Abstract
In this work, a facile strategy to synthesize Cu–hemin metal-organic frameworks (MOFs) with peroxidase-like activity was reported. The prepared Cu–hemin MOFs were characterized by various techniques such as scanning electron microscopy, transmission electron microscopy, X-ray powder diffraction, Fourier transform infrared spectroscopy, UV–visible absorbance spectra, and so on. The results showed that the prepared Cu–hemin MOFs looked like a ball-flower with an average diameter of 10 μm and provided a large specific surface area. The Cu–hemin MOFs possessing peroxidase-like activity could be used to catalyze the peroxidase substrate of 3,3,5,5-tetramethylbenzidine in the presence of H2O2, which was employed to detect H2O2 quantitatively with the linear range from 1.0 μM to 1.0 mM and the detection limit was 0.42 μM. Furthermore, with the additional help of glucose oxidase, a sensitive and selective method to detect glucose was developed by using the Cu–hemin MOFs as catalyst and the linear range was from 10.0 μM to 3.0 mM and the detection limit was 6.9 μM. This work informs researchers of the advantages of MOFs for preparing biomimetic catalysts and extends the functionality of MOFs for biosensor application.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peroxidase is a natural enzyme which participates in the physiological metabolism in vivo (Filizola and Loew 2000; Rodríguez-López et al. 2001). In biological processes, peroxidase as a catalyst is capable of catalyzing other oxides or peroxides with highly efficient activation, such as H2O2. Generally, it is difficult to extract peroxidase because of suffering from many serious disadvantages such as high cost, storage difficulties and the activity of peroxidase is easily affected by environmental even to lose their activity (Wulff 2002; Shoji and Freund 2002). Therefore, synthesis and developing of peroxidase mimics instead of peroxidase to improve their activity are of great significance (Feng et al. 2012).
In order to overcome these disadvantages, researchers made a huge effort to develop enzyme mimics in recent years. As the result, many enzyme mimics, such as serine proteases mimetics (Qin et al. 2013), cytochrome P450 mimetics (Visser 2007) and so on, have been developed. Peroxidase mimetics, such as porphyrin (Wu et al. 2014), hematin (Hu et al. 2015a, b), hemin (Wang et al. 2007; Fruk and Niemeyer 2005) and so on, have attracted great attention for their promising potential in the detection of glucose and H2O2 (Ellis et al. 2009). However, compared with natural enzymes, the catalytic activity of these peroxidase mimics was relatively poor (Qin et al. 2013). Therefore, using synthetic systems to mimic natural enzymes with high catalytic activity is still required (Qin et al. 2013).
Hemin, also named as iron protoporphyrin IX, which is the cofactor for many enzyme/protein families, including cytochromes (Wade et al. 2004), peroxidases (Strittmatter et al. 2013), hemoglobins (Fox and Thomson 1964), and myoglobins (Monzani et al. 2000), can catalyze a variety of oxidation reactions like peroxidase enzymes (Zhang and Dasgupta 1992). Nevertheless, direct application of hemin as an oxidation catalyst is usually challenging because of the formation of catalytically inactive dimer and self-degradation in aqueous solution (Bruice 1991). One promising approach to solve this problem is to immobilize hemin on supports such as zeolites, clays, nanoparticles, hydrogels, or carbon materials. Unfortunately, this approach will decrease the density of active sites inevitably (Wang et al. 2007; Xue et al. 2012). An alternative approach is to protect the hemin center by modifying the porphyrin to produce dendrimers (Shema-Mizrachi et al. 2011) or molecular crystals (Bezzu et al. 2010), which is a synthetically demanding method.
Metal-organic frameworks (MOFs) have emerged as a new class of crystalline materials because of their fascinating structures with high thermal stability (Lee et al. 2009), permanent porosity (Horcajada et al. 2009), open metal sites in the skeleton (Jihyun et al. 2010), and huge surface area (Cadiau et al. 2013). These fascinating properties make them be widely used in many fields, such as gas storage (Liu et al. 2014), separations (Bux et al. 2011), catalysis (Pintado-Sierra et al. 2013), adsorption (Yang et al. 2014), and sensing (Hu et al. 2015a, b). In recent years, researchers proposed a new strategy by loading hemin just as guest molecule in the caves of Cu MOFs to protect the hemin center (Feng et al. 2012).
