Abstract
Improvement of electrical conductivity of poly ethylene oxide (PEO)–LiI polymer electrolytes is necessary for their use in solid state lithium ion battery. In this study a new kind of PEO–LiI-based polymer electrolytes embedded with CdO nanoparticles with improved electrical conductivity has been prepared and characterized. The electron microscopic studies confirm that CdO nanoparticles of average size 2.5 nm are dispersed in the PEO matrix. The glass transition temperature of the PEO–LiI electrolyte decreases with the introduction of CdO nanoparticle in the polymer matrix. X-ray diffraction, electron microscopic, and differential scanning calorimetry studies show that the amorphous phase of PEO increases with the introduction of CdO nanoparticle and that the increase in amorphous phase is maximum for 0.10 wt% CdO doping. The electrical conductivity of the sample with 0.10 wt% CdO increases by three orders in magnitude than that of the PEO–LiI electrolyte. The electrical conductivity of PEO–LiI electrolyte embedded with CdO nanoparticle exhibits VTF behavior with reciprocal temperature indicating a strong coupling between the ionic and the polymer chain segmental motions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ion conducting polymer electrolytes have drawn much attention because of their high energy density, good flexibility, mechanical strength, good cyclability, etc., for use as electrolytes in solid state lithium ion rechargeable batteries (Scrosati et al. 2001; Tarascon and Armand 2001; Dias et al. 2000). Mainly poly ethylene oxide (PEO)–lithium salt-based polymer electrolytes (Croce et al. 1998; Meyer 1998; Xi and Tang 2004; Gadjourova et al. 2001; Stoeva et al. 2003) have been studied extensively and the ionic conductivity is the key parameter of their performance. The dissolution of lithium salt in PEO electrolyte is due to the fact that PEO chains coil around the Li+ ions and thus separates the cations from the counter anions (Dias et al. 2000). It is well established (Mao et al. 2000) that at room temperature the nature of PEO is biphasic containing both crystalline and amorphous phases and the transport of Li+ cations in the polymer matrix is related to the local relaxation and segmental motion of PEO chains. These two mechanisms are achieved when the amorphous phase of PEO is dominant. Generally PEO tends to crystallize below its melting temperature (mp 60 °C) and thus shows much higher crystalline phases near room temperature and the conductivity of these electrolytes is much lower than 10−5 Ω−1 cm−1, which restricts its application in rechargeable batteries.
Over the last two decades, many efforts have been made to increase the conductivity of these polymer electrolytes. The addition of various plasticizers, such as tetraethylene glycol (TEG), ethylene carbonate (EC), propylene carbonate (PC) etc., to the polymer matrix increases their conductivity considerably (Frech and Chintapalli 1996; Kim and Smotkin 2002). However, they suffer from several drawbacks, such as decomposition, volatilization, reaction toward lithium metal electrode and also the deterioration of the mechanical properties. The addition of ceramic nanoparticles like Al2O3, TiO2 in PEO–LiClO4 electrolyte (Croce et al. 1998, 1999, 2001) and inclusion of inorganic nanoparticles as fillers in the PEO–lithium salt-based polymer electrolytes (Wang et al. 2009; Bhattacharya and Ghosh 2008; Fan et al. 2003; Ribeiro et al. 2001; Scrosati et al. 2000; Nan et al. 2003) improves the conductivity considerably but below 10−4 Ω−1 cm−1.
In this study synthesis of PEO–LiI polymer nancomposite electrolyte embedded with different concentrations of CdO nanoparticles of average size 2.5 nm has been reported. It has been observed that the increase of amorphous phase of PEO–LiI electrolytes and hence the electrical conductivity is maximum for 0.10 wt% CdO doping concentration.
Experimental
First, CdO nanoparticle was synthesized by dissolving cadmium chloride (CdCl2) in distilled water and then adding NaOH bead into the solution under stirring condition. A white precipitation of Cd(OH)2 was produced. Then it was filtered and washed for several times to remove the insoluble residue to find Cd(OH)2 colloid. It was then heat treated at 400 °C for 24 h to obtain the CdO nanoparticles powder.
To prepare PEO–LiI electrolyte, PEO (MW = 400000, Aldrich) and appropriate amount of vacuum dried anhydrous LiI (Aldrich) were dissolved together in acetonitrile. The molar ratio of the ethylene oxide segments to the lithium ions was maintained at [EO]/[Li] = 12. After stirring for 24 h at room temperature, the solutions became very thick after solvent evaporation. Then it was cast in PTFE containers and dried in a vacuum oven at 55 °C for 36 h to form the free standing homogeneous films of about 0.01 cm thick.
