Abstract
In order to clarify the intrinsic reason for the smaller remanence (M r)-to-saturation (M s) magnetization ratio M r/M s than that expected by the Stoner–Wohlfarth model in CoFe2O4/CoFe2 nanoparticles in the previous report, we first prepared well-dispersed CoFe2O4 nanoparticles, and then they were diluted in the SiO2 matrix followed by reduction in H2 as far as possible to exclude or reduce disadvantageous variables (such as the growth and aggregation of particles and the exchange coupling between soft magnetic particles in the process of reducing) affecting magnetic properties. Such an idea has not been taken into account before to our knowledge. The analyses on the magnetic results indicate that the CoFe2O4/CoFe2 nanoparticles herein reported are a pure dipolar system, in which the coercivity (H c) and M r/M s ratio are very sensitive to the anisotropy and the strength of dipolar interaction. These results signify that it is important to maintain the CoFe2O4/CoFe2 nanoparticles with higher anisotropy and weaker dipolar interaction for improving M r/M s and H c. This suggestion was further confirmed by our another result wherein an M r/M s value of 0.64 was obtained even though no exchange coupling was observed in the CoFe2O4/CoFe2 nanoparticles, and further work is in process.
Graphical abstract
Numerous efforts have devoted to improve the values of M
s and M
r/M
s by compositing hard CoFe2O4 (CFO) ferrite with soft CoFe2 (CF) alloy, which unfortunately give the low M
r/M
s value (<0.5) even in presence of the exchange coupling. Key issues involve the preparation of CFO/CF composite. Previously the preparation of CFO/CF undergoes the synthesis of CFO and the subsequent reducing in the H2 ambient, as shown in Figure (a), while in this work well dispersed CFO nanoparticles were first prepared , and then diluted in the SiO2 matrix followed by reducing in H2 to exclude or reduce disadvantageous variables, such as the growth and aggregation of particles and the exchange coupling between soft magnetic particles in the process of reducing, as shown in Figure (b). Our results suggest that higher anisotropy and weaker dipolar interaction favor the larger M
r/M
s value, as shown in Figure (c).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Well-dispersed magnetic nanoparticles (NPs) have been widely investigated, because they not only possess unique properties differing from their bulk polycrystalline counterparts but also have potential applications in data storage and biotechnology. Furthermore, they provide a highly controlled experimental system for studying fundamental phenomena in physics (Coşkun et al. 2012; Topkaya et al. 2013; Xu et al. 2014a, b). Specially, nano-scaled CoFe2O4 (CFO) NPs are very attractive because of their potentials in high-density information storage, electromagnetic wave absorption, ferrofluids, catalysts, drug targeting, magnetic separation, magnetic resonance imaging and gas sensor (Whitney et al. 1993; Carlà et al. 2013; Leite et al. 2012; Wu et al. 2011; Topkaya et al. 2013; Song and Zhang 2004). CFO is a cubic anisotropy crystal with the first crystalline anisotropy constant K 1 > 0, and therefore its theoretical remanence (M r)-to-saturation (M s) ratio M r/M s is 0.832 in the case of noninteracting single domain particles with the randomly oriented easy axis according to the Stoner–Wohlfarth model (Stoner and Wohlfarth 1948; Vargas et al. 2005). However, CFO has low M s value because of its ferrimagnetism. Recently, numerous efforts have devoted to improve the values of M s and M r/M s by compositing CFO with CoFe (CF) alloy.
