Abstract
This work concerns rheological and frictional behaviour of lubricating oils containing platelet molybdenum disulfide (MoS2) nanoparticles (average diameter ~50 nm; single layer thickness ~3 nm). Stable nano-MoS2 lubricants were formulated and measured for their rheological behaviour and tribological performance. Rheological experiments showed that the nano-MoS2 oils were non-Newtonian following the Bingham plastic fluid model. The viscosity data fitted the classic Hinch–Leal (H–L) model if an agglomeration factor of 1.72 was introduced. Tribological experiments indicated that the use of MoS2 nanoparticles could enhance significantly the tribological performance of the base lubricating oil (reduced frictional coefficient, reduced surface wear and increased stability). Scanning electron microscopy, laser confocal microscope and x-ray energy dispersive spectroscopy analyses suggested that the reduced frictional coefficient and surface wear be associated with surface patching effects. Such patching effects were shown to depend on the concentration of MoS2 nanoparticles, and an effective patching required a concentration over approximately 1 wt%. The increased stability could be attributed to the enhanced heat transfer and lubricating oil film strength due to the presence of nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Friction provides a driving force for many physical processes including rolling, shaping and cutting (Childs and Steadman 1977). Friction also causes energy and material losses during these processes. The use of lubricants is the most effective way to reduce these undesirable effects. Lubricants can be broadly divided into three categories of solid, liquid or gas (Stachowiak and Batchelor 2011). The most commonly used lubricants are liquid-based lubricating oils made from a base oil and various additives (Le Suer 1965). The additives play a crucial role to the performance of lubricating oils in terms of anti-corrosion, wear resistance and anti-oxidation (Wang et al. 2006). Lubricating oils containing a small amount of nanoparticles are also termed as nanofluids (Choi and Eastman 1995), and a considerable amount of work has been carried out over the last decade on heat transfer aspects of such fluids (Eastman et al. 2001; He et al. 2007; Das et al. 2008). Recent years have also seen an increased amount of work on the use of nanoparticles to reduce surface friction and surface wear; see for example Xu et al. (1996), Lee et al. (2007), (2009), Hwang et al. (2011) and Reeves et al. (2013). These studies investigated the effects of size (Reeves et al. 2013), morphology (Hwang et al. 2011) and concentration (Lee et al. 2007) of nanoparticles on tribological behaviour. Nanoparticles used in these studies include metals, metal oxides and carbon allotropes. Qiu et al. (2001) studied the tribological performance of oils containing Ni nanoparticles. They showed that the presence of the nanoparticles reduced the diameter of surface wear scar by 31 % and decreased the friction coefficient by 26 %. Hu and Dong (1998) investigated the effect on the lubricating behaviour of the addition of TiO2 nanoparticles to a base lubricant oil. They observed the existence of an optimal nanoparticle concentration of below 1 wt%. Several mechanisms have been proposed to explain the effects of nanoparticles on the friction and wear resistance. These include formation of films due to particle deposition (Qiu et al. 1999), ball bearing of nanoparticles between frictional surfaces (Xu et al. 1996) and surface patching (Liu et al. 2004).
As a commonly used solid lubricant with good thermal stability and corrosion resistance, MoS2 has found a wide range of applications (Savan et al. 2000). Several studies have been performed on the use of MoS2 as an additive of lubricating oils, including fullerene- and sphere-like MoS2 nanoparticles and micro- and nano-sized MoS2 slices (Huang et al. 2005; Hu et al. 2009, 2010; Zhu et al. 2011). However, little has been done on the rheological and thermal behaviour of oils containing MoS2 nanoparticles, or their effects on the tribological performance. Our early studies showed that the use of MoS2 nanoparticles could enhance thermal conductivity and heat dissipation of lubricating oils (Wan et al. 2013). In this paper, we report the use of platelet MoS2 nanoparticles as an additive of lubricating oils. Lubricating oils with different concentrations of MoS2 nanoparticles are fabricated, and their rheological and tribological behaviour are investigated. Our results show that the addition of MoS2 nanoparticles leads to a significant improvement on the tribological performance of the oil when the particle concentration is over 1 wt%, whereas little change is observed to the oil viscosity at temperatures over about 30 °C. Attempts have also been made to interpret the experimental observations.