In this work, we attempt to use copper as the metal ion and hemin as the ligand to construct novel MOFs with peroxidase-like catalytic property for the first time. This new Cu–hemin MOFs could be synthesized at room temperature avoiding both extra heat and solvothermal treatment. Besides, water was used as the solvent for a green productive process. In the Cu–hemin MOFs, abundant catalytic active center of hemin was still reserved and they could catalyze the oxidation of substrate efficiently. We also investigated the peroxidase-like catalytic property and found that it possessed good affinity with substrate. On the basis of these findings, we developed a colorimetric method for quantitative analysis of H2O2 and glucose.
Materials and methods
Materials and solutions
Cu(NO3)2·3H2O, uric acid (UA), 3,3,5,5-tetramethylbenzidine (TMB), H2O2, galactose, fructose, l-cysteine, mannose, glucose, and other reagents were purchased from Shanghai Aladdin Chemistry Co. Ltd. (Shanghai, China). Glucose oxidase (GOD) and hemin were purchased from Sigma-Aldrich. All reagents were of analytical grade and used without further purification. All solutions were prepared with ultra-pure water purified by a Millipore-Q system (18.2 MΩ cm−1).
Instruments
UV–visible absorbance spectra were performed on a Hitachi U-3900H spectrophotometer. Avatar 360 Fourier transform infrared spectra (FTIR) spectrometer (Nicolet, USA) was applied to record FTIR spectra. Scanning electron microscopy (SEM) image and energy dispersive X-ray spectroscopy (EDS) image were taken by HITACHI S-3400N scanning electron microscope with a Phoenix energy X-ray analyzer. Transmission electron microscopy (TEM) was measured by JEM-2100 transmission electron microscopy (JEM, JEOL, Japan). X-ray powder diffraction (XRD) spectrum was performed on a D/Max 2500 V/PC X-ray powder diffractometer using Cu Kα radiation (λ = 0.154056 nm, 40 kV, 200 mA). The N2 adsorption/desorption data were collected at 77 K using an ASAP 2020 instrument (Micromeritics).
Synthesis of Cu–hemin MOFs
Cu–hemin MOFs were synthesized according to the following procedures. 43 mM Cu(NO3)2·3H2O and 0.50 mM hemin (PBS solution, pH = 7) were mixed with volume ratio of 2:1, then stirred for 2 h at 25 °C. After that, the solid was obtained by centrifugation at 3000 rmp for 10 min and washed with ultra-pure water for 5 times and then dried in a vacuum oven at 40 °C for 24 h. The whole process was shown in Scheme 1. The mechanism of Cu–hemin MOFs formation might be like HKUST-1 and the Cu2+ coordinated with –COOH of hemin to form Cu–hemin MOFs (Jeong et al. 2012).
UV–visible absorbance measurements
The peroxidase-like activity of Cu–hemin MOFs was measured by using 200 μg mL−1 Cu–hemin MOFs in sodium citrate (NaAc) buffer (10 mM, pH 6) in the presence of TMB (500 μM) and H2O2 (400 μM) as the substrates, and the total reaction volume was 1.0 mL. The steady-state kinetic assays were carried out at 50 °C with 10 min incubation. The concentration of the oxidized TMB was quantified by UV–Vis absorption spectroscopy at 652 nm by using a molar extinction coefficient of ε = 3.9 × 104 M−1 cm−1 (Zhang et al. 2010). The Michaelis–Menten constant was calculated by using Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation:
where ν is the initial velocity, V m represents the maximal reaction velocity, C corresponds to the concentration of substrate, and K m is the Michaelis constant.
Detection of H2O2
The detection for H2O2 was carried out as follows. 50 μL solution that contained different amounts of H2O2 was added into a 0.1 mM NaAc buffer (pH 6.0) with 0.5 mM TMB and 0.2 mg mL−1 Cu–hemin MOFs in a total volume of 1 mL. The resulted solution was incubated at 50 °C before UV–Vis measurements. The sensing colorimetric mechanism for H2O2 was schematically illustrated in Scheme 1.