Finally, the PEO–LiI-x wt% CdO nanocomposite electrolytes were prepared by dissolving appropriate amount of PEO and vacuum dried anhydrous LiI together in acetonitrile. The molar ratio of the ethylene oxide segments to the lithium ions was kept same as stated above. Then different concentrations of CdO nanoparticles (x = 0.05–0.20 wt%) were dispersed in acetonitrile separately and added into the mixture under stirring condition. After stirring for 24 h at room temperature, the solutions became very thick and free standing homogeneous films were produced following the same procedure as for PEO–LiI electrolytes as stated above.
X-ray diffraction (XRD) pattern of the CdO powder, as-prepared PEO–LiI films and PEO–LiI–CdO nanocomposite films were recorded in an X-ray diffractometer (D8 ADVANCE, BRUKER AXS) using CuKα radiation (0.154 nm wavelength) at a scan rate of 1 degree min−1. The differential scanning calorimetry (DSC) experiments were performed at a heating scan rate of 10 °C min−1 in Perkin-Elmer DSC-7 in N2 atmosphere. The samples were mounted on a specimen stub and coated with platinum and a JSM-6700F field emission scanning electron microscope (FE-SEM) (JEOL Co.), equipped with a link elemental analyzer and a silicon detector, was used for studying the surface morphology of the films. For transmission electron microscopic (TEM) studies a few drops of gel samples just before casting were sonicated in acetonitrile for 15 min in an ultrasonic bath (EYELA) and were dropped in a 300 mesh copper grid. The TEM images were taken using a high resolution TEM (HR-TEM, JEOL, model JEM 2010). Ionic conductivity of the PEO–LiI and PEO–LiI–CdO films were determined at various temperatures by ac impedance spectroscopy in the anhydrous environment in the frequency range 10 Hz–2 MHz using a RLC meter (QuadTech, model 7600). A conductivity cell containing two stainless steel blocking electrodes was used for the ac impedance measurement.
Results and discussion
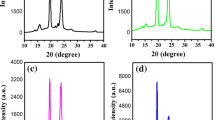
Figure 1a shows the XRD pattern of CdO powder. A perfect match of the diffraction peaks with those from JCPDS datasheet (JCPDS Datasheet No. 05-0640) indicates the absence of any impurity. The XRD patterns of the as-prepared PEO–LiI and PEO–LiI-x wt% CdO nanocomposite electrolytes are shown in Fig. 1b. No characteristic peaks for LiI have been observed in Fig. 1b, indicating that LiI forms complex with the ether oxygen of PEO (Wang et al. 2006). Two diffraction peaks (at 2θ = 19° and 23.5°) are observed for PEO–LiI electrolyte which are the characteristic peaks of PEO due to its crystalline phase. Figure 1b clearly indicates that the PEO–LiI electrolyte shows highest peak intensity, suggesting highest crystalline phase of PEO. With the addition of CdO in the PEO–LiI matrix, the peak intensity of crystalline PEO decreases and only trace of crystalline PEO has been detected for 0.10 wt% CdO nanoparticles in the PEO–LiI matrix, indicating that significant reduction in the crystalline phase of PEO and a homogeneous electrolyte with a high degree of dispersion of the CdO occurs for this composition. When the concentration of CdO is increased beyond the optimum value of 0.10 wt% the intensity of the two peaks tends to increase again, showing the increase of crystalline phase of PEO.
The DSC traces of PEO–LiI and PEO–LiI-x wt% CdO nanocomposite electrolytes are displayed in Fig. 2. The thermodynamic parameters such as glass transition temperature (T g), melting temperature (T m), and melting enthalpy (ΔH m) were obtained from the DSC traces and are summarized in Table 1. The percentage of crystalline phase of PEO (X c) has been calculated using the equation
where ΔH m is the melting enthalpy of the sample and ΔH PEO is the melting enthalpy (namely 213.7 J g−1) of completely crystallized PEO (Li and Hsu 1984). The inset of Fig. 2 shows the variation of T g with CdO doping concentration. It can be observed that when CdO nanoparticles is doped into PEO–LiI polymer electrolyte the glass transition temperature T g decreases from −36.4 °C to around −49 °C and does not change considerably with doping concentration. This result suggests improvement in the flexibility of the polymer host backbone. It is noted in Table 1 that the values of T m and ΔH m for all concentrations of CdO are lower than that for PEO–LiI electrolytes with lowest values for 0.10 wt%. A lower ΔH m value shows a reduced degree of crystalline phase. This decrease in crystallinity of PEO is due to the fact that the introduction of CdO nanoparticles lowers the reorganization tendency of polymer chain via the interaction with Li+ and PEO, promoting amorphous regions and leading to the formation of an amorphous interface area surrounding the nanoparticles (Croce et al. 1998; Nan et al. 2003). The lowest value of X c (11.8%) achieved for 0.10 wt% CdO suggests the formation of good complexation with a large ratio of amorphous phase to crystalline phase.
