Abstract
The sensitive and selective detection of mercury in aqueous solution is of paramount importance as the mercury concentration in drinking water above the threshold level set by world health organization can cause serious health issues to humans. We demonstrate a simple, facile, and cost-effective one pot synthesis route to synthesize MPA (3-mercaptopropionic acid) stabilized CdTe/CdS core-shell quantum dots and its application for detection of mercury using the fluorescence resonance energy transfer with a cationic dye, Rhodamine 6G. The quantum dots prepared via chemical reduction strategy using a combination of reducing agents, namely sodium borohydride and citric acid exhibit a high quantum efficiency (> 20% for solid state). Structural as well as luminescence studies of the prepared quantum dots were found to depend on the pH as well as the size of the quantum dots (hydrodynamic diameter ranging from 9 to 16 nm). Analysis of the fluorescence resonance energy transfer (FRET) between the prepared quantum dots and Rhodamine 6G elucidate that efficient energy transfer happens in the presence of a cetyl trimethyl ammonium bromide (CTAB) surfactant. Though the prepared CdTe/CdS quantum dots exhibit fluorescence quenching with an increase in mercury concentration and act as an “OFF-sensor,” the Rhodamine 6G-quantum dot pair employed here found to be a better approach as the inherent fluorescence of Rh6G is insensitive to mercury concentration. Our studies elucidate that the fluorescence ratio of Rh6G in a FRET pair follows a linear nature for the Stern-Volmer plot in the concentration range of Hg2+ ions (0.1 nM to 2 μM) and provide a LOD of 3.8 nM.

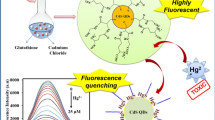
Schematic representation showing one-pot synthesis of CdTeCdS QDs for FRET based sensing of mercury ions in water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantum dots (QDs) based molecular probes have been popular in the design and development of biosensor (Ullah et al. 2018; Wang et al. 2018). Increased usage of QDs stems from their unique optoelectronic properties including size-dependent bandgap tunability, long fluorescence lifetimes, exceptional photostability, high quantum yield, and large surface area for molecular interactions (Frasco and Chaniotakis 2009; Resch-Genger et al. 2008). Amongst different kinds of QDs being used, cadmium-based semiconductor materials such as CdTe (Yao and Gou 2016; Zhang et al. 2012; Zhou et al. 2011), CdSe (Chu et al. 2012; Ulusoy et al. 2015), and CdS (Akshya et al. 2015; Li et al. 2007) are more popular, as their properties can be easily manipulated for the design and development of facile, sensitive, robust, and fast-response sensor probes (Vázquez-González and Carrillo-Carrion 2014). Furthermore, surface tailoring with biocompatible materials finds major application in pathogen detection (Ag et al. 2014), bioimaging (Ulusoy et al. 2015), and cell targeting (Zrazhevskiy and Gao 2013). In recent years, CdTe-based QDs have been successfully used as a fluorosensor probe for the detection of heavy metal ions (Ullah et al. 2018) such as mercury (Hg2+) (Kumar et al. 2017; Paim et al. 2017; Saikia et al. 2016; Zhu et al. 2017), copper (Cu2+) (Gong et al. 2016), silver (Ag+) (Chen et al. 2014), arsenic (As3+) (Tang et al. 2015), and toxic molecules like melamine (Tang et al. 2013) and trinitrotoluene (Chen et al. 2010).
The efficient interaction between QDs and the analyte molecule in an aqueous medium is a prerequisite for developing a sensor with high sensitivity and selectivity. Synthesis of water dispersible QDs with high quantum yield and surface passivation is an area of intense research. In general, synthesis of QDs by colloidal chemistry is simple and involves reduction of metal salts leading to nucleation followed by growth in nanoscale (Yao and Gou 2016; Zhou et al. 2011), CdSe (Chu et al. 2012; Ulusoy et al. 2015), and CdS (Akshya et al. 2015; Li et al. 2007). Several reported approaches to prepare water dispersible QDs (de Mello Donegá 2011; Jing et al. 2016) suffer from disadvantages such as wide size distribution, low quantum yield, broad emission bands and photobleaching. Owing to these, the sensitivity for analyte detection gets affected while QDs are being employed for sensing applications. Despite significant development in high quantum yield QD synthesis in a non-polar environment, most of these methods lack facile approach and need an additional energy drive for generating the nucleus and subsequent growth of QDs. For example, synthesis in non-polar solvent requires high boiling point solvent operated at high temperature using autoclaves (Chu et al. 2012) and even many of the aqueous organometallic routes utilize water-cooled condensers (Paim et al. 2017; Ulusoy et al. 2015), N2 protection (Ag et al. 2014; Gaponik et al. 2002), and high vacuum (Murray et al. 1993) to avoid the oxidation. Considering the commercial scale of synthesis and applications, establishing facile and cost-effective strategy is of paramount importance.
Of late, manipulation of surface properties by growing wide bandgap semiconductor shell materials such as CdS over CdTe core obtains increasing importance. The formation of such shell not only enhances the photostability but also reduces the frequency of non-radiative events by passivating the dangling bonds of trapped states (Bao et al. 2004) that prevent electron-hole delocalization towards outer surface (Ulusoy et al. 2015). Thus, core-shell QD offers added advantages such as enhancement in quantum yield, high photostability, and strong excitonic confinement dependent tailoring of optical properties. Similarly, chelating organic ligands anchored onto the QD plays a significant role in sensing applications. Thiols such as 3-mercaptopropionic acid (MPA), thioglycolic acid (TGA), glutathione, and l-cysteine (Chen et al. 2010; Li et al. 2008; Vázquez-González and Carrillo-Carrion 2014; Zhou et al. 2011) have been commonly used to decorate QD surface for various biomedical applications. The usage of short-chain length thiol ligands enable QD surface stabilization and provide better dispersibility in a polar solvent like water. In this case, MPA having thiol group link to QD surface via metal sulfide linkage and the free carboxylate group becomes available for interaction with water molecules.