Previously, the preparation of CFO/CF includes the synthesis of CFO and the subsequent reduction in the H2 ambience, aiming to achieve higher M s, M r, and coercivity H c values through exchange coupling between the hard and soft magnets. But almost all of the results give the following conclusions: (1) When the content of CF increases, the M s value increases rapidly but is accompanied with a large loss of H c. (2) In some previous reports, the exchange coupling between the hard and soft magnets was detected, but unfortunately the M r/M s ratio was not improved and even many reported M r/M s values were less than 0.5 (Quesada et al. 2014; Zan et al. 2013a, b, c; Li et al. 2015), the expected value theoretically for the uniaxial anisotropy crystal (Vargas et al. 2005). By carefully checking the results of the morphology characterizations in the previous reports, the following can be observed: (1) First of all, the prepared CFO particles aggregated seriously, and then they exhibited more severe conglomeration after they were reduced in the high-temperature condition. In such an undiluted or concentrated CFO/CF NP system, the exchange coupling effect may occur between hard/hard, hard/soft, and soft/soft grains, as shown in the schematic plot in Fig. 1a. It is possible that the detected exchange coupling in the previous reports occurs between the soft/soft CF grains because they remain in close contact after reduction, and such exchange coupling will be ineffective in improving M r/M s. (2) Besides the exchange coupling effect, the anisotropy also changes, because, on one hand, the relative content of hard and soft magnets varies with the reducing reaction, and on the other hand, reduction at high temperature gives rise to a change in the degree of aggregation and configuration which can be well understood with the results shown in Figs. 7, 9, 10 and 11 in our previous reports (Zan et al. 2013a, b, c). (3) In the concentrated or undiluted CFO/CF composite, the interparticle dipolar interaction cannot be neglected especially in the case of high content of soft CF with large M s value because the dipolar field H dip can be expressed as H dip = 2 μ/d 3, where μ (μ = M s × V, V is the particle volume) is the average magnetic moment of magnetic particle and d is the distance between particles (center to center). Hence one can see that in the case of concentrated CFO/CF system the exchange coupling, anisotropy and dipolar interaction synergistically affect the magnetic properties, and such the system is unfit for revealing the intrinsic origin of the magnetic properties.
In this work, we first prepared well-dispersed CFO NPs, and then they were diluted in the SiO2 matrix, followed by reduction in H2 ambience in order to prevent magnetic NPs from aggregating during the process. This strategy has not been reported before as far as we know. The purposes of this strategy are as follows: (1) It can prohibit not only the agglomeration and growth of magnetic NPs but also the exchange coupling effect between soft CF particles as shown in Fig. 1b. (2) By changing the mass ratio of CFO/SiO2, we can obtain magnetic NPs with different strengths of dipolar interactions. (3) By changing the reduction temperature, the relative content of CFO/CF can be altered, which consequently leads to the variation in anisotropy and dipolar interaction. Therefore such a system is a good model for studying the effect of dipolar interaction and anisotropy on magnetic properties. The results show that H c and M r are very sensitive to the dipolar interaction and anisotropy, making us understand that magnetic particles must be endowed with high anisotropy and weak dipolar interaction in order to improve the magnetic performance.
Experimental procedures
The synthesis and characterization of CFO/SiO2 were described elsewhere (Xu et al. 2015). The samples with the CFO/SiO2 mass ratio of 50:50 and 5:95 are hereafter referred to as CFO50 and CFO5. The CFO50 and CFO5 samples were, respectively, reduced in the H2 ambience (500 sccm, 96 % N2 + 4 % H2) for 4 h at temperatures T A = 300, 400, 500, 600, 700 and 800 °C to obtain two groups of samples.
Results and discussion
Crystal structures and morphology analysis of all samples
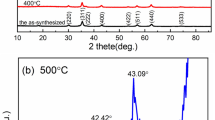
The crystal structures of all samples were characterized by the X-ray diffraction (XRD) method. Figures 2c–h, representatively, show the XRD patterns of samples CFO5 and CFO50 reduced at T A = 300, 500 and 700 °C. In the case of the CFO50 sample, it exhibits the pure cubic spinel CFO phase after reduction at 300 °C (Fig. 2c) according to the standard PDF card (Fig. 2a) of CoFe2O4 (No. 22-1086). Subsequently, reduction at 500 °C distinctly weakens the diffraction intensity from CFO (Fig. 2d), and two additional clear diffraction peaks can be indexed to the diffraction from CF phase as compared with the PDF card of CoFe2 (No. 65-4131) in Fig. 2b (Xiang et al. 2013). After reduction at 700 °C, the diffraction from CFO cannot be detected and the sample exhibits the pure CF phase (Fig. 2e) due to the reduction reaction: CoFe2O4 + 14H2 → CoFe2 + 4H2O. In the case of the sample CFO5, as shown in Figs. 2f–h, the phase evolves in the same trend as in the sample CFO50 except that the broad peak below 2θ = 30° results from the amorphous SiO2 (Nadeem et al. 2014). Figure 3 shows the normalized diffraction intensities from CFO (311) and CF (110) for the samples CFO50 (a) and CFO5 (b), more distinctly illustrating the phase evolution with the reducing temperature T A.
