analysed data were expressed as number of spores/100 g of dry soil. Healthy spores were counted. Each spore type was mounted sequentially in PVLG (polyvinyl alcohol–lactic acid–glycerol) (Koske and Tessier 1983) and a mixture of PVLG and Melzer’s reagent (Morton 1988) to identify and to obtain permanent slide specimens.
The morphological properties and subcellular structures were observed under a light microscope at 100× to 1000× magnifications. Identification was based on spore colour, size, surface ornamentation and wall structure, with reference to the descriptions provided by Schenck and Pérez (1988), International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi (INVAM, West Virginia, USA, http://invam.caf.wvu.edu) and the original species descriptions. Spore numbers were square rooted transformed and statistically analyzed. The data were subjected to one-way ANOVA using MINITAB software version 13.2, and means were compared by Tukey’s test (P < 0.05).
The frequency of occurrence of each AMF species was computed with the formula: X i /X 0 × 100, where X i = the number of spores for an individual species and X 0 = the total number of spores. The frequency of occurrence of each species was used to calculate the Shannon diversity index (H), species richness (S) and evenness (E), according to Magurran (1988). Differences in AM diversity among treatments were determined with ANOVA using the MINITAB software version 13.2, and means were compared by Tukey’s test (P < 0.05).
Results
Soil and climate characteristics
The sandy loam soil at the research site was acid with a pH ranging between 5.9 and 6.4 in the topsoil (Table 1). Soil pH was similar among the experimental area soils (~6). Textural composition was as follows: coarse sand = 49%, fine sand = 35.4%, silt = 0.6% and clay = 15%. The highest levels of silt were detected in the inoculated mixed model. CEC was high in the experimental area and the percentage of BS was medium. Soil phosphorus content was higher in E. camaldulensis monocultures than in the other treatments and reference sites.
The higher pluviometric index (Fig. 1) was found in January in contrast to the drought observed between March and October.
Plant growth
When inoculated with Rhizobium and AMF, both in monoculture and in mixed stands with E. camaldulensis and T. heptaphylla, the legume P. reticulata showed higher height growth than non-inoculated plants (Fig. 2a). With respect to P. reticulata diameter growth, the inoculated monoculture differed from uninoculated mixed plantation (Fig. 2b). Thus, after 2 years of cultivation, there was an effect of inoculation on P. reticulata height growth, both in monoculture and mixed plantation.
Height (a) and diameter (b) growth of Plathymenia reticulata under different treatments after 2 years of field plantation at Jaíba. Treatments: ♦ single plantation of P. reticulata, ■ single plantation of P. reticulata inoculated with Rhizobia and AMF, △ mixed plantation of P. reticulata + E. camaldulensis + Tabebuia heptaphylla, × mixed plantation of P. reticulata (Rhizobia + AMF) + E. camaldulensis (AMF) + T. heptaphylla. Different letters indicate significant differences as determined by Tukey’s HSD test (P ≤ 0.05)
Root colonization by AM fungi
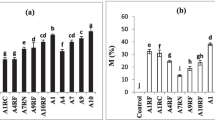
Plants in monocultures presented lower hyphae, vesicles and arbuscular colonization than in mixed plots (Fig. 3).
Mycorrhizal colonization during dry and rainy periods at monocultures and mixed plantations at Minas Gerais, Brazil. Treatments: ○ monoculture, ● monoculture (Rhizobium + AMF), □ mixed plantation, ■ mixed plantation (Rhizobium + AMF), ▲ E. camaldulensis monoculture (AMF), ◊ mixed plantation (AMF). Dry 2003 and 2004 (dry seasons), and rainy 2004 (rainy season). Different letters indicate significant differences as determined by Tukey’s HSD test (P ≤ 0.05)
Plathymenia reticulata did not show significant differences in hyphal colonization. During the rainy period an increase in vesicles production was observed in mixed plantations. The percentage of arbuscules in P. reticulata roots increased significantly in the rainy season, especially in mixed and inoculated plants, compared to the non inoculated monoculture (Fig. 3).
The roots of T. heptaphylla (which was not inoculated with AMF) showed significantly higher hyphal colonization levels (83.33%) when intercropped with inoculated plants of P. reticulata and E. camaldulensis in the last dry season (Fig. 3). Hyphae, vesicles and arbuscules increased during the rainy season and continued to increase till the last dry period. In general, T. heptaphylla mixed with inoculated plants presented significantly higher values of root colonization in comparison with plants mixed with non-inoculated intercropping plants.