Broad tunable absorption band of QDs allows its potential use as an energy donor for fluorescence resonance energy transfer (FRET)-based applications in biosensing, bioimaging, and bioassays (Clapp et al. 2004; Medintz et al. 2003; Shamirian et al. 2015). A typical FRET system operates by energy transfer that occurs when a donor dipole interacts with an acceptor dipole in proximity, ranging between 1 and 10 nm (Basheer et al. 2013; John et al. 2015; Kurian et al. 2005). Forster’s theory explains the efficiency of FRET depends on the extent of spectral overlap of donor emission spectrum with an absorption spectrum of an acceptor, quantum yield of the donor, and relative orientation of respective dipoles in solution. Several reports describing FRET-based sensing of small molecules, biomolecules, and metal ions using metal nanoparticles as energy donors such as gold nanoparticles (John et al. 2015; Rodzik-Czałka et al. 2017), silver nanoparticles (Tang et al. 2018), and CdTe (Li et al. 2008; Tang et al. 2013; Tang et al. 2015; Wang et al. 2018) have been reviewed elsewhere (Chen et al. 2013). However, to date, there are no reports available on FRET between CdTe/CdS QDs and a cationic dye, Rh6G, as a donor-acceptor pair that are used for the detection of Hg2+ ions. Therefore, we report, the mechanism of energy transfer from CdTe/CdS QDs to Rh6G mediated by cetyltrimethylammonium bromide (CTAB) in aqueous media.
Mercury ion is one of the major environmental toxicants that pose a health risk due to its serious role in drinking water contamination. Mercury binds to thiol groups of enzymes and proteins causes serious problems associated with the brain, kidney, and central nervous system (Saikia et al. 2016). According to Environmental Protection Agency (EPA), the recommended the tolerance limit for mercury in drinking water is 10 nM (Duan et al. 2009; Vázquez-González and Carrillo-Carrion 2014). Therefore, it is important to detect aqueous Hg2+ ions above recommended tolerant concentration. Herein, we report a simple and cost-effective one pot experimental strategy to synthesize water dispersible and photostable core-shell QDs (CdTe/CdS QDs stabilized with MPA and citric acid) with high quantum yield (> 20%) for sensing applications (Scheme 1). Further, we demonstrate the sensitive detection of Hg2+ ions using FRET between the prepared core-shell CdTe/CdS QDs and a well-known cationic dye molecule, Rhodamine 6G (Rh6G). Our studies elucidate a linear nature of Stern-Volmer plot for the concentration range of Hg2+ ions (0.1 nM to 2 μM) with a LOD of (3.8 nM). The choice of FRET pair stems from the fact that direct interaction of Hg2+ ions does not alter the emission properties of Rh6G and tailor the emission properties of QDs only. Such a selective interaction of Hg2+ ions towards QDs and not to Rh6G provides a sensitive and accurate route for the measurement of Hg2+ ions via FRET mechanism. Aside from the sensitive detection of Hg2+ ions, the FRET pair found to be insensitive to other divalent impurities.
Experimental
Chemicals
CdCl2, citric acid anhydrous (99.5%), CTAB (Cetyl trimethyl ammonium bromide) and NaOH were purchased from Merck and were of AR grade. MPA (3-mercaptopropionic acid, ≥ 99%), K2TeO3 (Potassium tellurite hydrate, ≥ 95%), Rh6G and NaBH4 (Sodium borohydride, AAS ≥ 99%) were purchased from Sigma-Sigma, MO, USA.
Preparation of CdTe/CdS QDs
High quantum yield aqueous core-shell CdTe/CdS QDs were synthesized by a simple one-pot chemical reduction method using the mixture of sodium borohydride and citric acid as reducing agents. Briefly, CdCl2 (10 mM), MPA (20 mM), K2TeO3 (2 mM), NaBH4 (32 mM), and citric acid (52 mM) in water were serially added in the molar ratio of 1:2:0.2:3.2:5.2 respectively, into a glass beaker. The colloidal solution was mixed thoroughly and sonicated for 5 min. For tuning the QD size, pH of samples was varied from 9.5 to 12.5 by adding sodium hydroxide (1 M). The final clear dark brown colloidal solution was heated and incubated overnight at 80 °C in a furnace. Highly fluorescent CdTe/CdS QDs powder sample was collected and stored at 4 °C. Later, the required amounts of QDs were dispersed in water for characterization and optimization study involving tuning the QD size, fluorescence emission, aqueous stability, and quantum yield that were performed. Bandgap was calculated using the well-known effective mass model equation (Brus 1986; Mathew et al. 2012).
where E is the bandgap of core-shell QDs, h is Planks constant, εr is the dielectric constant of the semiconductor, ε0 = 8.854 × 10−12C2N−1m−2 is the permittivity of free space, e the charge of an electron, \( {m}_{\mathrm{e}}^{\ast }={m}_{\mathrm{e}}{m}_{\mathrm{o}} \), and \( {m}_{\mathrm{h}}^{\ast }={m}_{\mathrm{h}}{m}_{\mathrm{o}} \). Here me (0.11) and mh (0.63) are the effective masses of electron and hole respectively for CdTe (Geyer et al. 2010), and m0 is the mass of an electron. Using dot radius (r) of CdTe/CdS QDs (synthesis pH 9.5) measured from HR-TEM image, bandgap energy E was calculated. Substituting the value for diameter (D) of the CdTe/CdS QDs in Eq. (2), molar extinction coefficient (ϵ) and concentration of CdTe/CdS were calculated (Yu et al. 2003).
Instrumentation and characterization of CdTe/CdS QDs
Photoluminescence of CdTe/CdS QD powders was observed through the naked eye after illuminating with a diode laser (405 nm, 5 mW) onto the powder samples prepared at pH 9.5, 10.5, 11.5, and 12.5. The corresponding fluorescent colors observed were green, yellow, orange, and pink, respectively. The powder X-ray diffraction patterns with CuKα radiation (λ = 0.154 nm) of CdTe and CdTe/CdS nanocrystals were investigated using a Rigaku Ultima-IV Powder X-Ray Diffractometer. Bragg’s equation was used to evaluate the lattice spacing of (111) of nanocrystals which is represented as d = nλ/(2 sin θB), where λ is the X-ray wavelength (CuKα = 0.154 nm), n is an integer, and θB is the half scattering angle of the (111) diffraction peak on the 2θ scale.