Detection of glucose
31.5 μL 2 mg mL−1 glucose oxidase was added into a 0.1 M phosphate buffer solution (PBS, pH 7.0) containing different amounts of glucose and incubated at 37 °C for 20 min. After that, the above solution was added into a 0.1 mM NaAc buffer (pH 6.0) containing 0.5 mM TMB and 0.2 mg mL−1 Cu–hemin MOFs in a total volume of 1 mL. The resulted solution was incubated at 50 °C before UV–Vis measurements. Selectivity analysis for glucose detection was carried out by using 10 mM urea acid, galactose, fructose, l-cysteine, and mannose instead of 1 mM glucose. The sensing colorimetric mechanism for glucose was schematically illustrated in Scheme 1.
Results and discussion
Characterization of Cu–hemin MOFs
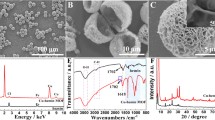
Different characterization techniques were used to investigate the morphology and structure of the as-prepared Cu–hemin MOFs. From the SEM image (Fig. 1a), we could see that the as-prepared Cu–hemin MOFs looked like a ball-flower with an average diameter of 10 μm. The inset of Fig. 1a and the TEM (Fig. 1b) image showed that the surface of Cu–hemin MOFs was very rough, which could provide a large specific surface area (18.937 m2 g−1, Fig. S1, Supporting Information). The ball-flower-like morphology and rough surface might provide many active sites, which was in favor of the catalytic oxidation of substrate. According to the N2 adsorption–desorption isotherms experiment, the mean diameter of the pore in Cu–hemin MOFs was about 25.7 nm (Fig. S1, Supporting Information).
FTIR spectra of Cu–hemin MOFs and hemin were shown in Fig. 1d. The bands at 1610 and 1135 cm−1 belonged to stretching of C=N bond and C–N bond of hemin, respectively (Qin et al. 2013). These bands could also be found in the curve of Cu–hemin MOFs. The peak at 1384 cm−1 belonged to –CH3 of hemin (Deng et al. 2013), which was stronger in curve of Cu–hemin MOFs due to the ordered assembly of hemin in the Cu–hemin MOFs. Hence, FT-IR spectra also proved the formation of Cu–hemin MOFs. As we all known, hemin has three feature elements (Fe, N, and Cl), and as shown in Fig. 1c, the peaks of Fe, N, Cl, and Cu were observed in the solid products, which declared the formation of Cu–hemin MOFs. Both the FTIR spectra and the EDS suggested that the Cu–hemin MOFs were successfully synthesized. Besides, the formation of Cu–hemin MOFs was also confirmed by XRD (Fig. S2, Supporting Information).
Comparison of the peroxidase-like activity between hemin and Cu–hemin MOFs
The oxidation of TMB in the presence of H2O2 was used to test the peroxidase-like activity of Cu–hemin MOFs. TMB is colorless, and it could be oxidized by peroxidase or compounds with peroxidase-like activity in the presence of H2O2 into the blue oxidation stated TMB (oxTMB), which could be proven by UV–Vis adsorption spectroscopy (Tan et al. 2014). The UV–Vis absorption curve of the produced blue solution showed two absorption peaks at 375 nm and 652 nm, which were typical adsorption peaks of oxTMB (Nirala et al. 2015; Zhao et al. 2015; Lin et al. 2015). The two typical adsorption peaks of oxTMB could be used to explore the activity of peroxidase or compounds with peroxidase-like activity.
In order to compare the peroxidase-like activity between hemin and Cu–hemin MOFs, the same amount of hemin and Cu–hemin MOFs (0.2 mg mL−1) was used as catalyst to oxidize TMB in the presence of H2O2. As shown in Fig. S3 (Supporting Information) and Scheme 1, in the Cu–hemin MOFs–H2O2 system, the hemin in the Cu–hemin MOFs could be oxidized by H2O2 in which the H2O2 was reduced into H2O. And then the oxidation stated hemin in the Cu–hemin MOFs could oxidize TMB into blue oxTMB which was used to explore the peroxidase-like activity of hemin and Cu–hemin MOFs. As shown in Fig. 2, for both hemin–TMB–H2O2 (Fig. 2a) and Cu–hemin MOFs–TMB–H2O2 solution (Fig. 2b), with the increasing of H2O2 concentration, the concentration of produced oxidation stated hemin was increased and more TMB was oxidized into oxTMB, and thus the UV–Vis absorbance at 652 nm increased accordingly. Although the UV–Vis absorbance also increased in the hemin–TMB–H2O2 solution, it always was much lower than that in the Cu–hemin MOFs–TMB–H2O2 solution (Fig. 2c, d). The result clearly indicated that the peroxidase-like activity of hemin in Cu–hemin MOFs was greatly enhanced. Besides, this method to improve the peroxidase-like activity of hemin is convenient without many synthetic demanding and very friendly to environment.
