Abstract
Manual handling of nanoparticles is a fundamental task of most nanomaterial research; such handling may expose workers to ultrafine or nanoparticles. Recent studies confirm that exposures to ultrafine or nanoparticles produce adverse inflammatory responses in rodent lungs and such particles may translocate to other areas of the body, including the brain. An important method for protecting workers handling nanoparticles from exposure to airborne nanoparticles is the laboratory fume hood. Such hoods rely on the proper face velocity for optimum performance. In addition, several other hood design and operating factors can affect worker exposure. Handling experiments were performed to measure airborne particle concentration while handling nanoparticles in three fume hoods located in different buildings under a range of operating conditions. Nanoalumina and nanosilver were selected to perform handling experiments in the fume hoods. Air samples were also collected on polycarbonate membrane filters and particles were characterized by scanning electron microscopy. Handling tasks included transferring particles from beaker to beaker by spatula and by pouring. Measurement locations were the room background, the researcher’s breathing zone and upstream and downstream from the handling location. Variable factors studied included hood design, transfer method, face velocity/sash location and material types. Airborne particle concentrations measured at breathing zone locations were analyzed to characterize exposure level. Statistics were used to test the correlation between data. The test results found that the handling of dry powders consisting of nano-sized particles inside laboratory fume hoods can result in a significant release of airborne nanoparticles from the fume hood into the laboratory environment and the researcher’s breathing zone. Many variables were found to affect the extent of particle release including hood design, hood operation (sash height, face velocity), work practices, type and quantity of the material being handled, room conditions, and the adequacy of the room exhaust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The laboratory fume hood is one of the most important pieces of equipment for protecting laboratory personnel from exposure to hazardous substances that they may be working with. A properly designed and operated fume hood should isolate the worker from any gases or aerosols released from the operation inside the hood, and reduce his/her exposure to close to zero. A hood that is not designed and/or operated properly, on the other hand, could expose the worker to hazardous levels of those materials. In fact, for materials such as airborne nanoparticles, which are not visible and have no other warning properties, the worker may have a false sense of security in feeling that the hood is offering good protection when it in fact is not.
Research involving nanoparticles is increasing dramatically, meaning that the risk of worker exposure is increasing at the same rate. Many organizations, including ours, have published Best Practices documents containing recommendations for working safely with nanoparticles (Ellenbecker 2007; DOE 2007). All of these documents recommend that operations be carried out in fume hoods whenever possible. Most do not caution the user about potential problems associated with hood use.
A recent survey of nanotechnology work practices published by the International Council on Nanotechnology (ICON) stated that two-thirds of the reporting organizations reported using fume hoods in the handling of nanomaterials. Over half (32 of 51) of companies reported using fume hoods, while two-thirds (4 of 6) of research labs and all university labs reported their use. Also, the report indicated that the larger organizations with greater than 250 people directly handling nanomaterials all use fume hoods, while only half (53%) of organizations with less than nine persons handle nanomaterials in fume hoods (Gerritzen, et al. 2006).
For handling dry powder nanomaterials, an appropriate ventilation system can significantly reduce inhalation exposure to nanomaterials. Fume hoods are the most popular ventilation systems available in most laboratories. Ideally, airborne nanoparticles will quickly be captured and removed through the air flow when nanoparticle powder is manipulated in the fume hood. The high surface to volume ratio of a nano-sized particle causes it to react almost instantaneously to the drag force of the hood air flow (Hinds 1999). Airborne nanoparticles should move exactly with the air around them, so that any particles could only escape if air is escaping the hood. Several standard methods have been developed to evaluate hood performance and the potential for contaminant escape. These methods (BSI 1994; CEN 2003; ANSI/ASHRAE 1995; ANSI 2003) typically rely on a tracer gas with no worker present, so their applicability to the actual handling of nanoparticles in a fume hood is uncertain. Therefore, a test of handling nanomaterials in the fume hood seems necessary to evaluate the efficiency of fume hoods under actual working conditions.