Plants inoculated (P. reticulata) and noninoculated (T. heptaphylla) had higher percentage of vesicles and arbuscules than those of noninoculated treatments in the rainy season but no significative difference was seen in the second dry season for the legume.
Eucalyptus camaldulensis presented both types of root colonization, by AMF and ECM. During the rainy season the AMF colonization decreased in all treatments, in monocultures as well as mixed plantations inoculated or not (Fig. 3), while the native ECM colonization levels improved (attaining 72%; Fig. 4). In contrast, in the dry seasons ECM was reduced up to 16.6% and AMF colonization increased, especially vesicle colonization, which reached 46.6% in the inoculated intercropping plot during the last dry season (Fig. 3).
Ectomycorrhizal colonization of E. camaldulensis monocultures and mixed plantations at Minas Gerais, Brazil, during dry and rainy periods. Treatments: ○ monoculture, ▲ monoculture (AMF), □ mixed plantation, ◊ mixed plantation (AMF). Different letters indicate significant differences as determined by Tukey’s HSD test (P ≤ 0.05)
Spore number and species richness of AM fungi
Among AMF species in the studied areas a total of 14 taxa was found. Of these, one belongs to the genus Glomus, eight to Acaulospora, three to Gigaspora and two to Scutellospora (Table 2).
Mean spore number was found to be significantly higher in the rainy season compared with the dry season (Fig. 5; Table 2).
Frequency of occurrence for the members of Acaulosporaceae, Gigasporaceae and Glomeraceae in rhizosphere of P. reticulata in treatments (1, 2, 5, 6), and in rhizosphere of E. camaldulensis (3 and 4) at Minas Gerais, Brazil. Treatments: (1) P. reticulata monoculture, (2) P. reticulata monoculture (Rhizobium + AMF), (3) Eucalyptus monoculture, (4) Eucalyptus monoculture (AMF), (5) mixed plantation, (6) mixed plantation (Rhizobium + AMF). Glomeraceae (hatched), Gigasporaceae (grey) and Acaulosporaceae (white). Different letters indicate significant differences as determined by Tukey’s HSD test (P ≤ 0.05)
The dominant Glomeromycota was Glomeraceae with only one species, Glomus brohultii, found during all samplings, in all treatments (Fig. 5; Table 2). During the dry season the spore numbers of this species did not differ; whereas during the rainy season, the highest spore density was found in the mixed plantation inoculated with Rhizobium and AMF (treatment 6).
Species of Gigasporaceae (Fig. 5), especially Gigaspora margarita (Table 2), were dominant in the rhizosphere of P. reticulata in the mixed plantation inoculated with AMF and Rhizobium in both studied seasons: rainy and dry.
Acaulosporaceae did not show significant differences among treatments in either of the studied seasons (Fig. 5), presenting the highest species richness (seven) despite not being inoculated (Table 2).
The AMF spore number was high in the P. reticulata inoculated plants in monoculture as well as in mixed plantations. This pattern, however, was not observed in the E. camaldulensis monoculture inoculated with AMF.
In the rhizospheres of Eucalyptus, sporulation was observed to increase in the rainy season when compared to the dry period (Table 2). During the rainy season, the total spore numbers in treatments 3 and 4 (E. camaldulensis and E. camaldulensis + AMF) were higher than in other treatments, except in the inoculated mixed plantation; these treatments, however, presented the lowest species richness and diversity.
Species richness ranged from three to eight. The highest species richness was found in the mixed plantations. The lowest species richness was found in Eucalyptus monocultures, presenting only three to six species (Table 2). The rooting-soil of P. reticulata showed more AMF species richness than Eucalyptus (Table 2) and AMF diversity was higher in mixed plantations (Table 2).
Discussion
The soil analyses revealed low organic matter content (mean 0.8%) in the experimental area. These levels are comparables to those of foredunes (0.8%) in Brazil (Córdoba et al. 2001). The low soil fertility seems to account for the plants having a high AM dependency which minimizes hydric stress and nutrient deficit as found by Tarafdar and Praveen-Kumar (1996). Lower CEC results from the sandy texture and low soil clay content.