Experimental
Preparation and characterization of nano-MoS2 oil samples
Commercial lubricating oil (SE 15W-40, Sinopec Lubricants, China) and analytical reagent grade platelet-like MoS2 nanoparticles (Beijing DK Nano S and T, China) were used to formulate the nano lubricants. The MoS2 nanoparticles had an average diameter of ~50 nm and a single layer thickness of ~3 nm. Span80 (chemical reagent grade, Tianjin Fuchen Chemical Reagent Ltd, China) was used as the dispersant for the formulation to ensure suspension stability. For all nano-MoS2 containing samples, a mass ratio of Span80 to MoS2 nanoparticles of 1:1 was used. The formulation process involved mixing the base oil, the nanoparticles and the dispersant with a high shear homogenizer (Model T18, IKA, Germany). The mixer was operated at 7,500 r/min, and the mixing time was 30 min for all samples. Using the above method, nano-MoS2 oils containing 0.1, 0.5, 1.0, 2.0 and 5.0 wt% were prepared for the work and they were denoted as M01, M05, M1, M2 and M5 for subsequent discussion, respectively. The stability of the nano-MoS2 oils was characterized through the settling method, and no sedimentation was observed within two weeks after the preparation. The morphology of the MoS2 nanoparticles was analyzed by a JEM-2100 transmission electron microscopy (TEM). Figure 1a shows a TEM image of the particles. One can see that the MoS2 nanoparticles have a platelet-like structure with a fairly uniform particle size distribution. The size distribution of MoS2 nanoparticles in the sample M1 was also analyzed by the dynamic light scattering method (Zetasizer Nano ZS, Malvern, UK). Figure 1c shows the results, which indicates an average particle diameter of 49.21 nm. This is larger than the equivalent particle diameter of single nanoparticles indicated by the TEM analyses. This is due to agglomeration of the nanoparticles due to van der Waals force. More discussion will be done late. A PANalytical X-ray Diffractometer (XRD) was used to examine the crystal structure of MoS2 nanoparticles. Figure 1b shows the results. The diffractive peaks at 14.4°, 32.7°, 39.5°, 49.8° and 58.4° in the figure correspond, respectively, to the diffraction of (002), (100), (103), (105), (110) planes of MoS2 crystals.
Rheological and tribological experiments
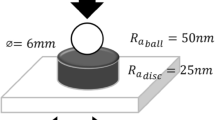
The rheological behaviour of the nano-MoS2 oils was measured by an Anton Paar MCR302 rheometer over a temperature of 20–90 °C and a shear rate between 0.1 and 1,000 s−1. Each sample was tested at least five times, and an average of the results was used for the analyses. The tribological experiments were performed using a frictional testing machine (Model MSG-10G, Shandong, China). A disc-on-disc configuration was used, and the frictional pairs were made of No. 45 stainless steel consisting of 97.53–98.91 % Fe, 0.42–0.50 % C, 0.17–0.37 % Si, 0.50–0.80 % Mn, 0.25 % Cr, 0.30 % Ni and 0.25 % Cu. The load, rotational speed and test duration were 1,500 N, 500 r/min and 180 s, respectively. Each of the frictional experiments was repeated at least three times from ambient temperature, and the averages of them were taken for analyses. A Mahr profilometer (Marsurf PS1, Germany) was used to measure linear roughness of the testing samples, whereas the 3-dimensional (3-D) surface topography of the samples was measured with an Olympus laser confocal microscope (LEXT OLS4000, Japan). Surface morphology and elemental distribution of the surfaces were analyzed using a Zeiss Auriga scanning electron microscopy (SEM) and an energy dispersive X-ray spectrometer (EDS).
Results and discussion
Rheological behaviour of nano-MoS2 oils
It is well known that the temperature of a frictional system changes with time on stream. This is likely to affect the rheology of the lubricating oil, leading to changes in the flow and the film thickness of the oil, and hence the tribological performance. We first look at the viscosity–temperature relationships of the nano-MoS2 oils. Figure 2a shows the dynamic viscosity of the base oil and nano-MoS2 oils as a function of temperature. One can see that the shear viscosities of the base oil and nano-MoS2 oils decrease rapidly with increasing temperature. The addition of nanoparticles changes the viscosity of the oil particularly at low temperatures of 20–30 °C. For extremely dilute suspensions (samples M01 and M05), the viscosities are strangely lower than the base oil at 20–30 °C. Exact mechanisms for such unusual observation need further investigation. However, the platelet-like structure of the nanoparticles and associated lubricating effect may have contributed to the decrease of the viscosities (Chen et al. 2008). At high concentrations and low temperatures, enhancements of viscosity of 1.16, 2.36 and 6.05 % are observed, respectively, for samples M1, M2 and M5. At temperatures over 30 °C, the viscosity difference between the base oil and nano-MoS2 oils becomes vanishingly small due to a combined effect of the Brownian motion and convection (Chen et al. 2007).