Fume hood design has evolved, so that several different basic types can now be found in laboratories. All hoods have a movable sash (usually vertical but sometimes horizontal), and most have adjustable back baffles. Most ventilation researchers consider sash position, hood face velocity, and airflow patterns inside the hood to be important variables affecting hood performance. If face velocities are too low, cross-drafts outside the hood can disrupt performance and allow nanoparticles given off inside the hood to escape. Excessively high velocities greater than 0.8 m/s can also be problematic (ANSI 2003).
Kim and Flynn (1991a, b, 1992) and Flynn and Ljungqvist (1995) concluded that counterrotating eddies occur on the downstream side of the worker as air flows around the worker toward the hood, and the reverse flow can pull the airborne nanoparticles back into the worker’s breathing zone. The end of the reverse flow zone reaches at least two body widths downstream of the worker and implies that a hand-held contaminant source cannot escape the influence of the re-circulating flow. Kim and Flynn (1991a, b, 1992), Flynn and Ljungqvist (1995), and Tseng et al. (2007) have studied wake effects associated with exposure and the leakage of contaminants in hood use. The deficit of those studies is the lack of consideration for actual dynamic motions of workers’ arms.
Air flow patterns inside the hood can also affect particle release. Pathanjali and Rahman (1996) used numerical modeling to demonstrate that air flow entering a hood moves to the back wall, proceeds upward to the top surface, moves forward to the sash, and moves downward once again in a vertical eddy. When the sash in their model hood was only 25% open, this re-circulating air eddy was maximized and reached the sash opening. In this case the vertical eddy could interact with the worker wake eddy, causing particle release.
Since many researchers at the NSF-funded Center for High-rate Nanomanufacturing (CHN)Footnote 1 use fume hoods when working with nanoparticles, a study was initiated to investigate the effectiveness of typical fume hoods in protecting against nanoparticle exposure. The purpose of this article is to report on the results of that study, and to recommend additional steps to improve hood performance.
Experimental conditions
Materials and hoods
Aluminum oxide and silver nanoparticles were used for this study. Aluminum oxide (Al2O3) nanoparticles, grade Al-015-003-025 (Nanophase Technologies Corporation, Romeoville, IL), also called nanoalumina, were manufactured using physical vapor synthesis (PVS).Footnote 2 They appear roughly spherical in shape, and have a reported density of 3,600 kg/m3 and primary particle size ranging from 27 to 56 nm; when dried, these particles formed agglomerates in the bulk material with a nominal size of 200 nm.
The silver (Ag) nanoparticles (Nano Dynamics Inc.) used in this study were engineered for increased electrical conductivity in low-temperature processing scenarios.Footnote 3 Nanosilver particles were manufactured as a grayish-white powder, appear roughly spherical in shape, have density of 10,500 kg/m3 and an average particle size of 60 nm.
Particle handling was studied using a conventional hood, a by-pass hood, and a constant velocity hood (ACGIH 2007). With no special features to help control the hood face velocity, a conventional hood has a constant air flow, and the hood face velocity varies inversely with the height of the sash opening. The studied conventional hood has a full open sash face dimension of 62 cm H × 130 cm W.
A by-pass hood attempts to maintain a constant velocity through the use of a by-pass grille located above the hood opening and attached to the hood exhaust fan. When the sash is wide open it blocks the by-pass grille, and as the sash is lowered the grille is uncovered, allowing increasing amounts of air to flow through the by-pass grille instead of the hood face, thus helping to maintain a constant face velocity. The full open sash face dimensions of the studied by-pass hood are 71 cm H × 218 cm W.
A constant velocity hood, also called a variable air volume (VAV) hood, uses a motor controller to vary the fan speed as the sash is moved in order to maintain a constant hood face velocity. The sash of the studied constant velocity hood has dimensions of 69 cm H × 163 cm W.