Plathymeniareticulata plants grew better when inoculated with Rhizobium and AMF as reported by Manjunath et al. (1984), Faria et al. (1995), Marques et al. (2001), Gross et al. (2004), and Pagano et al. (2007). It is known that dual inoculation generally increases plant growth to a greater extent than inoculation with only one symbiont (Chalk et al. 2006), and we suggest that P. reticulata be inoculated with Rhizobium and AMF for growth improvement. The lowest growth of P. reticulata uninoculated under mixed plantation is in agreement with other reports (Marques et al. 2001; Tian et al. 2003).
The dominance of hyphae and vesicles in the roots of T. heptaphylla in inoculated treatment suggests a nurse effect of inoculated plant species, like P. reticulata, on the non-inoculated T. heptaphylla. Leguminous plants have a much greater infectivity potential (Tarafdar and Praveen-Kumar 1996). Carneiro et al. (1998) found high root colonization in the same Tabebuia species and in Tabebuia serratifolia Rolfe, which was reported as responsive to P when mycorrhizal, presenting high arbuscular and vesicular colonization (Siqueira and Saggin-Júnior 2001). Similar AM colonization in Tabebuia roseo-alba (Ridl.) Sand. seedlings in greenhouse was showed by Zangaro et al. (2003).
We note that there was a seasonal effect related to mycorrhizal colonization and AM species diversity in the study area.
The increase in the percentage of arbuscules in the rainy season (in the native plants) may be related to the improvement of soluble nutrients released through litter decomposition (Scotti and Correa 2004). Mycorrhizal root colonization was always higher in P. reticulata plants inoculated in the mixed model than in other treatments, and the presence of arbuscules was observed in the three sampled periods.
Eucalyptus camaldulensis showed a lower colonization (hyphae, vesicles and arbuscules) during the rainy season, unlike the colonization of the other two plant species analyzed. However, during the rainy season, ECM increased. This may be explained by the periodical death of ECM when water is limited and the demand by the trees is high, which have to form again when drought ends before being able to resume nutrient uptake (Courty et al. 2006).
This suggests that Eucalyptus could obtain nutrients mainly from litter decomposition through ECM effect during the rainy season. This corroborates results from Santos et al. (2001), who observed an increase in ECM colonization and AMF reduction in E. camaldulensis. However, few arbuscules were found in E. camaldulensis roots as also confirmed by Santos et al. (2001), probably due to the presence of tannin, which interferes with arbuscules and vesicles observation. Eucalyptus usually presents a 40–50% ECM on root apical zones (Marschner and Dell 1994). The decrease of AMF colonization of E. camaldulensis in the rainy season and the continuous increasing of AMF colonization on T. heptaphylla suggests that the native species could help the AMF re-colonization of E. camaldulensis. Eucalyptus plants supply the reduction of ECM in the dry periods, increasing AMF colonization. This indicates the importance of AMF in this semi-arid region. The AM colonization of the Eucalyptus species studied is in accordance with data showed by Santos et al. (2001) and Dhar and Mridha (2006), who found a similar percentage of colonization in E. camaldulensis.
AMF spores were recovered at varying numbers from all treatments investigated, regardless of the inoculation treatment with AMF. The overall AMF spore number in the individual plants ranged from 106 to 214 during the rainy period and 129–183 in the dry season in single plantations reaching up to 310 in mixed and inoculated plantations during the rainy season, while in the dry season 240 spores were found. The number of spores was similar to those previously found. Souza et al. (2003), in the Caatinga region, documented a spore number varying between 34 and 850 spores/100 g soil. Yano-Melo et al. (1997) recorded similar results of 44–271 spores/100 g soil in the São Francisco valley. Albuquerque (2008) identified 29 species of AMF in three areas of Caatinga with different physiognomies (Serra Talhada-Sertão with a hyperxerophytic Caatinga; Caruaru—Agreste with hyperxerophytic Caatinga; Araripina—Chapada with Cerrado). The number of species ranged from 14 to 21, with Acaulospora being the most diversified genus. Also, variations were observed on spore number and richness promoted by seasonal changes. Dry periods favored the sporulation of a great number of species, increasing the populations of AMF. The results suggest that AMF spore number depends on the seasonal effect and on the diversity of vegetal species cover. Also, interspecific differences in the quality of litter produced by individual plant species can influence the mycorrhizal community (Conn and Dighton 2000), increasing its diversity.