Attention is now paid to the relationship between the shear stress and shear rate of the nano-MoS2 oils. Figure 2b shows the results for sample M1. One can see that the shear stress relates to the shear rate in a linear manner at all temperatures studied. However, the linear relationships at different temperatures do not extend to the origin, indicating that the nano lubricants are non-Newtonian but following the Bingham plastic fluid model (Bingham 1916-1917). A further inspection of the data shows that, for a given temperature, the slope of the lines changes little over the whole range of the shear rate investigated (0.1–1,000 s−1), indicating little shear dependence (Astarita and Marrucci 1974). The results for other nano-MoS2 oils are similar, which suggest that the nano-MoS2 oils could be used at high shear frictional conditions.
The data of nano-MoS2 oils at the low temperature of 20 °C are further investigated by comparing with classic theories for suspension viscosity. The first theory on the effect of particle concentration on suspension viscosity is due to Albert Einstein (Einstein 1906, 1911):
where \( \mu_{\text{nf, Einstein}} \) is the effective suspension viscosity, \( \varphi \) is the particle volume fraction and \( \mu_{\text{f }} \) is the base fluid viscosity. This equation is only applicable to dilute suspensions of non-interacting rigid spherical particles. A number of equations have subsequently developed for predicting the suspension viscosity under different conditions. Among these is the Hinch–Leal (H–L) equation for dilute suspensions of oblate spheroid particles (Hinch and Leal 1972):
where \( \mu_{{{\text{nf, H}}{-}{\text{L }}}} \) is the effective suspension viscosity, \( \varphi \) is the particle volume fraction and p is the shape factor of suspending particles. Figure 3 compares the experimental data with the above two equations, where the shape factor is calculated on the basis of single layers of MoS2. One can see that the Einstein equation underestimates the measured viscosity, whereas the H–L equation gives an overestimation. The reasons for the deviation are identified in the following. First, MoS2 nanoparticles used in this work are platelet shaped, and the Einstein equation and H–L equation are developed, respectively, for spherical and oblate spheroid particles. Second, both the Einstein and the H–L equations work for non-interacting particles, whereas MoS2 nanoparticles are expected to be interacting due to interparticle forces in the suspension. As the shape of MoS2 nanoparticles is closer to the oblate shaped particles, efforts have been made to analyse the data using the H–L equation and considering the interparticle interactions. Due to the van der Waals force, the platelet MoS2 nanoparticles tend to stack to form multilayer aggregates as illustrated in the inset of Fig. 3 (Dallavalle et al. 2012). On this basis, we assume that MoS2 nanoparticles form aggregates in the form of stacks of single layer of MoS2 nanoparticles. These aggregates are further assumed to have the same number of layers with the same height and diameter. The shape factor, p, in Eq. 2 can then be calculated and hence the effective viscosity as a function of the shape factor (and the layer number). We find that, under the conditions of this work, a corrected shape factor of \( p^{'} = 1.72p \) fits the experimental data very well as shown by the solid line in Fig. 3. Here, \( p^{'} = 1.72p \) implies that, on average, the aggregates of MoS2 nanoparticles have 1.72 layers. As a result, the H–L equation could be modified to give
Tribological performance of nano-MoS2 oils
Figure 4a compares the tribological performance of the base oil and nano-MoS2 oils. One can see that the frictional coefficient of the base oil decreases with time in the first 30 s. This followed by a rapid increase at time = 40 s. The increase in the frictional coefficient with time could be attributed to the temperature increase due to friction. As discussed above, an increase in temperature decreases the oil viscosity, which leads to the thinning of the lubricant oil film, and hence direct contact of the frictional pairs and the friction coefficient increase. The picture for the nano-MoS2 oils is very different. The tested samples can be divided into three groups: Group 1 (M01), Group 2 (M05) and Group 3 (M1 and M2). These are discussed in the following.