Measurements taken in the three hoods were taken at conditions of similar relative humidity as shown in Table 1. Crossdraft velocities were detected in the vicinity of both the by-pass and constant velocity hoods (Table 1). The crossdraft at the by-pass hood was caused by an air conditioner installed on the side wall which caused the air flow across the open sash with a velocity up to 0.18 m/s. The crossdraft at the constant velocity hood, up to 0.13 m/s, was less intense than the cross flow at the by-pass hood. Conditioned air supplied from the ceiling, located in front of the hood, caused air to flow periodically across the open sash.
Methods
Particle handling and cleaning
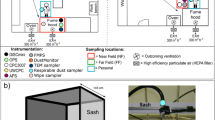
Particle handling was performed in each hood by pouring or transferring particles from beaker to beaker, as shown in Fig. 1. Experiments were performed using 100 g and 15 g of nanoalumina and 15 g nanosilver. The transferring task was performed by using a spatula to transfer nanoparticles from one beaker to another beaker; 0.7–1.2 g of nanoalumina were loaded at the open top of beaker for each spatula transfer. For the pouring task, nanoparticles were poured directly from one beaker into a second beaker at the center of the open top, so that the feeding and receiving beakers were adjacent to each other at the open edge. For handling 100 g nanoalumina, particles were transferred between 400 ml beakers. Pouring 100 g nanoalumina took about 1 min and transferring took about 4.5 min. For 15 g nanoalumina handling, 100 ml beakers were used. Pouring 15 g nanoalumina took about 20 s and transferring took about 90 s. For handling 15 g of nanosilver, 50 ml beakers were used because the higher-density particles had less volume. Nanoparticle-contaminated surfaces inside the hood were cleaned after completion of all handling tasks. A wet paper towel saturated with water was used to wipe up the spilled nanoparticle powder.
Measurement locations
Measurements were taken at a background location, source locations, and the researcher’s breathing zone under different sash locations. Background particle concentration was measured 1 m in front of the hood. Breathing zone concentration was measured near the researcher’s nose. Nanopowder handling tasks were performed in the hood on the work platform 15 cm back from the sill. Particle concentrations at the source were measured at upstream and downstream sides of releasing source. Upstream and downstream measuring locations were at 8 cm vertically above the beaker at the upstream and downstream edge, at respectively. Measurement locations are illustrated in Fig. 2.
For the conventional hood, sash locations for measurement were at full open, half open, and low chest height, which were 62, 44, and 16.5 cm open sash, respectively. The sash locations for the by-pass hood were at full open and half open which were 71 and 42 cm open, respectively (the low chest height was not tested because this sash position was beyond the range of the compensating grille). The sash locations of the constant velocity hood were at half open and low chest height of 38 and 17 cm open, respectively (the full open position was not tested because the constant face velocity could not be maintained at that position).
Real-time particle measurement
The concentrations of airborne nanoparticles were measured using the Fast Mobility Particle Sizer (FMPS®) spectrometer (Model 3091, TSI) in the range from 5.6 to 560 nm, with a total of 32 channels of resolution (16 channels per decade). The FMPS spectrometer performs particle size classification based on differential electrical mobility classification. Particle concentration and size distribution were recorded every second. Normalized particle number concentrations were calculated in each size channel based on the average concentration during each measuring time period. The background concentration measured before each experiment was used as the baseline for subtraction from the source concentration. A three meter length of Tygon® tubing was connected to the air inlet of FMPS to reach measuring locations.
Filtration sampling method
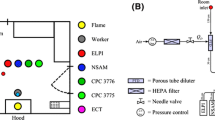
The FMPS measurements yield detailed information about airborne particle size distribution, but give no information about particle morphology and elemental composition. To provide this information, a new nanoparticle aerosol filter sampler was developed and used in these experiments. A schematic layout of the sampling setup is shown in Fig. 3. Transmission electron microscope (TEM)-copper grids (400 mesh with a titanium dioxide film) and carbon and copper tapes were taped onto 47 mm diameter polycarbonate membrane filters (0.2-μm pore size). Fiber backing filters were used to support the polycarbonate filters. Air flow was driven by a personal sampling pump at a flow rate of 0.3 L/min. This relatively low air flow was used for two reasons: the low total sample volume ensured that collected samples were not overloaded with larger particles, and the low filtration velocity increased particle residence time in the filter cassette and enhanced the collection of smaller particles by Brownian motion.