In diverse and stable plant ecosystems Gigasporaceae occurs at low spore densities and high species richness (Siqueira et al. 1989; Lovelock et al. 2003; Zhao et al. 2003). In our study, we found three to four species of Gigasporaceae, whereas an increased spore number of Gigaspora in the mixed plantation inoculated with AMF and Rhizobium were found in both studied seasons. This can be attributed to the fact that G. margarita was included in the inoculum and also the preference of P. reticulata for this AMF species.
Within a regional context, AMF species richness at our sites was lower in comparison to natural Caatinga areas. The lowest AMF species richness was found in E. camaldulensis stands followed by P. reticulata. The highest species richness was found in mixed plantations. These values are considerably lower than those reported by Souza et al. (2003), who found 24 taxa in Caatinga, and Yano-Melo et al. (1997, 2003), who recorded similar results of 14–15 AMF species in the São Francisco valley (Brazil).
Glomeraceae was dominant in our field experiments with a single species Glomus brohultii. This predominance is frequently observed under semi-arid and arid habitats (Stutz et al. 2000; Kennedy et al. 2002; Muthukumar and Udaiyan 2002; Panwar and Tarafdar 2006). Glomus has been considered as the best adapted genus for habitats subjected to drought (Haas and Menge 1990). Glomus fasciculatum proved to be the most effective AMF under arid conditions (Tarafdar and Praveen-Kumar 1996). Glomus promoted the growth of Eucalyptus dives and Eucalyptus viminalis (Adjoud et al. 1996). Moreover, Chen et al. (2007) found mostly Glomus species from 155 different eucalypt species plantations.
The literature shows that Glomus and Acaulospora are more competitive in the infection process because they are able to colonize the roots from all inoculum types (spores, colonized root fragments and hyphae), whereas Gigaspora and Scutellospora do it mainly from spores (colonization strategy) and poorly by extraradical hyphae (Klironomos and Hart 2002). This would explain in part the predominance of these two genera (Glomus followed by Acaulospora) observed in this work. On the other hand, these genera may be selected by host tree species effect.
In this study, Eucalyptus monocultures, especially in the rainy period, decreased AMF diversity, enhancing the dominance of Glomus brohultii. Carrenho et al. (2008) stated that Eucalyptus is ecologically damaging to many native plant species and its litter inhibits the annual understory vegetation. Our findings support the hypothesis that ECM develop more intensively in the organic residues of their autotrophic partners (Read 1991) and degrade the recalcitrant litter. The composition and properties of plant litter are essential controlling factors for organic matter formation and soil humification (Kögel-Knabner 2002).
The different colonization patterns of Eucalyptus during both rainy and dry periods may be explained in part by the lesser tolerance of ECM to dry conditions. Soil moisture is an important control of soil microbial activity through osmosis or controlling nutrient supply (Killham 1994). On the other hand, AMF usually increase host growth rates during drought by affecting nutrient acquisition and possibly hydration (Giri et al. 2003); however, colonization by different fungi affects water use efficiency differently (Simpson and Daft 1990). Moreover, according to other authors, the AMF-ECM succession is simply related to spatial competition for infection sites (Chilvers et al. 1987).
In this study, mixed plantations inoculated with rhizobia and AMF promote the growth of P. reticulata, supporting the observation that mycorrhizal plants acquire more nutrients, and are able to share them via an underground network of hyphal connections linking individuals within and between species (Simard et al. 2003). Co-occurring plant species respond differently to each AMF species and plant biomass increases due to their presence (van der Heijden 2003). Moreover, AMF are efficient in improving growth and nitrogen (N) content in legumes (Barea et al. 2002), which can transfer N to eucalypt plants by AMF hyphae interconnection (Rodrigues et al. 2003).
Our findings corroborate the hypothesis that vegetal community influences the community of AMF (van der Heijden et al. 1998) and mixed plantations that increase plant diversity also increase AMF spore number and diversity. On the other hand, mycorrhizal associations are potential factors determining diversity in ecosystems (Read 1990) that warrant ecosystem sustainability (Hart and Klironomos 2003).
Conclusion
The present study constitutes a first evaluation of mycorrhizal distribution for the successful establishment of the native species P. reticulata on cutaway dry forest in Brazil.