-
Group 1 (M01)—the friction coefficient of this group of samples is always higher than that of the base oil particularly in the first 80 s. After 80 s, the friction coefficient of M01 is only slightly higher and closely follows the trend of the base oil. Although there are MoS2 nanoparticles, due to very low concentration, they are unable to prevent the direct contact of the frictional pairs; see late for more discussion.
-
Group 2 (M05)—overall, the friction coefficient of this group of samples is lower than that of the base oil and Group 1 samples over the testing duration. However, serious fluctuations are observed in the friction coefficient, which could cause instability in longer operating durations. In addition, there is an increasing trend with time.
-
Group 3 (M1 and M2)—the friction coefficient of this group of samples shows a slow and steady decreasing trend with increasing time, which despite higher than that of the base oil in the initial stage, becomes lower after 60–70 s and much lower after 80–100 s.
The above discussion suggests the existence of a critical nanoparticle concentration above which improved frictional performance can be achieved; and for MoS2 nanoparticles, this concentration is approximately 1 wt%. The effects of particle concentration may also be partially associated with heat transfer as the presence of nanoparticles enhances the thermal conductivity of the base oil (Wan et al. 2013). This is supported by experimentally observed temperature rise of the nano-MoS2 oils in the frictional experiments, which are 52.5, 49.5, 47.6 and 43.3 °C, respectively, for M01, M05, M1 and M2 samples. The corresponding viscosity (obtained from Fig. 2a) is 0.025, 0.027, 0.029 and 0.033 Pa s, respectively, for M01, M05, M1 and M2. The high viscosity values of M1 and M2 could have contributed to the observed tribological stability (Heidenkummer et al. 1991).
Figure 4b shows the linear roughness of the frictional surfaces tested with the base oil and the nano-MoS2 oils. The first bar on the left is the linear roughness before testing with the base oil. The linear roughness of the frictional surfaces before testing with nano-MoS2 oils is similar and is, therefore, not shown in the figure for conciseness. As expected, due to the polishing effect (Furey 1961), the linear roughness of all samples is decreased after tests. The linear roughness of the tested surface with the base oil is much lower than that with M01, but is slightly higher than that of M05. The linear roughness of the tested surfaces with M1 and M2 are similar, which is considerably lower than that of the tested surfaces with other oil samples. These results agree well with the friction coefficient results discussed above.
Further discussion of the results
A schematic illustration of the effect of MoS2 nanoparticles on a friction process is given in Fig. 5. The base oil tends to form a thin layer of oil film on the frictional surface (Fig. 5a). With the addition of MoS2 nanoparticles, deposition of the particles on the frictional surface would occur (Winer 1967). The structure of the deposited layers may depend on the particle concentration. At low particle concentrations (e.g. M01 and M05), the amount of MoS2 nanoparticles is insufficient to form a low frictional surface (Fig. 5b). These nanoparticles could deposit on edges and corners of the surface structures, leading to an even rougher surface. This is supported by the experimental results of M01 and M05 (Fig. 4a). As particle concentration increases, the rough surface features could be effectively filled (patched) by the deposited nanoparticles (Fig. 5c), leading to a reduced friction coefficient. This is supported by the data for M1 and M2 samples shown in Fig. 4a. Similar results have also been observed by Lu et al. (2007). Although our experimentally observed phenomena are similar to the observations of Lu et al. (2007), they proposed a mechanism based on the roller bearing effect of nanoparticles, whereas we proposed the surface patching effect of nanoparticles as we believe platelet shaped particles are less likely to roll on the friction surface.
To validate the proposed patching mechanism, 3-dimensional morphologies of the frictional surfaces before and after the frictional tests are measured and the results are shown in Fig. 6a1–c1. One can see clear ridges of about 4 μm height on the surface before the frictional tests (Fig. 6a1). The surface becomes smoother with ridge height reduced to about 2 μm after frictional tests with the base lubricating oil (Fig. 6b1). The surface roughness is further reduced to <1 μm after testing with M1 (Fig. 6c1). These observations agree with the proposed patching mechanisms.