The cell lattice parameter a of CFO and CF can be obtained from sin2 θ = λ 2(H 2 + K 2 + L 2)/4a 2, where θ is the diffraction angle, λ the wavelength of the Cu Kα irradiation, and (HKL) the crystal plane index. The obtained lattice parameter a is equal to 0.838 and 0.287 nm, so the X-ray density is calculated to be 5.43 and 8.03 g/cm3 for CFO and CF, respectively.
Figure 4a shows the low-magnification TEM image of the as-prepared CoFe2O4 with the inset being the TEM image for the sample CFO5. The as-prepared CoFe2O4 is composed of well-dispersed NPs. For the CFO5 sample, the TEM image is not so clear as that for the as-prepared CoFe2O4 sample because CoFe2O4 NPs in the CFO5 sample were highly diluted by the amorphous SiO2 matrix. The particle size histogram and the Gaussian-fitting curve (solid line) are shown in Fig. 4b, giving the particle size of 16.3 ± 4 nm. In the case of the sample CFO50 after reduction at 500 °C, the high-resolution transmission electron microscopy (HRTEM) (Fig. 4c) shows that CFO/CF NPs are separated from each other even though they are treated at 500 °C due to the isolation of SiO2 matrix, which is different from the previous results in literature; the upper-left and lower-right insets show the magnified details of fringes of the CFO (111) and CF (110) crystalline planes, respectively, indicating the formation of CFO/CF composite; in these isolated NPs, at least the exchange interaction between CF grains, which is a short-range one in nature, can be excluded. Figure 4d shows the selected area electron diffraction (SAED) pattern of CFO50 reduced at 500 °C, also illustrating the formation of the composite.
The low-magnification TEM image of the as-prepared CFO (a) with the inset being the TEM image of the CFO5 sample; the particle size histogram and the Gaussian-fitting curve (solid line) (b); the HRTEM image (c) with the magnified details of fringes corresponding to the CFO (111) (upper-left inset) and CF (110) (lower-right inset) crystalline planes, and the SAED image (d) for the CFO50 reduced at 500 °C
The magnetic properties
Figure 5 shows the magnetization (M) dependence on the applied magnetic field (H) (−40 kOe < H < +40 kOe) measured at room temperature, i.e. M(H) loops of the CFO5 samples reduced at different temperatures from 300 to 800 °C. The magnetic parameters, including H c, M s as well as M r/M s drawn from loops, are listed in Table 1. The sample reduced at 300 °C, almost the pure CFO as shown in Fig. 2, has the largest H c (2321 Oe) and M r/M s (0.46) values but the smallest M s (18.04 emu/g) value. Upon increasing T A to 800 °C, both H c and M r/M s decrease monotonously to 169 Oe and 0.05, which vary in the same trend as reported before (Soares et al. 2011), while M s increases to 43.44 emu/g. These behaviors phenomenally can be assigned to the increase of CF phase and the intrinsic origin will be further discussed below.
Figure 6 shows the room temperature M(H) loops of the CFO50 samples reduced at different temperatures from 300 to 800 °C. The loops exhibit similar characteristics as shown in Fig. 5. The values of H c, M s, and M r/M s are listed in Table 2. These parameters change with T A in the same trend as occured in the sample CFO5. The M s value is obviously greater than that for the sample CFO5 because of the higher concentration of magnetic NPs, and the highest M s value of 183.80 emu/g is attained after reduction at 700 °C.
Analysis on the magnetic properties
The anisotropy field H K
As reported before (Laureti et al. 2010), the splitting point, where the magnetizing and demagnetizing branches separate (ΔM), corresponds to the irreversibility field which can be regarded as an estimate of anisotropy field H K . Herein H K is artificially regulated to be the magnetic field corresponding to the splitting point, where ΔM/M s is equal to 5 %. The obtained H K values for the CFO50 (solid circles) and CFO5 (empty circles) are shown in Fig. 7. With the increasing T A, they both decrease monotonously, and the reasons are as follows: (1) The increase of CoFe2 phase enhances the M s value, because CoFe2 is a typical soft ferromagnetic material with a high M s value of about 230 emu/g (Mohan et al. 2008), consequently diminishing the H K value due to H K = 2 K/M s, where K is the effective anisotropy constant. (2) For the soft and hard composite system, the effective anisotropy constant K is expressed by K = f s K s + f h K h, where f s and f h are the volume fraction, and K s and K h are anisotropy constant of soft and hard phases with K s << K h, respectively (Skomski and Coey 1993). The increase of T A raises the volume fraction f s of the soft magnetic CoFe2 phase, diminishing the K value and consequently resulting in the decrease of H K . Additionally the H K of CFO50 is smaller than that of CFO5, partially because CFO50 has a higher M s value.