It demonstrated that the introduction of P. reticulata and E. camaldulensis inoculated and mixed with T. heptaphylla promotes a higher AMF diversity, percentages of AMF colonization and native plant growth regardless of the season. Stem height was found a better indicator of inoculation response than diameter for P. reticulata plants.
This study also contributed to our understanding of the basis for observed response of different plant species to the same site conditions, showing that AM and ECM colonization of E. camaldulensis varies with seasons.
References
Adjoud D, Plenchette C, Halli-Hargas R et al (1996) Response of 11 Eucalyptus species to inoculation with three arbuscular mycorrhizal fungi. Mycorrhiza 6:129–135. doi:10.1007/s005720050117
Adjoud-Sadadou D, Halli-Hargas R (2000) Occurrence of arbuscular mycorrhiza on aged Eucalyptus. Mycorrhiza 9:287–290. doi:10.1007/PL00009993
Albuquerque PP (2008) Diversidade de Glomeromycetes e atividade microbiana em solos sob vegetação nativa do Semi-Árido de Pernambuco. Doctor’s thesis. Universidade Federal do Pernambuco, Recife
Aristizábal C, Rivera EL, Janos DP (2004) Arbuscular mycorrhizal fungi colonize decomposing leaves of Myrica parvifolia, M. pubescens and Paepalanthus sp. Mycorrhiza 14:4. doi:10.1007/s00572-003-0259-0
Augé RM, Schekel KA, Wample RL (1987) Leaf water and carbohydrate status of VA mycorrhizal rose exposed to drought stress. Plant Soil 99:291–302. doi:10.1007/BF02370876
Barea JM, Toro M, Orozco MO et al (2002) The application of isotopic (32P and 15N) dilution techniques to evaluate the interactive effect of phosphate solubilizing rhizobacteria, mycorrhizal fungi, and Rhizobium to improve agronomic efficiency of rock phosphate for legume crops. Nutr Cycl Agroecosyst 63:35–42. doi:10.1023/A:1020589732436
Binkley D, Dunkin KA, DeBell D et al (1992) Production and nutrient cycling in mixed plantations of Eucalyptus and Albizia in Hawaii. For Sci 38:393–408
Brundrett MC, Bougher NL, Dell B (1996) Working with mycorrhizas in forestry and agriculture. Monograph 32. ACIAR, Canberra
Cardoso IM, Kuyper TW (2006) Mycorrhizas and tropical soil fertility. Agric Ecosyst Environ 116:72–84. doi:10.1016/j.agee.2006.03.011
Cardoso IM, Boddington C, Janssen BH et al (2003) Distribution of mycorrhizal fungal spores in soils under agroforestry and monocultural coffee systems in Brazil. Agrofor Syst 58:33–43. doi:10.1023/A:1025479017393
Carneiro MAC, Siqueira JO, Moreira FMS et al (1998) Micorriza arbuscular em espécies arbóreas e arbustivas nativas de ocorrência no Sudeste do Brasil. Cerne 4:129–145
Carrenho R, Barbosa FF, Araújo CVM, Alves IJ, Santos OM (2008) Mycorrhizal associations in Eucalyptus spp.: status and needs. Tree For Sci Biotechnol 2(1):57–67
Chalk PM, Souza RF, Urquiaga S et al (2006) The role of arbuscular mycorrhiza in legume symbiotic performance. Soil Biol Biochem 38:2944–2951. doi:10.1016/j.soilbio.2006.05.005
Chen YL, Liu S, Dell B (2007) Mycorrhizal status of Eucalyptus plantations in south China and implications for management. Mycorrhiza 17:527–535. doi:10.1007/s00572-007-0125-6
Chilvers GA, Lapeyrie FF, Horan DP (1987) Ectomycorrhizal vs endomycorrhizal fungi within the same root system. New Phytol 107:441–448. doi:10.1111/j.1469-8137.1987.tb00195.x
Codevasf (2004) Distrito de Irrigação de Jaíba, total de precipitação mensal dos últimos dez anos. Writed Report
Conn C, Dighton J (2000) Litter quality influences on decomposition, ecto-mycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–496. doi:10.1016/S0038-0717(99)00178-9
Córdoba AS, Mendonça MM, Stürmer SL et al (2001) Diversity of arbuscular mycorrhizal fungi along a sand dune stabilization gradient: a case study at Praia da Joaquina, Ilha de Santa Catarina, South Brazil. Mycoscience 42:379–387. doi:10.1007/BF02461221