High-resolution transmission electron microscopy (HR-TEM) measurements and the corresponding selected area diffraction (SAED) patterns were investigated using Field Emission Gun-Transmission Electron Microscope 300 kV (Make, FEI; model, Tecnai G2, F30). HR-TEM images were analyzed for determining the dot size using ImageJ software. Fourier transform infrared spectroscopy (FT-IR) was performed using a Jasco FT/IR6200 spectrometer. Particle size and zeta potential measurements (QD concentration 1 mg/ml) were performed using nanoparticle Analyzer (HORIBA Scientific-The SZ-100 nanopartica series instruments, Japan). Absorption spectra were taken using a UV-visible spectrophotometer (Jasco V-650). The fluorescence emission spectra were recorded using laser-induced fluorescence (LIF) system. The LIF system consists of a He-Cd laser (5 mW, Model-IK5351R and KR1801C), a Czerny-Turner spectrograph (Model-ARC-SP150i, Acton, USA) with CCD (Model-DU920N-BU, Andor, Ireland), and a fiber-optic probe for fluorescence collection. The quantum yield of the powder samples was analyzed using a Jasco FP8300 Photoluminescence spectrometer equipped with a 450-W xenon lamp and a high-speed chopper. For quantum yield calculations, a measuring system was equipped with ILF-835 100mmF Integrating sphere unit, 1 mm path length solution cell, and FWQE-880 program.
FRET and detection of Hg2+ ions in aqueous media
FRET system was constructed by mixing the water dispersible CdTe/CdS QDs synthesized at pH 9.5 (2 μM), with varying concentrations of Rh6G (180–900 nM) in the presence of a cationic surfactant CTAB (50 μM). For fluorescence measurement, all samples were excited at 325 nm. Limit of detection (LOD) was determined using the slope from the Stern-Volmer linear plot of F0/F that corresponds to FRET or QD system versus Hg2+ concentration (0.1 nM to 2 μM). The concentration corresponds to F0/(F0-3SD) is the LOD of the system (Martin and Narayanaswamy 1997), where F0 and F are the normalized fluorescence intensities of either QDs or Rh6G emission peaks in the absence and presence of Hg2+ ions respectively, SD is the standard deviation (n = 3) of the blank sample without the analyte. Further, a control experiment involving the effect of Hg2+ on CdTe/CdS QDs (pH 9.5, 2 μM) in the presence of CTAB was independently conducted and analyzed. Additionally, interference of various cations influencing the detection of Hg2+ was studied. In brief, the fluorescence intensity of QD sample in the presence of Na+ (500 nM), Cu+ (200 nM), Ca2+ (500 nM), and Ag+, Co2+, Cu2+, Zn2+, Fe2+, Fe3+, and Cr3+ ions (100 nM each) independently as well as in a mixture against Hg2+ ions (100 nM and 500 nM) was determined quantitatively.
Results and discussion
Optimization of the synthesis of CdTe/CdS QDs
MPA-stabilized core-shell CdTe/CdS QDs were prepared in one-pot chemical reduction method. Chemical reagents were added serially in the order from Cd2+/water/MPA/Te\( {\mathrm{O}}_3^{2-} \)/NaBH4/citric acid in a glass beaker and heated at 80 °C in a hot furnace and incubated overnight. The reaction conditions were optimized by varying the concentration, molar ratio of metal ions and stabilizer, pH, and temperature. After 2 h, fluorescence emission was started to appear which may be due to the formation of CdTe QDs as reported earlier (Zhou et al. 2011). Overnight incubation in the presence of an excess of cadmium ions formed the Cd-S-MPA/citrate shell over CdTe seed nanoparticles. During the synthesis, MPA was added prior to tellurium ion to prevent the hydrolysis of K2TeO3; otherwise, it would lead to the formation of a precipitate of CdTeO3 and Cd (OH)2. As reported previously, under alkaline pH environment, the addition of sodium borohydride reduces the tellurium ion (Te4+ to Te2−) followed by complexing with cadmium ion results in CdTe QDs (Mathew et al. 2012; Yao and Gou 2016). Combination of two reducing agents has also shown to influence the particle size as well as distribution of nanoparticles in colloidal solutions (Piella et al. 2016). Therefore, for the first time, the present study reports the use of sodium borohydride and citric acid in combination for preparation of high-quality QDs in one pot. Further, citric acid is commonly used as capping agent during nanoparticle synthesis. Heating the reactants at 80 °C provides energy for the growth of QDs. Though the chemical reduction yields CdTe QDs, the unreacted cadmium ions react with thiol groups of MPA forming Cd-S-MPA shell layer over CdTe core and thus enhance photostability and quantum yield of QDs.
Characterization of CdTe/CdS QDs
Figure 1a showing the HR-TEM image of homogenously distributed core-shell CdTe/CdS QDs prepared at pH 9.5. The average particle size of spherical QDs was found to be 3.8 ± 0.5 nm (Fig. S1a). SAED analysis and a corresponding fast Fourier transformation (FFT) profile analysis of CdTe/CdS nanocrystals (Fig. 1b, c) indicated the crystalline nature of CdTe/CdS with well-resolved (111), (220), and (311) crystal planes. The lattice spacing of (111) corresponds to zinc blend structure of CdTe/CdS nanocrystals was evaluated to be 0.34 nm which appeared in between the standard inter planar distances of cubic CdTe (0.37 nm) and CdS (0.34 nm) (Bao et al. 2004; Ulusoy et al. 2015; Zhang et al. 2012). It was also verified using X-ray diffraction analysis where the d spacing values for the corresponding (111), (220), and (311) of CdTe/CdS and CdTe QDs were estimated and compared (Fig. 1d). Using the Bragg’s formula, d spacing values that correspond to (111) of CdTe/CdS nanocrystals prepared at pH 9.5, 10.5, 11.5, and 12.5 were determined to be 0.36, 0.36, 0.36, and 0.34 nm respectively. This shift in lattice spacing of XRD peaks with respect to CdTe may be due to high lattice strain induced by the epitaxial growth of CdS shell over CdTe core (Bao et al. 2004; Ulusoy et al. 2015). SAED pattern, FFT profile, and XRD data infer that the QD nanocrystals mostly adopt the structural details of CdS shell when the thickness of the shell is large enough to compress the CdTe core (Smith et al. 2009). Due to close lattice matching of CdTe and CdS, a boundary between CdTe core and CdS shell could not be distinguished. However, the lattice stretches across the crystal (Fig. 1a, inset image), suggesting the epitaxial growth of CdS shell over CdTe core without crystalline defects.