The dipolar field H dip
To test the interactions between the grains, the δm curves were measured. The δm curves were built using the magnetizing M r(H) and demagnetizing M d(H) remanent magnetizations. The measuring methods of M r(H) and M d(H) are identical with those reported in reference (Soares et al. 2011). M r(H) and M d(H) are normalized by the saturation remanence M r(H sat): m r(H) = M r(H)/M r(H sat) and m d(H) = M d(H)/M d(H sat), where H sat is an applied field to saturate the magnetization, and the δm is expressed as δm(H) = m d(H) − [1 − 2m r(H)]. Figure 8 representatively shows the δm curves of the samples CFO50 and CFO5 reduced at 300, 500, and 800 °C. Several main points are summarized as follows: (1) All δm curves exhibit the negative peak. The negative peak is an evidence of the predominance of dipole−dipole interactions, while the positive peak can be attributed to the predominance of exchange interactions. The δm plots indicate that the thermal treatments up to 800 °C do not induce exchange coupling between particle surfaces due to the isolation of SiO2, or in other words all samples are pure dipolar systems. (2) The δm amplitudes (i.e. strength of interparticle interactions) are 0.23, 0.33, and 0.49 for the CFO5 samples, and 0.33, 0.34, and 0.73 for the CFO50 samples reduced at 300, 500, and 800 °C, respectively. These data suggest that reduction at higher temperature enhances the strength of interparticle dipolar interactions. (3) The δm amplitudes of the CFO5 samples are smaller than those of the corresponding CFO50 samples, because the concentration (average distance) of CoFe2O4 NPs is smaller (longer) in the CFO5 samples. These conclusions can be better understood by analyzing the dipolar field, as discussed below.
In a sample of randomly distributed NPs with average magnetic moment μ (μ = M s × V, V is the particle volume), the maximum dipolar field H dip between nearest-neighboring particles is H dip = 2 μ/d 3, where d is the distance between particles (center to center). The H dip curves are shown in Fig. 9. It can be noticed that (1) after reduction above 500 °C, H dip increases rapidly resulting from the increase of μ due to the appearance of CoFe2 phase, and (2) the H dip values of CFO50 samples are higher than those of the CFO5 samples because of the smaller d and larger M s in CFO50 samples.
Figure 10 shows the H K and H dip for the samples CFO5 (a) and CFO50 (b). In the case of CFO5 samples, H K is larger than H dip at all reducing temperatures, suggesting that anisotropy dominates the magnetic properties because the CoFe2O4 NPs are highly diluted in the SiO x matrix, leading to the weak dipolar interaction. In the case of CFO50 samples, H K is larger than H dip as T A ≤ 600 °C but H K < H dip as T A > 600 °C, suggesting that the dipolar interaction becomes the non-negligible energy to affect magnetic properties in samples reduced at T A > 600 °C.
Effects of dipolar interaction and anisotropy on the magnetic properties
As shown in Fig. 11a, for both the CFO5 and CFO50 samples, H c monotonously decreases with the increasing T A, but the underlying mechanism is somewhat different in both the samples. In the CFO5 samples with H K >> H dip at all T A values, the decrease in H c can be assigned to the decrease of H K with T A due to the appearance of soft magnetic CoFe2 alloys, as discussed in Fig. 10a. In the sample CFO50, the H c is determined by anisotropy at T A ≤ 600 °C and the synergistic effect between the anisotropy and the dipolar interaction at T A > 600 °C based on the discussion for Fig. 10b. Additionally, the H c value of the CFO50 sample is less than that of the CFO5 sample, mainly because CFO50 has the smaller H K value (Fig. 7) than CFO5.
