Abstract
Spinel Li4Ti5O12 nanoparticles were prepared via a high-temperature solid-state reaction by adding the prepared cellulose to an aqueous dispersion of lithium salts and titanium dioxide. The precursors of Li4Ti5O12 were characterized by thermogravimetry and differential scanning calorimetry. The obtained Li4Ti5O12 nanoparticles were characterized using X-ray diffraction, transmission electron microscopy (TEM) and electrochemical measurements. The TEM revealed that the Li4Ti5O12 prepared with cellulose is composed of nanoparticles with an average particle diameter of 20–30 nm. Galvanostatic battery testing showed that nano-sized Li4Ti5O12 exhibit better electrochemical properties than submicro-sized Li4Ti5O12 do especially at high current rates, which can deliver a reversible discharge capacity of 131 mAh g−1 at the rate of 10 C, whereas that of the submicro-sized sample decreases to 25 mAh g−1 at the same rate (10 C). Its reversible capacity is maintained at ~172.2 mAh g−1 with the voltage range 1.0–3.0 V (vs. Li) at the current rate of 0.5 C for over 80 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that lithium ion batteries with high energy and power density have attracted much attention due to be extensively used for consumer electronic devices, portable power tools and vehicle electrifications (Kim et al. 2008). In the present commercial lithium ion batteries, graphite has been widely used as the lithium ion battery anode due to its even discharge–charge potential profile and low cost. Unfortunately, graphite has some disadvantages and cannot meet satisfactorily the performance requirements of some important applications, especially in the safety and rate performance (Qiao et al. 2008; Ge et al. 2008; Shen et al. 2006; Wang et al. 2006). As a result, alternative anode materials have attracted much interest. One promising candidate is spinel Li4Ti5O12, which has a theoretical capacity of 175 mAh g−1, a flat discharge potential of 1.56 V versus Li/Li+ and other advantages, such as low cost, environmental friendliness, excellent electrochemical properties and high safety. In addition, this spinel material, as a zero-strain insertion material, was proposed for anodes in lithium secondary batteries since there is no structural change during the insertion/extraction of lithium ions (Liu et al. 2009; Gao and Tang 2008; Gao et al. 2006). In recent years, spinel anode material Li4Ti5O12 has been extensively and deeply studied as an attractive anode material for lithium ion batteries.
However, as an insulator, the low rate capacity of Li4Ti5O12 resulting from poor electronic conductivity and its low tap density is the main impediment to commercialization. In fact, many new approaches have suggested solutions to improve the conductivity as follows (Jin et al. 2008; Gaberscek et al. 2007; Qi et al. 2009; Wang et al. 2006): (1) coating with an electron-conducting phase; (2) ionic substitution to enhance the electrochemical properties; and (3) particle size minimization while synthesizing the nano-sized particles as one of the most effective methods. Synthesizing the nano-sized Li4Ti5O12 could heighten the electrochemical performance of electrode materials, because small particle size will not only obviously shorten Li+ diffusion path and broaden the electrode/electrolyte contact surface, but also decrease the charge-transfer resistance of the electrodes (Jiang et al. 2006; Croce et al. 1998; Yi et al. 2010). However, it is very difficult to control the Li4Ti5O12 sizes via an easy way of solid-state reaction at high temperature.
We report a facile method with simple raw materials to synthesize Li4Ti5O12 nanoparticles. The rate capability of the nano-sized Li4Ti5O12 has been improved significantly compared to that of submicro-sized Li4Ti5O12 prepared by the same method. Although Yuan et al. (2009a, b, 2010) have indeed used cellulose during the preparation process, the obtained particle size is 0.2–0.8 μm and its discharge capacity is around 120 mAh g−1 at 10 C. In our paper, the transmission electron microscopy (TEM) image of the as-prepared Li4Ti5O12 used cellulose shows that all the particles are nano-sized and well distributed; the particle diameter is in the range of 20–30 nm, and discharge capacity is about 130 mAh g−1 at the same current rate (10 C).