Courty PE, Pouységur R, Buée M et al (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol Biochem 38:1219–1222. doi:10.1016/j.soilbio.2005.10.005
Cuenca G, De Andrade Z, Escalante G (1998) Arbuscular mycorrhizae in the rehabilitation of fragile degraded tropical lands. Biol Fertil Soils 26:107–111. doi:10.1007/s003740050351
DeBell D, Whitesell CD, Schubert TH (1985) Mixed plantation of Eucalyptus and leguminous tree enhance biomass production. Pacific Southwest forest and Range Experiment Station Forest Service. US Department of Agriculture, Res Paper PSW-175. Berkeley, CA
Dhar PP, Mridha MAU (2006) Biodiversity of arbuscular mycorrhizal fungi in different trees of Madhupur forest, Bangladesh. J For Res 17:201–205. doi:10.1007/s11676-006-0047-8
Empresa Brasileira de Pesquisa Agropecuária (1979) Manual de Análises Químicas de solos, plantas e fertilizantes. Embrapa, Brasília
Empresa Brasileira de Pesquisa Agropecuária (1999) Sistema brasileiro de classificação de solos. Centro Nacional de Pesquisa de Solos. Embrapa, Brasília
Faria MP, Vale FR, Siqueira JO et al (1995) Crescimento de leguminosas arbóreas em resposta a fósforo, fungo micorrízico e rizóbio. II. Peltophorum dubium (Spreng.) Taub. R Árv 19:433–446
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 84:679–684
Giri B, Giang PH, Kumari R (2003) Mycorrhizosphere: strategies and functions. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin
Gross E, Cordeiro L, Caetano FH (2004) Nodulação e micorrização em Anadenanthera peregrina var. falcata em solo de cerrado autoclavado e não autoclavado. R Bras Ci Solo 28:95–101
Haas JH, Menge JA (1990) VA mycorrhizal fungi and soil characteristics in avocado (Persea americana Mill.) orchard soil. Plant Soil 127:207–212. doi:10.1007/BF00014427
Hart MM, Klironomos JN (2003) Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299. doi:10.1038/35095041
Kennedy LJ, Tiller RL, Stutz JC (2002) Associations between arbuscular mycorrhizal fungi and Sporobolus wrightii in riparian habitats in arid south-west North America. J Arid Environ 50:459–475. doi:10.1006/jare.2001.0899
Khanna PK (1997) Nutrient cycling under mixed-species tree systems in southeast Asia. Agrofor Syst 38:99–120. doi:10.1023/A:1005952410569
Killham K (1994) Soil ecology. Cambridge University Press, UK, 242 p
Kirk JL, Beaudette LA, Hart M et al (2004) Methods of studying soil microbial diversity. J Microbiol Methods 58:169–188. doi:10.1016/j.mimet.2004.04.006
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184. doi:10.1007/s00572-002-0169-6
Kögel-Knabner I (2002) The macromolecular organic composition of plants and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. doi:10.1016/S0038-0717(01)00158-4
Koske RE, Tessier B (1983) A convenient, permanent slide mounting medium. Mycol Soc Am Newsl 34:59
Lovelock CE, Andersen K, Morton JB (2003) Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment. Oecol 135:268–279
Magurran AE (1988) Ecological diversity and its measurement. Croom Helm, London
Maia LC, Trufem SFB (1990) Fungos micorrízicos vesículo-arbusculares em solos cultivados no Estado de Pernambuco, Brasil. Rev Bras Bot 1:89–95
Manjunath A, Bagyaraj DJ, Gopala Gowda HS (1984) Dual inoculation with VA mycorrhiza and Rhizobium is beneficial to Leucaena. Plant Soil 78:445–448. doi:10.1007/BF02450380
Marques MS, Pagano MC, Scotti MR (2001) Dual inoculation of woody legume (Centrolobium tomentosum) with rhizobia and mycorrhizal fungi in southeastern Brazil. Agrofor Syst 52:107–117. doi:10.1023/A:1010637401475
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Matsumoto LS, Martines AM, Avanzi MA et al (2005) Interactions among functional groups in the cycling of carbon, nitrogen and phosphorus in the rhizosphere of three successional species of tropical woody trees. Appl Soil Ecol 28:57–65. doi:10.1016/j.apsoil.2004.06.008