UV–Vis absorption spectra of TMB in the presence of a hemin, b Cu–hemin MOFs and H2O2 with different concentrations (100, 200, 400, 600, 800 μM and 1 mM). c Peroxidase-like activity of hemin and Cu–hemin MOFs under different H2O2 concentrations determined by the absorption of oxTMB at 652 nm. The concentrations of hemin and Cu–hemin are 0.2 mg mL−1. d UV–Vis absorption spectra of TMB (0.5 mM) reaction solutions catalytically oxidized by (blue) no catalyst, (red) hemin (0.2 mg mL−1) and (green) Cu–hemin MOFs (0.2 mg mL−1) in the presence of 0.4 mM H2O2
Detection of H2O2
Since the UV–Vis absorbance at 652 nm was increased with the increasing of H2O2 concentration, the Cu–hemin MOFs could be used to determine H2O2 in the presence of TMB. As shown in Fig. 3a, the UV–Vis absorbance increased linearly with the increase of H2O2 concentration from 1.0 μM to 1.0 mM (Fig. 3b) and the color of solution gradually became blue (Inset in Fig. 3a). The detection limit was calculated to be 0.42 μM based on the ratio of signal-to-noise of 3. As shown in Table S1 (Supporting Information), the detection limit was much lower than many other H2O2 sensors (Lu et al. 2011; Liu et al. 2015; Li et al. 2015; Zhang et al. 2014). The linear range also was wider than some previous results (Dong et al. 2012; Hao et al. 2013; Shi et al. 2011; Zhang et al. 2014, 2015; Tian et al. 2012). Furthermore, we used the Cu–hemin MOFs which was stored for 0, 1, 2, 3, 6, 9, 12, 15 days as catalyst to test the stability of colorimetric sensor in the Cu–hemin MOFs–TMB–H2O2 system (concentration of H2O2 is 0.4 mM). The results showed that the absorbance at 652 nm almost did not vary, which indicated the good stability of the colorimetric sensor (Fig. S4, Supporting Information).
a UV–Vis absorption spectra and b linear calibration plot of oxTMB in the presence of Cu–Hemin MOFs and different concentrations of H2O2 ranging from 1.0 μM to 1.0 mM. Inset in a is the color changes of TMB–H2O2–Cu–hemin MOFs system with different H2O2 concentration: a–q contains 0, 1, 2, 4, 6, 8, 10, 20, 40, 60, 80, 100, 200, 400, 600, 800, and 1000 μM H2O2, respectively
Then the effects of the amount of Cu–hemin MOFs (Fig. 4a), pH (Fig. 4b) and reaction temperature (Fig. 4c) on the catalytic reaction were explored. As shown in Fig. 4, the 0.2 mg mL−1 Cu–hemin MOFs at pH 6.0 and 50 °C showed the optimal results. It should be noted that using hemin as the ligand to construct MOFs, the optimal temperature was increased by 10 °C as compared with hemin itself (Qin et al. 2013), which suggested the constructed Cu–hemin MOFs was an effective means to improve the thermal stability of hemin.