Experimental
Synthesis of cellulose
20 mL concentrated H2SO4 (98%) and 10 mL concentrated HNO3 (68%) were mixed together and stirred, and then absorbent cotton was added and immersed in this mixture for 30 min. Thereafter, the product was washed with distilled water several times to remove other ions and the acid, and then dried at 60 °C for 12 h. The obtained cellulose was milled for further use (Shen et al. 2006).
Li4Ti5O12 preparation
42 mmol LiAc·H2O, 50 mmol TiO2 and the prepared cellulose (2 g) were put in deionized water and stirred for 6 h. Excess Li was added to compensate for lithium volatilization loss during the high temperature of synthesis (Qi et al. 2009; Huang et al. 2011). The mixture was dried at 100 °C to remove water and the solid precipitated product was obtained. Then the solid precipitated product was preheated at 300 °C for 2 h to produce powder, which was put into an agate mortar and ground with a pestle for 0.5 h to get a well-mixed powder, and then it was calcined in air at 800 °C for 10 h in a muffle furnace. The Li4Ti5O12 nanoparticles were obtained. The submicro-sized Li4Ti5O12 was synthesized as above but without adding cellulose.
Characterization
The thermal decomposition behavior of the Li4Ti5O12 precursor powders was examined by means of thermogravimetry and differential scanning calorimetry (TG-DSC; STA449F3, NETZSCH, Germany) at a heating rate of 10 °C min−1. Powder X-ray diffraction (XRD; MXP18AHF, MAC, Japan) using Cu Kα radiation (λ = 1.54056 Å) was used to identify the phase composition of the synthesized powders. The grain size and structure of the samples were observed using TEM (H-600, HITACHI, Japan) with an accelerating voltage of 100 kV. The electronic conductivity of the samples was measured by using 4-point probes resistivity measurement system (RTS-9; PROBES, China). The surface areas of the samples were estimated by Brunauer-Emmett-Teller (BET; JW-BK, China).
Electrochemical measurements
The anodes were prepared by mixing 75 wt% Li4Ti5O12 powders with 15 wt% acetylene black, and 10 wt% polyvinylidene fluoride (as binder) in N-methylpyrrolidone and with Cu foil as the current collector. Lithium foil was used as the counter electrode, and the separator was a Celgard 2400 microporous polyethylene membrane. The electrolyte was 1 M LiPF6/EC + DEC (1:1, v/v). CR2032-type coin cells were assembled in an argon-filled glove box. Galvanostatic charge and discharge were controlled between 1.0 and 3.0 V on a cell test instrument (LAND CT2001, KINGNUO, China) at room temperature. The cyclic voltammetry was carried out on a CHI600B Electrochemical Workstation (CHI, 600B, CHENHUA, China) in the potential range of 1.0–3.0 V at a scan rate of 0.1 mV s−1. Electrochemical impedance spectroscopy measurements were performed using an impedance system (IM6ex, ZAHNER ELEKTRIK, Germany). The spectrum was potentiostatically measured by applying an ac voltage of 5 mV over the frequency range from 0.01 Hz to 100 kHz.
Results and discussion
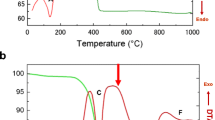
Figure 1 exhibits the result of simultaneous TG-DSC of the Li4Ti5O12 precursors obtained by adding cellulose or not. Figure 1a exhibits the result of simultaneous TG-DSC of a mixture containing LiAc·H2O and TiO2. Two significant weight losses can be detected in Fig. 1a: the first one occurs between 60 and 260 °C (∆m = 4.57%) and is associated with release of physically adsorbed water process; the second one (∆m = 16.41%) occurs between 260 and 600 °C and can be related to release of the water of crystallization and thermal decomposition of the partial reactants. Figure 1b exhibits the result of simultaneous TG-DSC of a mixture containing LiAc·H2O, TiO2 and the prepared cellulose. The TG curve of Fig. 1b presents two steps of weight loss. Release of an amount of physically absorbed water and cellulose occurs between 60 and 260 °C (∆m = 19.62%), and because of loss of cellulose, there is more weight loss than that shown in Fig. 1a. A continuous weight loss between 260 and 600 °C can be related to the release of the water of crystallization and thermal decomposition of the partial reactants. Judging from the difference of the weight loss between Fig. 1a and b, the decomposition of cellulose can be confirmed by the TG-DSC curves between 60 and 260 °C as shown in Fig. 1b. There is an obvious exothermic DSC peak at 650 °C, suggesting that the crystallization of Li4Ti5O12 forms above this temperature. Therefore, it is necessary to calcine the Li4Ti5O12 precursor above 650 °C to obtain the crystallized phase (Shen et al. 2006; Wang et al. 2006). In this study, thermal conversion into Li4Ti5O12 and subsequent crystalline growth was finished by preheating the precursor at 300 °C for 2 h to produce powder. The obtained powder was then calcined at 800 °C in air for 10 h. Figure 1c shows a small amount of weight loss of carbon in Li4Ti5O12 synthesized with cellulose. The presence of carbon can improve the electrical conductivity of Li4Ti5O12.