May BM, Attiwill PM (2003) Nitrogen-fixation by Acacia dealbata and changes in soil properties 5 years after mechanical disturbance or slash-burning following timber harvest. For Ecol Manage 181:339–355
McGonigle TP, Miller MH, Evans DG et al (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Miller RM, Jastrow JD (1992) The role of mycorrhizal fungi in soil conservation. In: Bethlenfalvay GJ, Linderman RG (eds) Mycorrhizae in sustainable agriculture. American Society of Agronomy, Madison, pp 29–44
Morton JB (1988) Taxonomy of VA mycorrhizal fungi: classification, nomenclature and identification. Mycotaxon 32:267–324
Muleta D, Assefa F, Nemomissa S et al (2008) Distribution of arbuscular mycorrhizal fungi spores in soils of smallholder agroforestry and monocultural coffee systems in southwestern Ethiopia. Biol Fertil Soils 44:653–659. doi:10.1007/s00374-007-0261-3
Muthukumar T, Udaiyan K (2002) Seasonality of vesicular arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecol 23:337–347. doi:10.1016/S1146-609X(02)01165-7
Nouaim R, Chaussod R (1996) Rôle des mycorhizes dans l’alimentation hydrique et minérale dês plantes, notamment des ligneux de zones arides. CIHEAM—Options Mediterraneennes
Pagano MC, Cabello MN, Scotti MR (2007) Phosphorus response of three native Brazilian trees to inoculation with four arbuscular mycorrhizal fungi. J Agric Technol 3:231–240
Pagano MC, Cabello MN, Bellote AF et al (2008) Intercropping system of tropical leguminous species and Eucalyptus camaldulensis, inoculated with rhizobia and/or mycorrhizal fungi in semiarid Brazil. Agrofor Syst 74:231–242. doi:10.1007/s10457-008-9177-7
Panwar J, Tarafdar JC (2006) Distribution of three endangered medicinal plant species and their colonization with arbuscular mycorrhizal fungi. J Arid Environ 65:337–350. doi:10.1016/j.jaridenv.2005.07.008
Parrota JA (1999) Productivity, nutrient cycling, and succession in single- and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta, and Leucaena leucocephala in Puerto Rico. For Ecol Manage 124:45–77
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Picone C (2000) Diversity and abundance of arbuscular-mycorrhizal fungus spores in tropical forest and pasture. Biotropica 32:734–750. doi:10.1646/0006-3606(2000)032[0734:DAAOAM]2.0.CO;2
Prado DE (2003) As Caatingas de América do Sul. Seção I. Padrões de diversidade e distribuição das espécies em escala regional. In: Leal IR, Tabarelli M, da Silva JMC (eds) Ecologia e Conservação da Caatinga. Ed. Universitária da UFPE, Recife, pp 1–74
Read DJ (1990) Ecological integration by mycorrhizal fungi. Endocytobiology 4:99–107
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391. doi:10.1007/BF01972080
Rizzini CT (1997) Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. Âmbito Cultural Edições Ltda
Rodrigues MGV, Souto RF, Menegucci JLP (2001) Influência do ensacamento do cacho na produção de frutos da bananeira `Prata-Anã′ irrigada, na região Norte de Minas Gerais. Rev Bras Frutic 23:559–562
Rodrigues LA, Martins MA, Salomão MSMB (2003) Uso de micorrizas e rizóbio em cultivo consorciado de eucalipto e sesbânia. I—crescimento, absorção e transferência de nitrogênio entre plantas. Rev Bras Cienc Do Solo 27:583–591
Santos VL, Muchovej RM, Borges AC et al (2001) Vesicular-arbuscular-ecto-mycorrhiza succession in seedlings of Eucalyptus spp. Braz J Microbiol 32:81–86. doi:10.1590/S1517-83822001000200002
Schenck NC, Pérez Y (1988) Manual for the identification of VA mycorrhizal fungi, INVAM. University of Florida, Gainesville
Scotti MR, Correa EJA (2004) Growth and litter decomposition of woody species inoculated with rhizobia and arbuscular mycorrhizal fungi in Semiarid Brazil. Ann Sci 61:87–95. doi:10.1051/forest:2003088
Silva SO, Pires ET, Pestana RKN et al (2006) Evaluation of cavendish banana clones. Ciênc agrotec 30:832–837
Simard WS, Jones MD, Durall DM (2003) Carbon and nutrient fluxes within and between mycorrhizal plants. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin