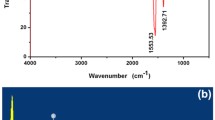
Structural characterization of CdTe/CdS QDs. a HR-TEM image with low and high magnifications (inset image, lattice fringe with scale bar 5 nm). b SAED pattern. c A corresponding fast Fourier transformation (FFT) profile of CdTe/CdS nanocrystals prepared at pH 9.5. d XRD spectra of CdTe/CdS QDs (pH 9.5–12.5). e FT-IR spectra of MPA and CdTe/CdS QDs (pH 9.5 and 11.5)
Table 1 shows the surface charge and particle size analysis of CdTe/CdS QDs determined by zeta potential and dynamic light scattering (DLS) measurements (Fig. S1b–e), respectively. Zeta potential values for CdTe/CdS QDs infer the highly negative surface charge of QDs which may be due to free carboxylate groups of MPA and citric acid present on the surface of QDs. Particle size analyses of CdTe/CdS QDs prepared at pH 9.5, 10.5, 11.5, and 12.5 were found to be 9 ± 0.5, 14 ± 0.5, 16 ± 0.8, and 19 ± 1 nm, respectively, with broad distribution indicated by polydispersity index. The average particle size determined by DLS is larger compared to HR-TEM. This is due to the formation of a hydrodynamic layer containing solvation layers and also influenced by physicochemical factors such as viscosity and concentration of the solution. On the other hand, HR-TEM shows particle size of nanocrystals alone (Choi et al. 2013).
FT-IR spectra in Fig. 1e of MPA, CdTe, and CdTe/CdS samples further corroborated the presence of free carboxylate groups on the surface of QDs. A broadband around 3400 cm−1 associated with free hydroxyl groups of citrate that may be adsorbed on the surface of QDs at 80 °C via hydroxyl group (De Sousa et al. 2013; Huang and Huang 2015), leaving one or free carboxylate groups on the surface. MPA molecules show weak S-H vibration peaks at 810 and 2570 cm−1 (Ag et al. 2014). These peaks disappeared in the FT-IR spectrum of CdTe and CdTe/CdS QDs inferring the oxidation of MPA followed by Cd-S bond formation. There is a significant shift in C=O asymmetric vibration peak corresponds to 1700 cm−1 (COOH group in citrate or MPA) to 1580 cm−1 (COO− group of citrate or MPA-stabilized QDs) implying deprotonation of COOH group in citrate or MPA stabilized QDs indicated as the symmetric vibration of COO− ion at 1400 cm−1 (Ag et al. 2014; Vale et al. 2016). Similarly, peaks 1074 and 1270 cm−1 were attributed to C-O stretching may be due to citrate adsorption on the surface of QDs (Huang and Huang 2015).
The critical factor in tuning the size of core-shell QDs was pH of the reactant solution. Addition of NaBH4 plus citric acid decreases the pH of the colloidal solution and causes black precipitation. Immediately, increasing the pH of the solution by adding the sodium hydroxide (1 M) dropwise with stirring forms a clear brown solution and facilitates the growth of QDs. With an increase in pH from 9.5 to 12.5, absorption and fluorescence emission spectra of QDs shifted towards higher wavelength region as shown in Fig. 2a, b, suggesting that pH of the reaction may influence the growth of QDs. Addition of citric acid has advantages such as it acts as a reducing agent and presence of tri-carboxylate groups may stabilize the QD surface by electrostatic interaction or adsorption; thereby, it prevents the particle agglomeration (Chu et al. 2012). Additionally, it has been observed that addition of citric acid narrowed the fluorescence emission, and thereby, it promoted monodispersed distribution of QDs in aqueous solution.
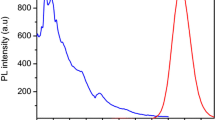
Optical characterization of CdTe/CdS QDs prepared at pH 9.5–12.5. a Absorption spectra. b Fluorescence emission spectra. c Normalized fluorescence spectra showing the FRET with varying concentrations of CdTe/CdS QDs (0.5–4 μM), Rh6G (180 nM), and CTAB (50 μM). d FRET with varying concentrations of Rh6G (180–720 nM), CdTe/CdS (2 μM), and CTAB (50 μM)
Optical characterization of QDs represented as absorption spectra (Fig. 2a) illustrates the pH-dependent shift in absorption edges. Absorption edge indicated the change in bandgap energy with an increase in the QD size (Aboulaich et al. 2012; de Mello 2011; Jing et al. 2016). Using the particle size value from HR-TEM and effective mass equation bandgap energy of CdTe/CdS QDs prepared at pH 9.5 was calculated, and it was found to be 2.8 eV. In contrast, bulk CdTe has bandgap energy of 1.54 eV (Kumar and Devadason 2013) suggesting that by tuning the particle size, the bandgap is altered which has a significant role in enhancing the oscillatory strength of the material. Further, Bohr exciton radius for CdTe QDs (7.3 nm) (Rajh et al. 1993) is higher than the particle size of CdTe/CdS QDs suggesting the strong quantum confinement effect. The growth of CdS shell passivates the core surface which may lead to enhancement in photoluminescence and quantum yield. In addition to passivation, shell material electrically insulates the core, whereas S2− group of CdS prevents the photo-oxidative degradation and surface defect formation (Smith et al. 2009). Elevating the pH of the solution during synthesis was found to alter the band edge of the QDs. As the QDs approach the bulk energy bandgap values, particle size distribution becomes wide which is evident from the increase in FWHM values of emission spectra suggesting that pH has a greater influence on particle size distribution. In this study, lowest FWHM value achieved for CdTe/CdS prepared at pH 9.5 (Fig. 2b) was 45 nm, suggesting a narrow size distribution (Duan et al. 2009; Vale et al. 2016). The absolute quantum yield in the solid state for CdTe/CdS QDs prepared at pH 9.5, 10.5, 11.5, and pH 12.5 was found to be 20 ± 0.5, 34 ± 1.5, 40 ± 2, and 54 ± 3% respectively, which is quite high for aqueous-based QD synthesis compared to previously reported values via absolute method (de Souza et al. 2016; Emin et al. 2010). The absolute quantum yield in the solid state has high significance in terms of future applications. The observed high quantum yield for the samples prepared implies that the synthesis route adopted here would yield high-quality aqueous semiconductor QDs that may find large scope for LED and biomedical applications. Similarly, stokes shift for QDs was found to be greater than 50 nm and it increased with the redshift in emission for different sized QDs, suggesting its potential applicability in the field of bioimaging. Stability of aqueous QDs in solution was determined as a function of its fluorescence emission after 6 months of storage at room temperature and 4 °C. QDs were mostly stable at 4 °C, whereas samples from room temperature showed shift in fluorescence emission (Fig. S2a). Nevertheless, the dry QD powders were highly stable even at room temperature and could be used by simply dispersing in water without sonication.