The M r/M s ratios of the samples CFO5 (empty circles) and CFO50 (solid circles) are shown in Fig. 11b. They both monotonously decrease with the increasing T A, which can be assigned to the synergistic effect of dipolar interaction and the anisotropy because the M r/M s ratio will be suppressed by increasing H dip (Zan et al. 2013a, b, c; Xu et al. 2015; Zan et al. 2013a, b, c) or decreasing H K (Xu et al. 2014a, b; Kurtan et al. 2013). Therefore, it is important to control the anisotropy and dipolar interaction for improving the M r/M s ratio. In order to support this viewpoint, the CoFe2O4 NPs with the size of about 30 nm were prepared, as shown in the TEM image in Fig. 12a. After reduction at 300 °C for 1.5 h, the M(H) loop of the sample was measured, as shown in Fig. 12b. A high M r/M s ratio (0.64) above 0.5 and a high H c value of 1660 Oe were obtained in the sample, even though exchange coupling was not observed in the δm curve (not shown here), which can be assigned to the high H K (3615 Oe) and weak H dip (~230 Oe) values, and further work is in process.
Conclusions
The CoFe2O4 NPs were synthesized by thermal decomposition of Fe(acac)3 and Co(acac)2. The as-prepared CoFe2O4 NPs exhibit good dispersity and uniform size of 16.3 ±4 nm. Then the CoFe2O4 NPs were dispersed in the SiO2 matrix with different concentrations of 5 % (CFO5) and 50 % (CFO50), followed by subsequently reducing in the 4 % H2 + 96 % N2 ambience at different reducing temperatures, T A, of 300, 400, 500, 600, 700 and 800 °C to obtain the composite of hard CoFe2O4 and soft CoFe2 alloys, in order to change the anisotropy and the interparticle dipolar interaction. The anisotropy and the dipolar interaction in the CFO5 and CFO50 samples were compared in detail, and their effects on the magnetic properties of CoFe2O4/CoFe2 composite were discussed on which much attention has not been paid before. Several suggestions are as follows:
(1) The reasons for the smaller M r/M s ratio in the CoFe2O4/CoFe2 composite than 0.5 in the previous reports can be assigned to the weak anisotropy and/or strong dipolar interaction in the concentrated magnetic NPs.
(2) The M r/M s ratio was not improved even if the exchange coupling was observed in undiluted CoFe2O4/CoFe2 composite, in which the exchange coupling maybe occurs between CoFe2 particles instead of between hard CoFe2O4 and soft CoFe2 phases.
(3) In order to improve the H c and M r/M s values, several important factors were suggested as follows: preparing the CoFe2O4 particles with a suitable size for controlling the anisotropy; keeping the CoFe2O4 particles separated from each other for controlling the strength of the dipolar interaction; preventing magnetic particles from conglomerating in the process of reducing to prepare CoFe2O4/CoFe2 composite.
We also suggest that the M r/M s ratio can be further increased if the exchange coupling between hard and soft magnets can be successfully accomplished. Further related work is in process.
References
Carlà F, Campo G, Sangregorio C, Caneschi A, Fernández CDJ, Cabrera LI (2013) Electrochemical characterization of core@shell CoFe2O4/Au composite. J Nanopart Res 15:1813
Coşkun M, Can MM, Coşkun ÖD, Korkmaz M, Fırat T (2012) Surface anisotropy change of CoFe2O4 nanoparticles depending on thickness of coated SiO2 shell. J Nanopart Res 14:1197
Kurtan U, Topkaya R, Baykal A (2013) Sol–gel auto-combustion synthesis of PVP/CoFe2O4 nanocomposite and its magnetic characterization. Mater Res Bull 48:4889–4895
Laureti S, Varvaro G, Testa AM, Fiorani D, Agostinelli E, Piccaluga G et al (2010) Magnetic interactions in silica coated nanoporous assemblies of CoFe2O4 nanoparticles with cubic magnetic anisotropy. Nanotechnology 21:315701