Particle characterization
Scanning Electron Microscope (SEM) and Scanning Transmission Electron Microscope (STEM) images of the samples were taken using a JSM-7401F Field emission scanning electron microscope (JEOL, Peabody, MA). The STEM images were obtained using a Transmitted Electron Detector (TED) attachment to the JSM-7401F and with the microscope operated at an accelerating voltage of 20 kV. The SEM images were obtained at an accelerating voltage of 1–15 kV of electron beam energy. Elemental analysis was performed using an Energy Dispersive Spectroscopy (EDS) attachment of the SEM (EDAX, Mahwah, NJ) and a primary electron beam excitation energy of 10 kV. In addition, samples were coated with a thin layer of Au using a Denton Vacuum Desk IV cold sputter unit to decrease sample charge build up during analysis by SEM.
Experiments performed
Variables studied in this research include handling method, handling quantity, nanomaterial type, face velocity and hood type. Variable face velocities, either dependent or independent of sash location, depending on hood type, were studied as shown in Table 2.
Results
Effects of quantity and face velocity on particle release
Particle number concentration measured at the researcher’s breathing zone using the conventional hood increased significantly during 100 g nanoalumina particle handling. The particle number concentrations measured during handling 100 g nanoalumina particles in the conventional hood using transferring and pouring methods are plotted in Figs. 4a and b, respectively. In order to distinguish the increase in the particle concentration during the handling tasks, the particle number concentration at the background measured before experiment was subtracted from Figs 4a and b. The resulting increases in particle concentrations as a function of particle diameter are plotted in Figs. 4c and d. For measurements at various face velocities, all size distributions showed likely bimodal curves with one peak at particle size of 200 nm which was the most common size of agglomerated nanoalumina in the bulk material. The exposure concentration increase using the spatula transferring method showed similar size distribution and magnitude at all three velocities (Fig. 4c). While pouring (Fig. 4d), more particles were carried out of the hood during handling at the highest face velocity of 1.0 m/s when the sash was lowered to the researcher’s low chest height. The increase in particle number concentration measured at the breathing zone during pouring was as high as 13,000 particles/cm3.
Breathing zone concentration during handling100 g nanoalumina particles in the conventional hood: (a) Transferring, (b) Pouring; Concentration increase in the breathing zone: (c) Transferring, (d) Pouring. Y-axis: Relative normalized particle number concentration calculated using measured concentration subtracting average background concentration. X-axis: Diameter of the average particle size in each channel of the FMPS
For handling 15 g nanoalumina particles in the conventional hood, concentrations at the breathing zone were consistently lower compared to handling of 100 g nanoalumina particles, as can be seen in Fig. 5. For both transferring and pouring, the highest exposure occurred at the face velocity of 1.0 m/s when the concentration peaked at 500- 600 particles/cm3 above background levels.
Typical particles collected on copper tapes during handling 100 and 15 g nanoalumina are shown in Figs. 6a and b, respectively. Particle sizes were found in a broad range from submicrometer to several micrometers. Many nanoparticles in the form of large agglomerates were collected at the worker’s breathing zone during handling 100 g nanoalumina as can be seen in Fig. 6a, while fewer nanoalumina particles were collected during handling 15 g nanoalumina particles, as shown in Fig. 6b.
For handling 100 g nanoalumina particles in the by-pass hood, the increase in particle concentration as a function of particle diameter is plotted in Fig. 7. Measurements at the breathing zone were taken at two face velocities; the increased concentrations measured at the full open sash position were greater than the concentrations measured at the half-open sash position. The size distributions showed multiple peaking curves with one peak at particle size of 200 nm, which was the most common size of agglomerated nanoalumina in the bulk material.