Figure 3 shows the FE-SEM micrographs of PEO–LiI and PEO–LiI–CdO nanocomposite electrolytes for varying concentration of CdO nanoparticle. The electrolytes exhibit a spherulite like surface structure, which is a typical morphology of a crystalline PEO with flexible chains. The amorphous phase of PEO is also observed in the gap between spherulites. The small pores are caused by the fast evaporation of the acetonitrile solvent during the preparation process.
The average size (diameter) of spherulites for PEO–LiI electrolytes (Fig. 3a) is found to be 120 μm. Also the spaces in between the spherulites i.e., the amorphous regions of PEO in this electrolyte are very narrow. It is interesting to note that when CdO is inserted into PEO–LiI electrolyte the number of PEO spherulites increases and the average size of spherulites decreases to about 50 μm (Fig. 3b–e), which are both beneficial for the decreasing of the crystalline phase of PEO (Xi et al. 2005, 2006). CdO acts as a nucleation site of spherulite and provides an obstacle for the spherulite growth (Jeong et al. 2007). It is observed that the size of the spherulites does not change appreciably with the variation of CdO concentration, but the change of amorphous phase between spherulites is significant. A dramatic improvement of the amorphous phase of PEO is observed when 0.10 wt% CdO is doped into the matrix (Fig. 3c). However, further increase of CdO content decrease the amorphous region between the spherulites. The increased amount of amorphous phase in between the spherulites is very much important for conduction of Li+ ions.
The transmission electron micrographs for the two samples of PEO–LiI–CdO nanocomposite electrolytes with 0.05 and 0.10 wt% CdO are shown in Fig. 4a and b, respectively. The TEM images show that CdO nanoparticles are distributed in the polymer matrix. The histograms for the distribution of the particle size, calculated from TEM images, are shown in Fig. 4c and d, respectively, for these samples. The average size of the particles has been obtained from the fits of the data to the log normal distribution (Soderlund et al. 1998) given by the following relation
where r is particle diameter, ρ is the geometric mean diameter, and σ is the dimensionless geometric standard deviation. The average size of the particles obtained from the fits is 2.5 nm in both the cases. Similar distribution of particles has been observed with the same average particle size for other CdO contents.
TEM images of a PEO–LiI-0.05 wt% CdO polymer electrolytes and b PEO–LiI-0.15 wt% CdO polymer electrolytes. c and d are histograms for distribution of CdO nanoparticles shown for PEO–LiI-0.05 wt% CdO polymer electrolytes and PEO–LiI-0.15 wt% CdO polymer electrolytes, respectively. The solid curves are the fits to lognormal function (Eq. 2)
Figure 5 shows the variation of the dc conductivity at T = 303 K, obtained from complex impedance plot, as a function of CdO concentration. The dc conductivity first increases sharply with the increase of CdO concentration and attains a maximum value for 0.10 wt% CdO and then decreases beyond this concentration. It may be noted that the dc conductivity (3 × 10−4 S cm−1) for nanocomposite electrolyte with 0.10 wt% CdO is about three orders in magnitude higher than that of the PEO–LiI electrolyte. Basically, the ion transport in PEO–Li salt-based electrolyte occurs due to the movement of Li+ ions through the amorphous phase of PEO (Croce et al. 1998, 1999; Xi and Tang 2004). For the present PEO–LiI–CdO, XRD, SEM, and DSC studies show that when 0.10 wt% CdO nanoparticles is doped into the PEO–LiI matrix the amorphous phase of PEO attains its maximum value. For the present compositions the incremental amorphous phase increases the movement of Li+ ions through the matrix which in turn increases the dc conductivity and the highest conductivity of the composition with 0.10 wt% of CdO is due to the maximum amorphous phase for this composition. The decrease in the conductivity for higher CdO concentration is attributed to the fact that at higher concentration of CdO, the aggregation of the nanoparticles takes place due to inter particle interaction which in turn provides a blockage on the transport of charge carriers (Wang et al. 2009; Fan et al. 2003; Nan et al. 2003; Kumar et al. 2001).