Leite GCP, Chagas EF, Pereira R, Prado RJ, Terezo AJ, Alzamora M, Baggio-Saitovitch E (2012) Exchange coupling behavior in bimagnetic CoFe2O4/CoFe2 nanocomposite. J Magn Magn Mater 324:2711–2716
Li WX, Wang LC, Li GM, Xu Y (2015) Hollow CoFe2O4–Co3Fe7 microspheres applied in electromagnetic absorption. J Magn Magn Mater 377:259–266
Mohan M, Chandra V, Manoharan SS (2008) A new nano CoFe2 alloy precursor for cobalt ferrite production via sonoreduction process. Curr Sci India 94:473–476
Nadeem K, Krenn H, Sarwar W, Mumtaz M (2014) Comparison of surface effects in SiO2 coated and uncoated nickelferrite nanoparticles. Appl Surf Sci 288:677–681
Quesada A, Rubio-Marcos F, Marco JF, Mompean FJ, García-Hernández M, Fernández JF (2014) On the origin of remanence enhancement in exchange-uncoupled CoFe2O4-based composites. Appl Phys Lett 105:202405
Skomski R, Coey JMD (1993) Giant energy product in nanostructured two-phase magnets. Phys Rev B 48:15812
Soares JM, Cabral FAO, Araújo JHD, Machado FLA (2011) Exchange-spring behavior in nanopowders of CoFe2O4–CoFe2. Appl Phys Lett 98:072502
Song Q, Zhang ZJ (2004) Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J Am Chem Soc 126:6164–6168
Stoner EC, Wohlfarth EP (1948) A mechanism of magnetic hysteresis in heterogeneous alloys. Philos Trans R Soc Lond A 240:599–642
Topkaya R, Baykal A, Demir A (2013) Yafet–Kittel-type magnetic order in Zn-substituted cobalt ferrite nanoparticles with uniaxial anisotropy. J Nanopart Res 15:1359
Vargas JM, Nunes WC, Socolovsky LM, Knobel M, Zanchet D (2005) Effect of dipolar interaction observed in iron-based nanoparticles. Phys Rev B 72:184428
Whitney TM, Searson PC, Jiang JS, Chien CL (1993) Fabrication and magnetic properties of arrays of metallic nanowires. Science 261:1316–1319
Wu HX, Liu G, Wang X, Zhang JM, Chen Y, Shi JL, Yang H, Hu H, Yang SP (2011) Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomater 7:3496–3504
Xiang J, Zhang XH, Li JL, Chu YQ, Shen XQ (2013) Fabrication, characterization, exchange coupling and magnetic behavior of CoFe2O4/CoFe2 nanocomposite nanofibers. Chem Phys Lett 576:39–43
Xu ST, Ma YQ, Xu YF, Sun X, Geng BQ, Zheng GH, Dai ZX (2014a) The effects of surface spin on magnetic properties of weak magnetic ZnLa0.02Fe1.98O4 nanoparticles. Nanoscale Res Lett 9:545
Xu YF, Ma YQ, Xu ST, Zan FL, Zheng GH, Dai ZX (2014b) Obtainment of exchange coupling coefficient of Ni0.6Zn0.4Fe2O4/SrFe12O19 composites. Mater Lett 131:203–205
Xu ST, Ma YQ, Xu YF, Sun X, Geng BQ, Zheng GH, Dai ZX (2015) Pure dipolar-interacted CoFe2O4 nanoparticles and their magnetic properties. Mater Res Bull 62:142–147
Zan FL, Ma YQ, Ma Q, Xu YF, Dai ZX, Zheng GH, Wu MZ, Li G (2013a) Magnetic and impedance properties of nanocomposite CoFe2O4/Co0.7Fe0.3 and single-phase CoFe2O4 prepared via One-step hydrothermal synthesis. J Am Ceram Soc 96:3100–3107
Zan FL, Ma YQ, Ma Q, Xu YF, Dai ZX, Zheng GH (2013b) Giant exchange bias and exchange enhancement observed in CoFe2O4-based composites. J Alloy Compd 581:263–269
Zan FL, Ma YQ, Ma Q, Zheng GH, Dai ZX, Wu MZ, Li G, Sun ZQ, Chen XS (2013c) One-step hydrothermal synthesis and characterization of high magnetization CoFe2O4/Co0.7Fe0.3 nanocomposite permanent magnets. J Alloy Compd 553:79–85
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11174004 and 51471001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geng, B.Q., Ma, Y.Q., Xu, Y.F. et al. Separated CoFe2O4/CoFe nanoparticles by the SiO x matrix: revealing the intrinsic origin for the small remanence magnetization. J Nanopart Res 17, 281 (2015). https://doi.org/10.1007/s11051-015-3087-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3087-1