Simpson D, Daft MJ (1990) Interaction between water stress and different mycorrhizal inocula on plant growth and mycorrhizal development in maize and sorghum. Plant Soil 121:179–186. doi:10.1007/BF00012310
Siqueira JO, Saggin-Júnior OJ (2001) Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 11:245–255. doi:10.1007/s005720100129
Siqueira JO, Colozzi-Filho A, de Oliveira E (1989) Occurrence of vesicular-arbuscular mycorrhizae in agro- and natural ecosystem of Mina Gerais State. Pesquisa Agropecu Bras 24:1499–1506
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, Inc., London
Somasegaran P, Hoben HJ (1985) Methods in legume-Rhizobium technology. NIFTAL, Hawaii
Souza RG, Maia LC, Sales MF, Trufem SFB (2003) Diversidade e potencial de infectividade de fungos micorrízicos arbusculares em área de caatinga, na Região de Xingo, Estado de Alagoas, Brasil. Rev Brasil Bot 26:49–60
Stutz JC, Copeman R, Martin CA, Morton JB (2000) Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can J Bot 78:237–245. doi:10.1139/cjb-78-2-237
Subramanian KS, Charest C, Dwyer LM et al (1997) Effects of arbuscular mycorrhizae on leaf water potential, sugar content, and P content during drought and recovery of maize. Can J Bot 75:1582–1591. doi:10.1139/b97-870
Tarafdar JC, Praveen-Kumar (1996) The role of vesicular arbuscular mycorrhizal fungi on crop, tree and grasses in an arid environment. J Arid Environ 34:197–203. doi:10.1006/jare.1996.0101
Tian C, He X, Zhong Y et al (2003) Effect of inoculation with ecto- and arbuscular mycorrhizae and Rhizobium on the growth and nitrogen fixation by black locust, Robinia pseudoacacia. New For 25:125–131. doi:10.1023/A:1022675915627
van der Heijden MGA (2003) Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin
van der Heijden MGA, Boller T, Wiemkem A et al (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
Varma A (1995) Arbuscular mycorrhiza fungi: the state of the art. Crit Rev Biotechnol 15:179–199. doi:10.3109/07388559509147407
Walker C, Mize W, McNabb HS (1982) Populations of endogonaceous fungi at two locations in central Iowa. Can J Bot 60:2518–2529. doi:10.1139/b82-305
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal mycorrhizal fungi. Plant Soil 198:97–107. doi:10.1023/A:1004347701584
Yano-Melo AM, Maia LC, Morgado LB (1997) Fungos micorrízicos arbusculares em bananeiras cultivadas no vale do submédio São Francisco. Acta Bot Bras 11:115–121
Yano-Melo AM, Trufem SFB, Maia LC (2003) Arbuscular mycorrhizal fungi in salinized and surrounded areas at the São Francisco Submedium Valley, Brazil. Hoehnea 30:79–87
Zambolim L, Barros NF (1982) Constatação de micorriza vesicular-arbuscular em Eucalyptus spp. na região de Viçosa, MG. Rev Arvore 6:95–97
Zangaro W, Nisizaki SMA, Domingos JCB et al (2003) Mycorrhizal response and successional status in 80 woody species from south Brazil. J Trop Ecol 19:315–324. doi:10.1017/S0266467403003341
Zhao ZW, Wang GH, Yang L (2003) Biodiversity of arbuscular mycorrhizal fungi in a tropical rainforest of Xishuangbanna, southwest China. Fungal Divers 13:233–242
Acknowledgments
This research was supported by the Ministry of Environment: National Found of Environment (FNMA). The authors are grateful to the Council for the Development of Higher Education at Graduate Level, Brazil (CAPES) for a scholarship granted to Marcela C. Pagano. Marta N. Cabello is a researcher from Comisión de Investigaciones Científicas (CIC), Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagano, M.C., Scotti, M.R. & Cabello, M.N. Effect of the inoculation and distribution of mycorrhizae in Plathymenia reticulata Benth under monoculture and mixed plantation in Brazil. New Forests 38, 197–214 (2009). https://doi.org/10.1007/s11056-009-9140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-009-9140-0