The variation of logarithmic electrical conductivity for all compositions with reciprocal temperature is shown in Fig. 6. An Arrhenius type of behavior is obtained for PEO–LiI electrolyte with activation energy of 1.98 eV. Other authors (Itho et al. 2003; Ramesh et al. 2002) also observed such type of behavior in polymeric system below melting point. But the conductivity variation of CdO-doped electrolytes has been well described by the Vogel–Tamman–Fulcher (VTF) relation (Vogel 1921; Tamman and Hesse 1926; Fulcher 1925) expressed as
where σ 0 is the conductivity pre-exponential factor, k B is the Boltzman constant, E a is the Vogel activation energy, T 0 is the Vogel scaling temperature, and T is the absolute temperature. The conductivity data for the CdO-doped electrolytes were fitted to Eq. 3 using non linear least square fits. The fitting parameters σ0, E a, and T 0 are shown in Table 2. Generally, the value of (T g−T 0) is of the order of 50 K for polymeric system (Angell 1983; Natesan et al. 2006). The values of T 0 obtained from the fit are about 40 K lower than the values of T g. The VTF model proposes that the ionic conductivity is favored as a result of the segmental motion of the polymer chain in the matrix. A reasonably good fit of the conductivity to VTF relation over a wide range of temperature clearly indicates that a strong coupling exists between the ionic and the polymer chain segmental motions of the investigated CdO-doped electrolytes (Ribeiro et al. 2001; Fragiadakis et al. 2008).
Variation of dc conductivity with reciprocal temperature of PEO–LiI-x wt% CdO polymer electrolytes: square for x = 0; circle for x = 0.05; triangle for x = 0.10; inverted triangle for x = 0.15, and times symbol for x = 0.20. The solid lines are the VTF fits (Eq. 3)
Conclusions
In this study, novel PEO–LiI polymer nanocomposite electrolyte embedded with CdO nanoparticles with improved electrical conductivity has been synthesized. CdO nanoparticles of average size 2.5 nm have been prepared by thermal route. For a doping concentration of 0.10 wt% CdO into the PEO–LiI matrix, the increase of amorphous phase of PEO is maximum. At this doping level the nanocomposite electrolyte forms a good complexion and a significant enhancement of the dc conductivity has been observed. However, beyond this concentration the conductivity decreases due to blocking effect on the transport of ions resulting from aggregation of nanoparticles. The temperature dependence of the dc conductivity of the CdO-doped electrolytes is well explained by the VTF relation.
References
Angell CA (1983) Fast ion motion in glassy and amorphous materials. Solid State Ionics 9–10:3–16
Bhattacharya S, Ghosh A (2008) Effect of ZnO nanoparticles on the structure and ionic relaxation of poly(ethylene oxide)–LiI polymer electrolyte nanocomposites. J Nanosci Nanotech 8:1922–1926
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Croce F, Curini R, Martinelli A, Persi L, Ronci F, Scrosati B (1999) Physical and chemical properties of nanocomposite polymer electrolytes. J Phys Chem B 103:10632–10638
Croce F, Persi L, Scrosati B, Fiory FS, Plichta E, Hendrickson MA (2001) Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrchimica Acta 46:2457–2461
Dias FB, Plomp L, Veldhuis JBJ (2000) Trends in polymer electrolytes for secondary lithium batteries. J Power Sources 88:169–191
Fan L, Dang Z, Wei G, Nan CW, Li M (2003) Effect of nanosized ZnO on the electrical properties of (PEO)16LiClO4 electrolytes. Mater Sci Eng B 99:340–343
Fragiadakis D, Dou S, Colby RH, Runt J (2008) Molecular mobility, ion mobility, and mobile ion concentration in poly(ethylene oxide)-based polyurethane ionomers. Macromolecules 41:5723–5728
Frech R, Chintapalli S (1996) Effect of propylene carbonate as a plasticizer in high molecular weight PEO-LiCF3SO3 electrolytes. Solid State Ionics 85:61–66
Fulcher GS (1925) Analysis of recent measurements of the viscosity of glasses. J Am Ceram Soc 8:339–355
Gadjourova Z, Andreev YG, Tunstall DP, Bruce PG (2001) Ionic conductivity in crystalline polymer electrolytes. Nature 412:520–523