FRET: energy transfer between CdTe/CdS QDs and Rh6G
CdTe/CdS QDs prepared at pH 9.5 was used for FRET with Rh6G, as small-sized QDs have more surface electrons and therefore more co-ordination and reactivity with Hg2+ ions (Zhu et al. 2017). Further, CdTe/CdS QDs (pH 9.5) showed sharp fluorescence peak at 525 nm, which has maximum spectral overlap with an absorption spectrum of Rh6G. For FRET measurements, these CdTe/CdS QDs and Rh6G were mixed in water. However, simply mixing the two solutions do not induce FRET. Due to the weak electrostatic interaction between CdTe/CdS QDs and Rh6G in an aqueous medium, energy transfer is hindered. Addition of cationic surfactant (CTAB) reduces the surface tension of the solution (Li et al. 2008) and brings two species in close proximity and therefore favors the energy transfer from CdTe/CdS to Rh6G (Fig. 2c, d). The concentration of CTAB (50 μM) employed here was far below critical micelle concentration (Singh et al. 1980) and did not result in precipitation of QDs, as reported previously (Tang et al. 2013). Further, it was sufficient to form a bridge between donor and acceptor molecules. Nevertheless, fluorescence quenching due to FRET was clearly distinguished as two shoulder peaks compared to QD peak alone. Varying the concentration of one of the FRET pairs keeping the other constant altered the FRET efficiency, which further validates the FRET process as shown in Fig. 2c, d. In our studies for Hg2+ ion detection via FRET mechanism, we have chosen a minimum concentration of Rh6G (180 nM) and mixed with 2 μM of CdTe/CdS so that the emission peaks of both fluorophores are clearly visible. In addition, utilization of ultra-low concentration of Rh6G precludes the possibility of dye aggregation induced by quenching the fluorescence. It is worthwhile to mention here that Hg2+ has no influence on the emission properties of Rh6G, as shown in Fig. S2b.
Energy transfer efficiency (E) determines the effectiveness of the FRET system. The spectral overlap of the fluorescence emission spectrum of CdTe/CdS QDs (pH 9.5) and the absorption spectrum of Rh6G are shown in Fig. S2c. Energy transfer between CdTe/CdS QDs and Rh6G was quantified by calculating the area under the curve of donor emission spectra in the presence and absence of acceptor. E is expressed as (Clapp et al. 2004; Medintz et al. 2003; Tang et al. 2015)
where A and A0 are the area under the curve of donor emission spectra in the presence and absence of the acceptor respectively. In this study, E could reach > 50% according to Eq. (3).
The distance between the donor and acceptor (r) is the critical factor for energy transfer process. The distance r can be calculated using the following equation (Clapp et al. 2004; Li et al. 2008; Shamirian et al. 2015; Tang et al. 2015),
where R0 is the critical distance for 50% energy transfer efficiency and is expressed as
where K2 (2/3) and n (1.33) refer to the spatial orientation factor and the refraction index respectively. The quantum yield ΦD of CdTe/CdS QDs at pH 9.5 was measured to be 20%. The spectral overlap integral J (λ) is calculated from the following equation:
where ID is the fluorescence intensity of the donor and ∈A is the molar extinction coefficient of the acceptor. The J (λ) is calculated by integrating the spectra for 450–620 nm, and it was calculated to be 4.87 × 10−14 cm3 L mol−1. From Eq. 5, R0 was calculated to be 4.6 nm. Consequently, fitting the data into Eq. 4, the r value was determined which was found to be 4.8 nm which indicates the high probability of FRET from CdTe/CdS to Rh6G.