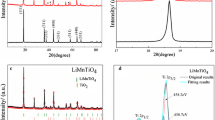
Figure 2 shows the XRD patterns for Li4Ti5O12 synthesized by adding cellulose or not, which were collected using Cu Kα radiation (λ = 1.54056 Å). The diffraction patterns confirm that both the crystal structures are coincident with the spinel Li4Ti5O12 standard in the Joint Committee on Powder Diffraction Standards database (JCPDS No. 49-0207). No impurity was detected from the XRD patterns of the samples, indicating that the two samples have the cubic spinel structure (Liu et al. 2009). From the XRD patterns, we can see that Li4Ti5O12 were synthesized successfully by adding cellulose or not, and the added cellulose does not change the cubic structure of spinel Li4Ti5O12.
TEM image of the synthesized Li4Ti5O12 without adding cellulose reveals that the particles are mainly submicro-sized, and the particle shapes are not regular as shown in Fig. 3a. Figure 3b shows the TEM image of the Li4Ti5O12 by adding cellulose, in which we can clearly see that all the particles are nano-sized, well-distributed and the particle diameter is in the range of 20–30 nm. Here cellulose works as an absorbing agent and a kind of dispersant, which could easily absorb metal ions so that the metal ions could be effectively distributed on the surface of cellulose. The aggregation of metal ions can be prevented (Shen et al. 2006). It is obvious that addition of cellulose avoids effectively the growing of crystal grain exceptionally to overcome the agglomeration of particles. The sample added cellulose can provide higher surface area and better electrical conductivity. In order to confirm this, we have carefully tested the electrical conductivity and BET surface area for comparison with both of the samples as shown in Table 1. It can been seen clearly that the surface area of the sample prepared by adding cellulose is almost three times of that of the sample without adding cellulose, and the electrical conductivity of the sample prepared by adding cellulose is higher than that of the sample without adding cellulose. The results indicate that the Li4Ti5O12 prepared with cellulose has ideal spinel structure, regular shape, narrow particle size distribution, higher surface area and electrical conductivity improved by residual carbon from calcining of cellulose.
Figure 4 shows the cyclic voltammograms (CVs) of nano-sized Li4Ti5O12 and submicro-sized Li4Ti5O12 in the potential range of 1.0–3.0 V at the scan rates of 0.1 mV s−1. Each curve clearly demonstrates that there is one pair of redox peaks in the range of 1.0–3.0 V, which is in accordance with the typical CV characteristics of spinel Li4Ti5O12. The redox peaks should be attributed to the oxidation and reduction of Ti3+/Ti4+ accompanied along with Li+ insertion/extraction in the materials (Wang et al. 2009; Yi et al. 2009). However, compared with submicro-sized Li4Ti5O12, the peak currents and integrated peak area of nano-sized Li4Ti5O12 are much higher, indicating that nano-sized Li4Ti5O12 have a higher capacity and reactivity. These confirm that the Li4Ti5O12 nanoparticles have good reversibility and the nano-sized structure is very advantageous for the transportation of Li+.