Elemental distributions on the frictional surfaces are also measured to further validate the proposed patching mechanism. The results are shown in Fig. 6a2–c2. One can see the existence of common elements of Fe, C, Si, Cr, Mn, Ni and Cu before and after the frictional tests. These are from the No. 45 stainless steel. The existence of O is likely to be from decomposition of the base oil during the test (Qi et al. 2012). Additional elements of Mo (0.58 wt%) and S (0.47 wt%) are found on the surface tested with M1 (Fig. 6c2), indicating the deposition of the MoS2 nanoparticles during the frictional process. The insets in Fig. 6a2–c2 are SEM images of the surfaces, which show that the roughness agrees with the 3-D morphology as shown in Fig. 6a1–c1.
Conclusions
We prepared lubricant oils containing platelet MoS2 nanoparticles and studied their rheological behaviour and tribological performance. Both the base oil and nano-MoS2 oils are non-Newtonian, showing the Bingham plastic fluid behaviour with the viscosity decreasing with increasing temperature. The viscosity data fitted the classic H–L model if an agglomeration factor of 1.72 was introduced. Tribological experiments indicated that the use of MoS2 nanoparticles could enhance significantly the tribological performance of the base lubricating oil. SEM, laser confocal microscope and x-ray energy dispersive spectroscopy analyses suggested that the enhanced tribological performance be associated with surface patching effects. Such patching effects were shown to depend on the concentration of MoS2 nanoparticles and an effective patching required a concentration over ~1 wt%.
References
Astarita G, Marrucci G (1974) Principles of non-Newtonian fluid mechanics. McGraw-Hill, New York
Bingham EC (1916–1917) An investigation of the laws of plastic flow. Bulletin of the Bureau of Standards (13): 309–353
Chen H, Ding Y, Tan C (2007) Rheological behaviour of nanofluids. New J Phys 9(10):367
Chen LF, Xie HQ, Li Y, Yu W (2008) Nanofluids containing carbon nanotubes treated by mechanochemical reaction. Thermochim Acta 477(1–2):21–24. doi:10.1016/j.tca.2008.08.001
Childs THC, Steadman R (1977) Friction cutting - useful wear process. Met Constr-Brit Weld 9(11):523–524
Choi SU, Eastman J (1995) Enhancing thermal conductivity of fluids with nanoparticles. Argonne National Lab., IL (United States), No. ANL/MSD/CP-84938; CONF-951135-29
Dallavalle M, Sandig N, Zerbetto F (2012) Stability, dynamics, and lubrication of MoS2 platelets and nanotubes. Langmuir 28(19):7393–7400. doi:10.1021/La300871q
Das SK, Choi SUS, Yu W, Pradeep T (2008) Nanofluid: science and technology. Wiley-Interscience. Hoboken, NJ, pp 123–165
Eastman JA, Choi SUS, Li S, Yu W, Thompson LJ (2001) Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett 78(6):718–720. doi:10.1063/1.1341218
Einstein A (1906) Eine neue Bestimmung der Molekuldimension (a new determination of the molecular dimensions). Ann der Phys 19(2):289–306
Einstein A (1911) Berichtigung zu meiner Arbeit: eine neue Bestimmung der Molekul-dimension (correction of my work: a new determination of the molecular dimensions). Ann der Phys 34(3):591–592
Furey M (1961) Metallic contact and friction between sliding surfaces. Asle Trans 4(1):1–11
He Y, Jin Y, Chen H, Ding Y, Cang D, Lu H (2007) Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int J Heat Mass Trans 50(11–12):2272–2281. doi:10.1016/j.ijheatmasstransfer.2006.10.024
Heidenkummer H-P, Kampik A, Thierfelder S (1991) Emulsification of silicone oils with specific physicochemical characteristics. Graefe’s Arch Clin Exp Ophthalmol 229(1):88–94
Hinch E, Leal L (1972) The effect of Brownian motion on the rheological properties of a suspension of non-spherical particles. J Fluid Mech 52(04):683–712
Hu ZS, Dong JX (1998) Study on antiwear and reducing friction additive of nanometer titanium oxide. Wear 216(1):92–96. doi:10.1016/S0043-1648(97)00252-4