To further investigate the catalytic property of Cu–hemin MOFs, the steady-state kinetic experiments were carried out (Fig. 5). The kinetic data were obtained by varying one substrate concentration while keeping the other substrates constant. Here, both TMB (Fig. 5a) and H2O2 (Fig. 5b) were followed the typical Michaelis–Menten behaviors. Both the maximum initial velocity (V m) and Michaelis–Menten constant (K m) were calculated according to Line weaver–Burk plots (Insets in Fig. 5a, b), and the result was listed in Table S2 (Supporting Information). It is known that K m is an indicator of enzyme affinity to substrates and a smaller K m value reflects a stronger affinity of enzyme for substrate. In this work, the Cu–hemin MOFs had smaller K m (2.18 mM) for H2O2 and larger K m (1.42 mM) for TMB as compared with those of HRP (3.73 mM for H2O2 and 0.434 mM for TMB) (Gao et al. 2007). The result indicated the Cu–hemin MOFs had higher affinity for H2O2. In contrast, the larger K m for TMB suggested the weaker affinity between Cu–hemin MOFs and TMB than HRP. The smaller K m also suggested that Cu–hemin MOFs exhibited an improved catalytic activity toward H2O2 as compared with HRP. Moreover, the Vm of the Cu–hemin MOFs for H2O2 (116 × 10−8 M s−1) and TMB (26.22 × 10−8 M s−1) was larger than that of HRP for H2O2 (8.71 × 10−8 M s−1) and TMB (10 × 10−8 M s−1) as shown in Table S2 (Supporting Information), also indicating an enhanced catalytic activity.
Detection of glucose
As discussed above, the Cu–hemin MOFs could be used to determine the concentration of H2O2 with the help of TMB. In the presence of O2, with the help of glucose oxidase, glucose would be catalyzed into gluconic acid companied by the reduction of O2 into H2O2. So we expanded the application of Cu–hemin MOFs to detect glucose. As shown in Fig. 6a, in a certain range of glucose concentration, the absorbance at 652 nm increased linearly with increasing glucose concentration. As illustrated in Fig. 6b, the linear relationship between the absorbance concentration and the glucose concentration was from 10.0 μM to 3.0 mM, and the detection limit was 6.9 μM. The results were superior to other materials as shown in Table S3 (Supporting Information). The good performance might be attributed to the porous structure of Cu–hemin MOFs for providing more contact sites between substrates and the peroxidase mimic.
a UV–Vis absorption spectra and b linear calibration plot of oxTMB in the presence of Cu–Hemin MOFs and different concentrations of glucose ranging from 10.0 μM to 3.0 mM. c UV–Vis absorbance response of the TMB–H2O2–Cu hemin MOFs system to glucose (1 mM) and different interferential substances (10 mM)
Furthermore, some possibly coexisted interfering substances were also tested in our work. 10.0 mM UA, galactose, fructose, l-cysteine, and mannose were detected to instead of 1.0 mM glucose, respectively. As shown in Fig. 6c, these chemicals almost have no obvious influence on the absorbance at 652 nm. Then we attempt to use this material to detect the glucose in human serum. As shown in Table S4 (Supporting Information), the glucose in human serum could be detected successfully and accurately by this method. The results indicated that this method might be promising to diagnose diabetes.
Conclusion
In summary, the novel peroxidase mimic of Cu–hemin MOFs was synthesized successfully via a simple and environment friendly method. The porous structure of Cu–hemin MOFs protected hemin from self-degrading and aggregating and enhanced the peroxidase-like activity. The peroxidase-like property of Cu–hemin MOFs was investigated and showed a higher affinity to H2O2 than the natural enzyme HRP. On this basis, a simple and visual colorimetric method to detect H2O2 and glucose was developed successfully. The result exhibited a reasonable linear range and detection limit with high sensitivity, stability and selectivity. Furthermore, this method was applied to detect glucose in real sample and showed good anti-interference ability, so it was promising in diagnosing diabetes.