Detection of Hg2+ ions
Though the efficacy of CdTe-based QDs as a fluoro-probe for Hg2+ ion concentrations has already been reported (Gong et al. 2016; Paim et al. 2017; Saikia et al. 2016; Vázquez-González and Carrillo-Carrion 2014; Yao and Gou 2016; Zhu et al. 2017), FRET-based Hg2+ ion detection that exploits energy transfer between QD and a dye molecules is less explored. As shown in Fig. S2b, the intrinsic fluorescence of Rh6G dye molecules is unaffected by the addition of Hg2+ ions. However, as shown in Fig. 3a, the fluorescence of QDs as well as Rh6G in FRET pair is decreased with increase in the concentration of Hg2+ ions. In comparison to the fluorescence quenching of QDs in the presence of Hg2+ ions, herein, the fluorescence quenching of Hg2+ ions in FRET pair depends upon less efficient energy transfer arising from the interaction of QDs with Hg2+ ions. The fluorescence intensity ratio of Rh6G emission in a FRET pair as a function of Hg2+ ion concentration (Stern-Volmer plot) follows a linear behavior, as shown in Fig. 3b. The Stern-Volmer equation for such a mixture can be written as (Ding et al. 2015; Duan et al. 2009; Martin and Narayanaswamy 1997; Paim et al. 2017; Saikia et al. 2016)
FRET-based detection of Hg2+ ions in water. a Fluorescence quenching of FRET system as a function of Rh6G fluorescence intensity in the presence or absence of varying concentrations of Hg2+ ions. b Stern-Volmer plot showing F0/F corresponds to fluorescence quenching intensity of Rh6G versus concentration of Hg2+ ions. c Fluorescence quenching of CdTe/CdS QDs in the presence or absence of varying concentrations of Hg2+ ions. d Stern-Volmer plot showing F0/F corresponds to fluorescence quenching intensity of CdTe/CdS QDs versus concentration of Hg2+ ions
where F0 and F are relative fluorescence intensities of Rh6G in the FRET samples in the absence and presence of the analyte (Hg2+) respectively. We have carried out a similar measurement for CdTe/CdS QDs (pH 9.5) alone in the presence of varying concentrations of Hg2+ ions, and the corresponding emission spectrum is shown in Fig. 3c. The Stern-Volmer plot corresponding to QDs-Hg2+ ions is shown in Fig. 3d. Similar to the case of Rh6G, here also the Stern-Volmer plot follows a linear trend with a regression coefficient value of 0.98 as compared to 0.99 for Rh6G. The Ksv and Stern-Volmer constant, estimated from the slope of the linear curve, are found to be 1.56 × 106 M−1 and 1.54 × 106 M−1, respectively for Rh6G of FRET system and CdTe/CdS QDs. While the regression coefficient and Stern-Volmer constant are of the same order, the fluorescence of QDs alone can be quenched due to many reasons that include surface modification, agglomeration, inner filter effects, electron transfer process, and non-radiative recombination pathways in the presence of heavy metals (Frasco and Chaniotakis 2009; Resch-Genger et al. 2008; Vázquez-González and Carrillo-Carrion 2014). On the other hand, the dependence of Rh6G florescence quenching only on the FRET process makes them as a more reliable candidate for the sensor fabrication. Moreover, the LOD concentration of Hg2+ corresponds to F0/(F0-3SD) is found to be 3.8 nM and 11 nM for Rh6G of FRET system and CdTe/CdS QDs respectively, suggesting that detection of as low as 1 ppb (parts per billion) concentration of Hg2+ could be achieved with the FRET pair used here.
The fluorescence quenching mechanism in the presence of analyte has been attributed to mainly two mechanisms. Firstly, Hg2+ ions in association with CTAB binds to QDs creating a surface trap that causes non-radiative energy transition due to electron transfer and hence the quenching of QDs fluorescence (Gong et al. 2016; Saikia et al. 2016). In contrast, the FRET system showed not only decrease in QD fluorescence but also decreased in FRET efficiency with increasing Hg2+ concentrations (Fig. 3a). It was pertinent to note that neither the CTAB nor the Hg2+ ions affected the Rh6G fluorescence emission. Therefore, in the presence of Hg2+ ions, decrease in fluorescence intensity of Rh6G in a FRET system is may be due to the influence of QD quenching by charge transfer to Hg2+ ions as described earlier (Gong et al. 2016; Saikia et al. 2016), which in turn reduces the FRET efficiency. Secondly, the presence of cations as in this case Hg2+ might influence the aggregation of negatively charged QDs. Hg2+ ion substitution for Cd2+ ion leads to the formation of less soluble alloyed Cdx Hg1 − x Te (Li et al. 2008). However, zeta potential measurement analysis showed a decrease in surface charge from − 71 ± 5 to − 45 ± 6 mV for FRET samples incubated with Hg2+ ions, supporting the charge transfer mechanism involving electron transfer from CdTe/CdS QDs to Hg2+ ions that induce fluorescence quenching (Gong et al. 2016; Saikia et al. 2016).
Literature data suggested mainly bivalent cations such as Ag+, Co2+, Cu2+, Zn2+ and Fe2+ interferes in the detection of Hg2+ ions (Gong et al. 2016; Vázquez-González and Carrillo-Carrion 2014; Zhu et al. 2017). Apart from these, we have also tested interference of other ions such as Na+ (500 nM), Cu+ (500 nM), Ca2+ (500 nM), trivalent ions like Fe3+ and Cr3+ (100 nM each), and mixture of ions on FRET system. The influence of these ions on fluorescence quenching of FRET system as a function of Rh6G fluorescence was tested as shown in Fig. S3. The percentage quenching efficiency (F/F0) was found to be below 5% for Na+, Cu+, Ca2+, Ag+, Co2+, Cu2+, and Zn2+ and < 10% for Fe2+, Fe3+, and Cr3+ ions. However, the small variation in the F/F0 observed for mixture may be due variation in pH due to salts or partial absorption by ions. In contrast, Hg2+ enhanced the quenching efficiency to 34 and 47% at 100 and 500 nM concentrations respectively, inferring the efficiency of CdTe/CdS and Rh6G based FRET system for selective sensing of Hg2+ ions in aqueous condition. The problems due to the interference of mixture of metal ions in high concentration can be resolved by dilution and changing the pH of sample mixtures before addition of FRET components.
Conclusions
In summary, an optimized cost-effective one step synthesis of core-shell CdTe/CdS QDs in aqueous media was demonstrated. The prepared QDs not only are highly photostable but also exhibit very high quantum yield (> 20%) in the solid state. The pH-dependent studies clearly demonstrate that the fluorescence emission can be red-shifted by increasing the pH and thus offer the possibility of maximum spectral overlap with an acceptor in a FRET pair. The FRET pair of CdTe/CdS and Rh6G bridged via cetyltrimethylammonium bromide has proven its efficiency for sensitive and selective detection of Hg2+ ions as compared to other bivalent metal ions. The LOD of the FRET pair-based sensor has a limit of detection of 3.8 nM which can detect the lowest level of allowed Hg2+ ion level in drinking water.