Figure 5a shows the rate performance of the nano-sized Li4Ti5O12 and submicro-sized Li4Ti5O12 at different current densities (0.1, 0.5, 1, 5 and 10 C). We have observed that nanostructured Li4Ti5O12 has a much higher discharge capacity with increasing in current density. At the lowest current rate of 0.1 C (from cycles 1–10), both the samples have a specific discharge capacity around 173 mAh g−1, which is close to the theoretical capacity, but with increasing current density from 0.5 to 10 C (from cycles 11–50), it leads to a large difference in performance, especially at 10 C; the specific discharge capacity of the nano-sized Li4Ti5O12 remains almost 128 mAh g−1, while that of the other sample decreases to 25 mAh g−1. This improvement might be attributable to the high surface area and high electrical conductivity of the nanostructured materials (Liu et al. 2009). The data indicate that the large surface-to-volume ratio of nano-sized particle enhances the kinetics of the Li4Ti5O12 electrodes. To evaluate the discharge capacity retention, the nano-sized Li4Ti5O12 was performed 80 cycles at 0.5 C (Fig. 5b). The sample shows very good discharge capacity retention and the discharge capacity retention after 80 cycles is about 99.4%. The results come from the nano-sized active materials which can supply larger electrode/electrolyte contact area. Therefore, the nano-sized Li4Ti5O12 material shows excellent discharge capacity retention through the reduction of particle size (Kim et al. 2008; Croce et al. 1998; Yi et al. 2010).
The electrochemical results are shown in terms of voltage versus discharge capacity (Fig. 6a) and voltage versus differential capacity (dQ/dV) (Fig. 6b) in the 1st and 80th discharge process at 0.5 C of nano-sized Li4Ti5O12. The 1st and 80th discharge capacities were 173.3 and 172.2 mAh g−1, respectively, with a voltage plateau at about 1.56 V versus Li/Li+. From the 1st cycle to the 80th cycle, the capacity loss is about 0.6%, which illustrates that discharge capacity retention remains at 99.4% throughout the cycling process. The voltage versus dQ/dV curves clearly display a spike peak at about 1.56 V (Fig. 6b), which corresponds to a flat discharge plateau (Fig 6a). The voltage versus dQ/dV curve over 80 cycles is nearly the same as that of the 1st cycle, which can be inferred that Li4Ti5O12 nanoparticles have better cycling behavior and stability.
The curves of Fig. 7 present typical Nyquist plots of the nano-sized Li4Ti5O12 and submicro-sized Li4Ti5O12. The plots are similar to each other in shape, with a semicircle line appearing in the high-frequency domain and a straight line in the low-frequency region. The depressed semicircle in the moderate frequency region is attributed to the charge-transfer process (Liu et al. 2009; NuLi et al. 2008). Compared with the results shown, it can be seen that the semicircle for the nano-sized sample is smaller. The charge-transfer resistance of the nano-sized Li4Ti5O12 electrode is around 140 Ω, while that of submicro-sized Li4Ti5O12 electrode is above 270 Ω. This indicates that the nano-sized particles decrease charge-transfer resistance (Maier 2007), which is in good agreement with the electrochemical test results above.
Conclusions
Nano-sized Li4Ti5O12 was obtained by adding cellulose to TiO2 and LiAc·H2O by a high-temperature solid-state reaction. The added cellulose does not affect the spinel structure. Moreover, due to the fine particle size and large surface area, nano-sized Li4Ti5O12 showed better electrochemical performance, with the good discharge capacity retention of 99.4% after the 80th cycle at 0.5 C and less inner resistance, higher reversibility and the discharge capacity of 131 mAh g−1 at 10 C, which is remarkably higher than that of the submicro-sized Li4Ti5O12.