References
Bezzu CG, Helliwell M, Warren JE, Allan DR, McKeown NB (2010) Heme-like coordinationchemistry within nanoporous molecular crystals. Science 327:1627–1630
Bruice TC (1991) Reactions of hydroperoxides with metallotetraphenylporphyrins in aqueous solutions. Acc Chem Res 24:243–249
Bux H, Chmelik C, Krishna R, Caro J (2011) Ethene/ethane separation by the MOF membrane ZIF-8: molecular correlation of permeation, adsorption, diffusion. Fuel Energy Abstr 369:284–289
Cadiau A, Brites CD, Costa PM, Ferreira RA, Rocha J, Carlos LD (2013) Ratiometric nanothermometer based on an emissive Ln3+-organic framework. ACS Nano 7:7213–7218
Deng S, Lei J, Huang Y, Cheng Y, Ju H (2013) Electrochemiluminescent quenching of quantum dots for ultrasensitive immunoassay through oxygen reduction catalyzed by nitrogen-doped graphene-supported hemin. Anal Chem 85:5390–5396
Dong Y, Zhang H, Rahman Z, Su L, Chen X, Hu J, Chen X (2012) Graphene oxide–Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 4:3969–3976
Ellis WC, Tran CT, Denardo MA, Fischer A, Ryabov AD, Collins TJ (2009) Design of more powerful iron-TAML peroxidase enzyme mimics. J Am Chem Soc 131:18052–18053
Feng D, Gu Z, Li J, Jiang H, Wei Z, Zhou H (2012) Zirconium-metalloporphyrin PCN-222: mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts. Angew Chem 124:10453–10456
Filizola M, Loew GH (2000) Role of protein environment in horseradish peroxidase compound I formation: molecular dynamics simulations of horseradish peroxidase–HOOH complex. J Am Chem Soc 122:18–25
Fox JB, Thomson JS (1964) The formation of green heme pigments from metmyoglobin methemoglobin and by the action of nitrite. Biochemistry 3:1323–1328
Fruk L, Niemeyer CM (2005) Covalent hemin–DNA adducts for generating a novel class of artificial heme enzymes. Angew Chem Int Ed 44:2603–2606
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N et al (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Hao J, Zhang Z, Yang W, Lu B, Ke X, Zhang B et al (2013) In situ controllable growth of CoFe2O4 ferrite nanocubes on graphene for colorimetric detection of hydrogen peroxide. J Mater Chem A 1:4352–4357
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T et al (2009) Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater 9:172–178
Hu D, Song Y, Wang L (2015a) Nanoscale luminescent lanthanide-based metal-organic frameworks: properties, synthesis, and application. J Nanopart Res 17:310
Hu Q, Hu W, Kong J, Zhang X (2015b) Ultrasentitive electrochemical DNA biosensor by hematin as efficient biomimetic catalyst toward in situ metallization. Biosens Bioelectron 63:269–275
Jeong NC, Samanta B, Lee CY, Farha OK, Hupp JT (2012) Coordination-chemistry control of proton conductivity in the iconic metal-organic framework material HKUST-1. J Am Chem Soc 134:51–54
Jihyun A, Geib SJ, Rosi NL (2010) High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine- and amino-decorated pores. J Am Chem Soc 132:38–39
Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT (2009) Metal-organic framework materials as catalysts. Chem Soc Rev 38:1450–1459
Li M, Xiong Y, Liu X, Bo X, Zhang Y, Han C et al (2015) Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 7:8920–8930
Lin X, Deng H, Wu G, Peng H, Liu A, Lin X et al (2015) Platinum nanoparticles/graphene oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst 140:5251–5256
Liu S, Qi X, Lin R, Cheng X, Liao P, Zhang J et al (2014) Porous Cu(I) triazolate framework and derived hybrid membrane with exceptionally high sensing efficiency for gaseous oxygen. Adv Funct Mater 24:5866–5872
Liu J, Bo X, Zhao Z, Guo L (2015) Highly exposed Pt nanoparticles supported on porous graphene for electrochemical detection of Hydrogen peroxide in living cells. Biosens Bioelectron 74:71–77
Lu W, Liao F, Luo Y, Chang G, Sun X (2011) Hydrothermal synthesis of well-stable silver nanoparticles and their application for enzymeless hydrogen peroxide detection. Electrochim Acta 56:2295–2298
Monzani E, Alzuet G, Casella L, Redaelli C, Bassani C, Sanangelantoni AM et al (2000) Properties and reactivity of myoglobin reconstituted with chemically modified protohemin complexes. Biochemistry 39:9571–9582
Nirala NR, Abraham S, Kumar V, Bansal A, Srivastava A, Saxena PS (2015) Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens Actuators B 218:42–50