References
Aboulaich A, Billaud D, Abyan M, Balan L, Gaumet J-J, Medjadhi G, Ghanbaja J, Rl S (2012) One-pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl Mater Interfaces 4:2561–2569
Ag D, Bongartz R, Dogan LE, Seleci M, Walter J-G, Demirkol DO, Stahl F, Ozcelik S, Timur S, Scheper T (2014) Biofunctional quantum dots as fluorescence probe for cell-specific targeting. Colloids Surf B: Biointerfaces 114:96–103
Akshya S, Hariharan P, Kumar VV, Anthony SP (2015) Surface functionalized fluorescent CdS QDs: selective fluorescence switching and quenching by Cu2+ and Hg2+ at wide pH range. Spectrochim Acta A Mol Biomol Spectrosc 135:335–341
Bao H, Gong Y, Li Z, Gao M (2004) Enhancement effect of illumination on the photoluminescence of water-soluble CdTe nanocrystals: toward highly fluorescent CdTe/CdS core-shell structure. Chem Mater 16:3853–3859
Basheer NS, Kumar BR, Kurian A, George SD (2013) Silver nanoparticle size-dependent measurement of quantum efficiency of Rhodamine 6G. Appl Phys B Lasers Opt 113:581–587
Brus L (1986) Zero-dimensional “excitons” in semiconductor clusters. IEEE J Quantum Electron 22:1909–1914
Chen G, Song F, Xiong X, Peng X (2013) Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind Eng Chem Res 52:11228–11245
Chen L, Zhao Q, Zhang X-Y, Tao G-H (2014) Determination of silver ion based on the redshift of emission wavelength of quantum dots functionalized with rhodanine. Chin Chem Lett 25:261–264
Chen Y, Chen Z, He Y, Lin H, Sheng P, Liu C, Luo S, Cai Q (2010) L-cysteine-capped CdTe QD-based sensor for simple and selective detection of trinitrotoluene. Nanotechnology 21:125502
Choi M-J, Pierson R, Chang Y, Guo H, Kang I-K (2013) Enhanced intracellular uptake of CdTe quantum dots by conjugation of oligopeptides. J Nanomater 2013:14
Chu VH, Nghiem THL, Le TH, Vu DL, Tran HN, Vu TKL (2012) Synthesis and optical properties of water soluble CdSe/CdS quantum dots for biological applications. Adv Nat Sci Nanosci Nanotechnol 3:025017
Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H (2004) Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J Am Chem Soc 126:301–310
de Mello DC (2011) Synthesis and properties of colloidal heteronanocrystals. Chem Soc Rev 40:1512–1546
De Sousa ME, Fernández van Raap MB, Rivas PC, Mendoza Zélis P, Girardin P, Pasquevich GA, Alessandrini JL, Muraca D, Sánchez FH (2013) Stability and relaxation mechanisms of citric acid coated magnetite nanoparticles for magnetic hyperthermia. J Phys Chem C 117:5436–5445
de Souza GC, Ribeiro DS, Rodrigues SSM, Paim APS, Lavorante AF, da Silva VL, Santos JL, Araújo AN, Montenegro MCB (2016) Clean photoinduced generation of free reactive oxygen species by silica films embedded with CdTe–MTA quantum dots. RSC Adv 6:8563–8571
Ding X, Qu L, Yang R, Zhou Y, Li J (2015) A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 30:465–471
Duan J, Song L, Zhan J (2009) One-pot synthesis of highly luminescent CdTe quantum dots by microwave irradiation reduction and their Hg2+-sensitive properties. J Nano Res 2:61–68
Emin S, Loukanov A, Wakasa M, Nakabayashi S, Kaneko Y (2010) Photostability of water-dispersible CdTe quantum dots: capping ligands and oxygen. Chem Lett 39:654–656
Frasco MF, Chaniotakis N (2009) Semiconductor quantum dots in chemical sensors and biosensors. Sensors 9:7266–7286
Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmüller A, Weller H (2002) Thiol-capping of CdTe nanocrystals: an alternative to organometallic synthetic routes. J Phys Chem B 106:7177–7185
Geyer S, Porter VJ, Halpert JE, Mentzel TS, Kastner MA, Bawendi MG (2010) Charge transport in mixed CdSe and CdTe colloidal nanocrystal films. Phys Rev B 82:155201
Gong T, Liu J, Liu X, Liu J, Xiang J, Wu Y (2016) A sensitive and selective sensing platform based on CdTe QDs in the presence of l-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem 213:306–312
Huang F, Huang B (2015) Aqueous synthesis of water-soluble citrate-modified cadmium selenide/cadmium sulfide/zinc sulfide quantum dots. Spectrosc Lett 48:422–426
Jing L, Kershaw SV, Li Y, Huang X, Li Y, Rogach AL, Gao M (2016) Aqueous based semiconductor nanocrystals. Chem Rev 116:10623–10730
John J, Thomas L, George NA, Kurian A, George SD (2015) Tailoring of optical properties of fluorescein using green synthesized gold nanoparticles. Phys Chem Chem Phys 17:15813–15821
Kumar MMD, Devadason S (2013) Structural and optical properties of CdTe/CdSe heterostructure multilayer thin films prepared by physical vapor deposition technique. S Appl Nanosci 3:453–459
Kumar P, Kim K-H, Bansal V, Lazarides T, Kumar N (2017) Progress in the sensing techniques for heavy metal ions using nanomaterials. J Ind Eng Chem 54:30–43
Kurian A, George SD, Nampoori V, Vallabhan C (2005) Study on the determination of molecular distance in organic dye mixtures using dual beam thermal lens technique. Spectrochim Acta A Mol Biomol Spectrosc 61:2799–2802
Li H, Shih WY, Shih W-H (2007) Synthesis and characterization of aqueous carboxyl-capped CdS quantum dots for bioapplications. Ind Eng Chem Res 46:2013–2019
Li J, Mei F, Li W-Y, He X-W, Zhang Y-K (2008) Study on the fluorescence resonance energy transfer between CdTe QDs and butyl-rhodamine B in the presence of CTMAB and its application on the detection of Hg(II). Spectrochim Acta A Mol Biomol Spectrosc 70:811–817