References
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Gaberscek M, Dominko R, Jamnik J (2007) Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem Commun 9:2778–2783
Gao F, Tang ZY (2008) Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim Acta 53:5071–5075
Gao J, Jiang CY, Ying JR, Wan CR (2006) Preparation and characterization of high-density spherical Li4Ti5O12 anode material for lithium secondary batteries. J Power Sources 155:364–367
Ge H, Li N, Li DY, Dai CS, Wang DL (2008) Electrochemical characteristics of spinel Li4Ti5O12 discharged to 0.01 V. Electrochem Commun 10:719–722
Huang Y, Qi Y, Jia D, Wang X, Guo Z, Cho WI (2011) Synthesis and electrochemical properties of spinel Li4Ti5O12−xClx anode materials for lithium-ion batteries. J Solid State Electrochem. doi:10.1007/s10008-011-1611-5
Jiang CH, Hosono EJ, Zhou HS (2006) Nanomaterials for lithium ion batteries. Nanotoday 1:28–33
Jin B, Jin EM, Park KH, Gu HB (2008) Electrochemical properties of LiFePO4-multiwalled carbon nanotubes composite cathode materials for lithium polymer battery. Electrochem Commun 10:1537–1540
Kim DK, Muralidharan P, Lee HW, Ruffo R, Yang Y, Chan CK, Peng HL, Huggins RA, Cui Y (2008) Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett 11:3948–3951
Liu H, Wang GX, Park JS, Wang JZ, Liu HK, Zhang C (2009) Electrochemical performance of α-Fe2O3 nanorods as anode material for lithium-ion cells. Electrochim Acta 54:1733–1736
Maier J (2007) Size effects on mass transport and storage in lithium batteries. J Power Sources 174:569–574
NuLi YN, Zeng R, Zhang P, Guo ZP, Liu HK (2008) Controlled synthesis of α-Fe2O3 nanostructures and their size-dependent electrochemical properties for lithium-ion batteries. J Power Sources 184:456–460
Qi YL, Huang YD, Jia DZ, Bao SJ, Guo ZP (2009) Preparation and characterization of novel spinel Li4Ti5O12−xBrx anode materials. Electrochim Acta 54:4772–4776
Qiao H, Xiao LF, Zhang LZ (2008) Phosphatization: a promising approach to enhance the performance of mesoporous TiO2 anode for lithium ion batteries. Electrochem Commun 10:616–620
Shen PZ, Jia DZ, Huang YD, Liu L, Guo ZP (2006) LiMn2O4 cathode materials synthesized by the cellulose–citric acid method for lithium ion batteries. J Power Sources 158:608–613
Wang YQ, Wang JL, Wang J, Nuli YN (2006) High-rate LiFePO4 electrode material synthesized by a novel route from FePO4·4H2O. Adv Funct Mater 16:2135–2140
Wang YG, Liu HM, Wang KX, EiJi H, Wang YR, Zhou HS (2009) Synthesis and electrochemical performance of nano-sized Li4Ti5O12 with double surface modification of Ti(III) and carbon. J Mater Chem 19:6789–6795
Yi TF, Shu J, Zhu YR, Zhu XD, Zhu RS, Yue CB, Zhou AN, Zhu RS (2009) High-performance Li4Ti5−xVxO12 (0 ≤ X ≤ 0.3) as an anode material for secondary lithium-ion battery. Electrochim Acta 54:7464–7470
Yi TF, Shu J, Zhu YR, Zhu XD, Zhu RS, Zhou AN (2010) Advanced electrochemical performance of Li4Ti4.95V0.05O12 as a reversible anode material down to 0 V. J Power Sources 195:285–288
Yuan T, Cai R, Wang K, Ran R, Liu S, Shao ZP (2009a) Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. Ceram Int 35:1757–1768
Yuan T, Wang K, Cai R, Ran R, Shao ZP (2009b) Cellulose-assisted combustion synthesis of Li4Ti5O12 adopting anatase TiO2 solid as raw material with high electrochemical performance. J Alloy Compd 477:665–672
Yuan T, Cai R, Gu P, Shao ZP (2010) Synthesis of lithium insertion material Li4Ti5O12 from rutile TiO2 via surface activation. J Power Sources 195:2883–2887
Acknowledgments
This work was supported by the Natural Science Foundation of Xinjiang Province (No. 200821121), the National Natural Science Foundation of China (Nos. 21161021 and 21061013), the Technological People Service Corporation (No. 2009GJG40028), the Science and Technology Foundation of Urumqi (Nos. y08231006 and ZD8113007), the Science and Technology Foundation of Xinjiang University (No. BS100114), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (No. IRT1081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, H., Huang, Y., Jia, D. et al. Preparation and characterization of spinel Li4Ti5O12 nanoparticles anode materials for lithium ion battery. J Nanopart Res 14, 713 (2012). https://doi.org/10.1007/s11051-011-0713-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-011-0713-4