Hu KH, Wang J, Schraube S, Xu YF, Hu XG, Stengler R (2009) Tribological properties of MoS2 nano-balls as filler in polyoxymethylene-based composite layer of three-layer self-lubrication bearing materials. Wear 266(11–12):1198–1207. doi:10.1016/j.wear.2009.03.036
Hu KH, Hu XG, Xu YF, Huang F, Liu JS (2010) The effect of morphology on the tribological properties of MoS2 in liquid paraffin. Tribol Lett 40(1):155–165
Huang HD, Tu JP, Zou TZ, Zhang LL, He DN (2005) Friction and wear properties of IF-MoS2 as additive in paraffin oil. Tribol Lett 20(3–4):247–250. doi:10.1007/s11249-005-8552-z
Hwang Y, Lee C, Choi Y, Cheong S, Kim D, Lee K, Lee J, Kim SH (2011) Effect of the size and morphology of particles dispersed in nano-oil on friction performance between rotating discs. J Mech Sci Technol 25(11):2853–2857. doi:10.1007/s12206-011-0724-1
Le Suer WM (1965) Process of preparing lubricant additives. US Patent
Lee J, Cho S, Hwang Y, Lee C, Kim SH (2007) Enhancement of lubrication properties of nano-oil by controlling the amount of fullerene nanoparticle additives. Tribol Lett 28(2):203–208. doi:10.1007/s11249-007-9265-2
Lee C-G, Hwang Y-J, Choi Y-M, Lee J-K, Choi C, Oh J-M (2009) A study on the tribological characteristics of graphite nano lubricants. Int J Precis Eng Manuf 10(1):85–90. doi:10.1007/s12541-009-0013-4
Liu G, Li X, Qin B, Xing D, Guo Y, Fan R (2004) Investigation of the mending effect and mechanism of copper nano-particles on a tribologically stressed surface. Tribol Lett 17(4):961–966
Lu HF, Fei B, Xin JH, Wang RH, Li L, Guan WC (2007) Synthesis and lubricating performance of a carbon nanotube seeded miniemulsion. Carbon 45(5):936–942. doi:10.1016/j.carbon.2007.01.001
Qi XW, Lu L, Jia ZN, Yang YL, Liu HR (2012) Comparative tribological properties of magnesium hexasilicate and serpentine powder as lubricating oil additives under high temperature. Tribol Int 49:53–57. doi:10.1016/j.triboint.2011.12.014
Qiu SQ, Dong JX, Chen GX (1999) Tribological properties of CeF3 nanoparticles as additives in lubricating oils. Wear 230(1):35–38
Qiu SQ, Zhou ZR, Dong JX, Chen GX (2001) Preparation of Ni nanoparticles and evaluation of their tribological performance as potential additives in oils. J Tribol-Trans Asme 123(3):441–443. doi:10.1115/1.1286152
Reeves CJ, Menezes PL, Lovell MR, Jen T-C (2013) The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol Lett 51(3):437–452. doi:10.1007/s11249-013-0182-2
Savan A, Pflüger E, Voumard P, Schröer A, Simmonds M (2000) Modern solid lubrication: recent developments and applications of MoS2. Lubr Sci 12(2):185–203
Stachowiak G, Batchelor AW (2011) Engineering tribology. Butterworth-Heinemann, Oxford, pp 1–9
Wan Q, Jin Y, Ding Y (2013) Tribological characteristics and heat transfer behavior of nano-MoS2 based lubricating oil. Lubr Eng 38(6):17–21. doi:10.3969/j.issn.0254-0150.2013.06.004
Wang XL, Xu BS, Xu Y, Yu HL, Shi PJ (2006) The antifriction and self-repairing performances of the nanometer additive in lubricating oil. In: Proceedings of the First International Conference on Maintenance Engineering, 798–802
Winer WO (1967) Molybdenum disulfide as a lubricant: a review of the fundamental knowledge. Wear 10(6):422–452
Xu T, Zhao JZ, Xu K (1996) The ball-bearing effect of diamond nanoparticles as an oil additive. J Phys D Appl Phys 29(11):2932–2937
Zhu GP, Sun JL, Wang B, Wang YZ (2011) Study on tribological properties of the rolling fluid containing nano-MoS2 for cold rolling of steel strip. China Pet Process Pe 13(1):64–69
Acknowledgments
This work was supported by a Focused Deployment Project of Chinese Academy of Sciences (KGZD-EW-302-1), Key Technologies R and D Program of China (No. 2012BAA03B03) and National Natural Science Foundation of China (No. 21106148).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, Q., Jin, Y., Sun, P. et al. Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. J Nanopart Res 16, 2386 (2014). https://doi.org/10.1007/s11051-014-2386-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2386-2