Martin A, Narayanaswamy R (1997) Studies on quenching of fluorescence of reagents in aqueous solution leading to an optical chloride-ion sensor. Sensors Actuators B Chem 39:330–333
Mathew S, Saran AD, Singh Bhardwaj B, Ani Joseph S, Radhakrishnan P, Nampoori V, Vallabhan C, Bellare JR (2012) Size dependent optical properties of the CdSe-CdS core-shell quantum dots in the strong confinement regime. J Appl Phys 111:074312
Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM (2003) Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat Mater 2:630–638
Murray C, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Paim APS, Rodrigues SSM, Ribeiro DS, de Souza GC, Santos JL, Araújo AN, Amorim CG, Teixeira-Neto É, da Silva VL, Montenegro MC (2017) Fluorescence probe for mercury (ii) based on the aqueous synthesis of CdTe quantum dots stabilized with 2-mercaptoethanesulfonate. New J Chem 41:3265–3272
Piella J, Bastús NG, Puntes V (2016) Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem Mater 28:1066–1075
Rajh T, Micic OI, Nozik AJ (1993) Synthesis and characterization of surface-modified colloidal cadmium telluride quantum dots. J Phys Chem 97:11999–12003
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775
Rodzik-Czałka Ł, Lewandowska-Łańcucka J, Gatta V, Venditti I, Fratoddi I, Szuwarzyński M, Romek M, Nowakowska M (2017) Nucleobases functionalized quantum dots and gold nanoparticles bioconjugates as a fluorescence resonance energy transfer (FRET) system—synthesis, characterization and potential applications. J Colloid Interface Sci
Saikia D, Dutta P, Sarma NS, Adhikary NC (2016) CdTe/ZnS core/shell quantum dot-based ultrasensitive PET sensor for selective detection of Hg(II) in aqueous media. Sensors Actuators B Chem 230:149–156
Shamirian A, Ghai A, Snee PT (2015) QD-based FRET probes at a glance. Sensors 15:13028–13051
Singh H, Saleem SM, Singh R, Birdi K (1980) Micelle formation of ionic surfactants in polar nonaqueous solvents. J Phys Chem 84:2191–2194
Smith AM, Mohs AM, Nie S (2009) Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat Nanotechnol 4:56–63
Tang G, Du L, Su X (2013) Detection of melamine based on the fluorescence resonance energy transfer between CdTe QDs and Rhodamine B. Food Chem 141:4060–4065
Tang G, Wang J, Li Y, Su X (2015) Determination of arsenic (III) based on the fluorescence resonance energy transfer between CdTe QDs and Rhodamine 6G. RSC Adv 5:17519–17525
Tang L, Mo S, Liu SG, Ling Y, Zhang XF, Li NB, Luo HQ (2018) A sensitive “turn-on” fluorescent sensor for melamine based on FRET effect between polydopamine-glutathione nanoparticles and Ag nanoparticles. J Agric Food Chem 66:2174–2179
Ullah N, Mansha M, Khan I, Qurashi A (2018) Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. TrAC Trends Anal Chem 100:155–166
Ulusoy M, Walter J-G, Lavrentieva A, Kretschmer I, Sandiford L, Le Marois A, Bongartz R, Aliuos P, Suhling K, Stahl F (2015) One-pot aqueous synthesis of highly strained CdTe/CdS/ZnS nanocrystals and their interactions with cells. RSC Adv 5:7485–7494
Vale BR, Silva FO, Carvalho MS, Raphael E, Ferrari JL, Schiavon MA (2016) Water-soluble CdTe/CdS Core/Shell semiconductor nanocrystals: how their optical properties depend on the synthesis methods. Crystals 6:133
Vázquez-González M, Carrillo-Carrion C (2014) Analytical strategies based on quantum dots for heavy metal ions detection. J Biomed Opt 19:101503
Wang Y-Y, Xiang X, Yan R, Liu Y, Jiang F-L (2018) Förster resonance energy transfer from quantum dots to rhodamine B as mediated by a cationic surfactant: a thermodynamic perspective. J Phys Chem C 122:1148–1157
Yao J, Gou X (2016) An investigation of preparation, properties, characterization and the mechanism of zinc blende CdTe/CdS core/shell quantum dots for sensitive and selective detection of trace mercury. J Mater Chem C 4:9856–9863
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Zhang Y-Y, Kim J-Y, Kim Y, Jang D-J (2012) Controlled optical properties of water-soluble CdTe/CdS/ZnS quantum dots. J Nanopart Res 14:1117
Zhou D, Lin M, Chen Z, Sun H, Zhang H, Sun H, Yang B (2011) Simple synthesis of highly luminescent water-soluble CdTe quantum dots with controllable surface functionality. Chem Mater 23:4857–4862
Zhu J, Zhao Z-J, Li J-J, Zhao J-W (2017) CdTe quantum dot-based fluorescent probes for selective detection of Hg(II): the effect of particle size. Spectrochim Acta, Part A 177:140–146
Zrazhevskiy P, Gao X (2013) Quantum dot imaging platform for single-cell molecular profiling. Nat Commun 4:1619
Acknowledgements
We gratefully acknowledge the (1) DST-FIST programme via approval letter SR/FST/PSI-174/2012 for providing Powder X-ray diffractometer; (2) SERB, DST Govt. of India through the project no. SB/S2/CMP-017/2014; (3) SAIF, IIT Bombay, for providing HR-TEM facility; (4) Dr. Sudarshan Kini, sincerely thanks Manipal University for providing the postdoctoral fellowship; and (5) SDG acknowledges Manipal University for Dr. T M A Pai Endowment Chair in Applied Nanosciences.
Funding
This study received financial support from Joint Manipal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1886 kb)
Rights and permissions
About this article
Cite this article
Kini, S., Ganiga, V., Kulkarni, S.D. et al. Sensitive detection of mercury using the fluorescence resonance energy transfer between CdTe/CdS quantum dots and Rhodamine 6G. J Nanopart Res 20, 232 (2018). https://doi.org/10.1007/s11051-018-4320-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4320-5