Pintado-Sierra M, Rasero-Almansa AM, Corma A, Iglesias M, Sánchez F (2013) Bifunctional iridium-(2-aminoterephthalate)–Zr–MOF chemoselective catalyst for the synthesis of secondary amines by one-pot three-step cascade reaction. J Catal 299:137–145
Qin F, Jia S, Wang F, Wu S, Song J, Liu Y (2013) Hemin@ metal-organic framework with peroxidase-like activity and its application to glucose detection. Catal Sci Technol 3:2761–2768
Rodríguez-López JN, Lowe DJ, Hernández-Ruiz J, Hiner ANP, García-Cánovas F, Thorneley RNF (2001) Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: identification of intermediates in the catalytic cycle. J Am Chem Soc 123:11838–11847
Shema-Mizrachi M, Pavan G, Levin E, Danani A, Lemcoff N (2011) Catalytic chameleon dendrimers. J Am Chem Soc 133:14359–14367
Shi W, Wang Q, Long Y, Cheng Z, Chen S, Zheng H et al (2011) Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun 47:6695–6697
Shoji E, Freund MS (2002) Potentiometric saccharide detection based on the pKa changes of poly (aniline boronic acid). J Am Chem Soc 124:12486–12493
Strittmatter E, Liers C, Ullrich R, Wachter S, Hofrichter M, Plattner DA, Piontek K (2013) First crystal structure of a fungal high-redox potential dye-decolorizing peroxidase. J Biol Chem 288:4095–4102
Tan H, Ma C, Gao L, Li Q, Song Y, Xu F et al (2014) Metal-organic framework-derived copper nanoparticle@ carbon nanocomposites as peroxidase mimics for colorimetric sensing of ascorbic acid. Chem Eur J 20:16377–16383
Tian J, Liu S, Luo Y, Sun X (2012) Fe(III)-based coordination polymer nanoparticles: peroxidase-like catalytic activity and their application to hydrogen peroxide and glucose detection. Catal Sci Technol 2:432–436
Visser SP (2007) Preferential hydroxylation over epoxidation catalysis by a horseradish peroxidase mutant: a cytochrome P450 mimic. J Phys Chem B 111:12299–12302
Wade RC, Winn PJ, Schlichting I, Sudarko (2004) A survey of active site access channels in cytochromes P450. J Inorg Biochem 98:1175–1182
Wang Q, Yang Z, Zhang X, Xiao X, Chang CK, Xu B (2007) A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew Chem Int Ed 46:4285–4289
Wu Z, Chen L, Liu J, Parvez K, Liang H, Shu J et al (2014) High-performace electrocatalysts for oxygen reduction derived from cobalt porphyrin-based conjugated mesoporous polymers. Adv Mater 26:1450–1455
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102:1–28
Xue T, Jiang S, Qu Y, Su Q, Cheng R, Dubin S et al (2012) Graphenesupported hemin as a highly active biomimetic oxidation catalyst. Angew Chem 124:3888–3891
Yang J, Liu Q, Sun W (2014) Shape and size control and gas adsorption of Ni(II)-doped MOF-5 nano/microcrystals. Microporous Mesoporous Mater 190:26–31
Zhang G, Dasgupta PK (1992) Hematin as a peroxidase substitute in hydrogen peroxide determinations. Anal Chem 64:517–522
Zhang X, Gong S, Zhang Y, Yang T, Wang C, Gu N (2010) Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J Mater Chem 20:5110–5116
Zhang T, Lu Y, Luo G (2014) Synthesis of hierarchical iron hydrogen phosphate crystal as a robust peroxidase mimic for stable H2O2 detection. ACS Appl Mater Interfaces 6:14433–14438
Zhang L, Peng D, Liang R, Qiu J (2015) Graphene quantum dots assembled with metalloporphyrins for “turn on” sensing of hydrogen peroxide and glucose. Chem Eur J 21:9343–9348
Zhao D, Chen C, Lu L, Yang F, Yang X (2015) A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified gold nanoparticles. Sens Actuators B 215:437–444
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (21465014 and 21465015), Natural Science Foundation of Jiangxi Province (20142BAB203101 and 20143ACB21016), The Ministry of Education by the Specialized Research Fund for the Doctoral Program of Higher Education (20133604110002), the Ground Plan of Science and Technology Projects of Jiangxi Educational Committee (KJLD14023), and the Open Project Program of Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Normal University (Nos. KLFS-KF-201410, KLFS-KF-201416).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, F., He, J., Zeng, M. et al. Cu–hemin metal-organic frameworks with peroxidase-like activity as peroxidase mimics for colorimetric sensing of glucose. J Nanopart Res 18, 106 (2016). https://doi.org/10.1007/s11051-016-3416-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3416-z