Itho T, Miyamura Y, Ichikawa Y, Uno T, Kubo M, Yamamoto O (2003) Composite polymer electrolytes of poly(ethylene oxide)/BaTiO3/Li salt with hyperbranched polymer. J Power Sources 119–121:403–408
Jeong SS, Lim YT, Choi YJ, Cho GB, Kim KW, Ahn HJ, Cho KK (2007) Electrochemical properties of lithium sulfur cells using PEO polymer electrolytes prepared under three different mixing conditions. J Power Sources 174:745–750
Kim YT, Smotkin ES (2002) The effect of plasticizers on transport and electrochemical properties of PEO-based electrolytes for lithium rechargeable batteries. Solid State Ionics 149:29–37
Kumar B, Scanlon LG, Spry RJ (2001) On the origin of conductivity enhancement in polymer–ceramic composite electrolytes. J Power Sources 96:337–342
Li X, Hsu SL (1984) An analysis of the crystallization behavior of poly(ethylene oxide)/poly(methyl methacrylate) blends by spectroscopic and calorimetric techniques. J Polym Sci Polym Phys Ed 22:1331–1342
Mao G, Perea RF, Howells WS, Price DL, Saboungi ML (2000) Relaxation in polymer electrolytes on the nanosecond timescale. Nature 405:163–165
Meyer WH (1998) Polymer electrolytes for lithium-ion batteries. Adv Mater 10:439–448
Nan CW, Fan L, Lin Y, Cai Q (2003) Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys Rev Lett 91:266104
Natesan B, Karan NK, Katiyar RS (2006) Ion relaxation dynamics and nearly constant loss behavior in polymer electrolyte. Phys Rev E 74:042801
Ramesh S, Yahaya AH, Arof AK (2002) Dielectric behaviour of PVC-based polymer electrolytes. Solid State Ionics 152–153:291–294
Ribeiro R, Silva GG, Mohallen NDS (2001) A comparison of ionic conductivity, thermal behaviour and morphology in two polyether–LiI–LiAl5O8 composite polymer electrolytes. Electrchimica Acta 46:1679–1686
Scrosati B, Croce F, Persi L (2000) Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J Electrochem Soc 147:1718–1721
Scrosati B, Croce F, Panero S (2001) Progress in lithium polymer battery R&D. J Power Sources 100:93–100
Soderlund J, Kiss LB, Niklasson GA, Granqvist CG (1998) Lognormal size distributions in particle growth processes without coagulation. Phys Rev Lett 80:2386–2388
Stoeva Z, Litas IM, Staunton E, Andreev YG, Bruce PG (2003) Ionic conductivity in the crystalline polymer electrolytes PEO6:LiXF6, X = P, As, Sb. J Am Chem Soc 125:4619–4626
Tamman G, Hesse W (1926) The dependence of viscosity upon the temperature of supercooled liquids. Z Anorg Allg Chem 156:245–257
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Vogel H (1921) Temperature dependence of viscosity of melts. Z Phys 22:645–646
Wang YJ, Pan Y, Wang L, Pang MJ, Chen L (2006) Characterization of (PEO)LiClO4-Li1.3Al0.3Ti1.7(PO4)3 composite polymer electrolytes with different molecular weights of PEO. J Appl Poly Sci 102:4269–4275
Wang L, Yang W, Wang J, Evans DG (2009) New nanocomposite polymer electrolyte comprising nanosized ZnAl2O4 with a mesopore network and PEO–LiClO4. Solid State Ionics 180:392–397
Xi J, Tang X (2004) Nanocomposite polymer electrolyte based on poly(ethylene oxide) and solid super acid for lithium polymer battery. Chem Phys Lett 393:271–276
Xi J, Qiu X, Zheng S, Tang X (2005) Nanocomposite polymer electrolyte comprising PEO/LiClO4 and solid super acid: effect of sulphated-zirconia on the crystallization kinetics of PEO. Polymer 46:5702–5706
Xi J, Qiu X, Wang J, Bai Y, Zhu W, Chen L (2006) Effect of molecular sieves ZSM-5 on the crystallization behavior of PEO-based composite polymer electrolyte. J Power Sources 158:627–634
Acknowledgments
One (AK) of the authors acknowledges the Council of Scientific and Industrial Research, Government of India, for providing him research fellowship (via Grant no. 09/080(0573)/2007-EMR-I) for the work. The support by the Department of Science and Technology, Government of India through its nanoscience initiative program is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karmakar, A., Ghosh, A. Poly ethylene oxide (PEO)–LiI polymer electrolytes embedded with CdO nanoparticles. J Nanopart Res 13, 2989–2996 (2011). https://doi.org/10.1007/s11051-010-0194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0194-x