Particle accumulation from hood leakage
The change in total concentration at the worker’s breathing zone during handling nanoparticles is plotted as a function of time in Fig. 8, after subtracting the breathing zone concentration at time t = 0 just before handling particles began. The four measurements were taken at a relative humidity of 48–63% (Table 3). The concentrations climb with time for experiments using 100 g handling in the conventional and by-pass hoods, while the concentrations during handling 15 g nanoalumina particles remained close to background concentrations throughout the experiments. Particle total concentration changes during handling tasks in three different hoods are shown in Fig. 8. Concentrations measured in the conventional hood gradually increased during both 100 g and 15 g nanoalumina handling and stayed at a high concentration level at the end of the 100 g handling task. Nanoparticle exposure was significant for all measurements during handling particles in the 20-year-old conventional hood. For handling 100 g nanoalumina in the by-pass hood, the concentration increase was less than that measured in the conventional hood. The 15 g handling tasks performed in the constant velocity hood did not generate accumulated particles as detected by the FMPS, but a concentration increase while handling in the conventional hood can be seen between 10 and 20 min after handling 15 g nanoalumina.
Results indicate that the breathing zone particle concentration was the highest when working in the conventional hood while handling 100 g nanoalumina. For the 100 g handling task, particle concentration at the worker’s breathing zone accumulated due to excess nanoparticles that were generated inside the hood and carried out of the hood resulting in a dramatic increase of nanoparticles at worker’s breathing zone. This also results in an increased particle concentration in the laboratory air right after completing handling experiments as can be seen in Fig. 9.
Cleaning the contaminated work surface inside the hood was the main task performed after completion of the nanoparticle handling experiments. Concentrations in the general laboratory air after completing handling tasks increased greatly while cleaning spilled particles at the hood workplace. Three experiments at different environmental conditions were performed in the conventional hood and the results showed that particle concentrations were reduced slightly at 8 min after completing experiment and rose quickly during cleaning between 14 and 21 min after experiment; concentrations declined after cleaning was complete.
Particle concentration during cleaning in a low relative humidity (19%) environment was much higher than in a high relative humidity (52%) environment for 100 g handling experiments. The 15 g handling experiment yielded significantly lower particle release into the laboratory air.
Effect of nanomaterial type
As shown in Fig. 10, significant numbers of agglomerated nanoalumina particles peaking at 200 nm appeared on the downstream side for all measurements. However, nanosilver particles with agglomerated size peaking at 150 nm were detected on just one of four measurements (Fig. 11). Although FMPS concentration data collected during handling 15 g nanosilver showed that only one measurement was significant, few nanosilver agglomerates were found among particles collected at worker’s breathing zone as can be seen in Figs. 12a and b. Since collected silver particles were several micrometers in diameter and no particles in the nanometer size range were found on the filters, most likely the bulk nanosilver material undergoes intense agglomeration as seen in Fig. 12c.
Discussion
The results of this series of experiments indicate the potential for significant exposures when handling nanoparticles in fume hoods. The magnitude of any such exposure is highly dependent on several variables, including hood type, hood face velocity/sash position, the actual material being handled, the quantity of that material being handled, the nature of the handling activity, external crossdrafts, and relative humidity.
Effect of sash height and face velocity on hood containment
These tests indicate that a standard hood design with a constant air flow, where the face velocity varies inversely with the sash height, presents a greater possibility for particle release compared to more modern hood designs incorporating features such as constant face velocity and improved baffle design. Our results suggest that sash heights both below and above the optimum range may lead to nanoparticle release. Greenley et al. (2000) concluded that the percent leakage increased by factors of 28–300 when the sash height is raised above the breathing zone; however, no correlation between average face velocity and containment was observed in their study. The containment problem at a lower sash height with higher velocities was not addressed in the Greenley study. Our study showed that the highest exposure during handling nanoalumina particles in the conventional hood occurred at the lowest sash height with a face velocity of 1.0 m/s. Exposures at half and full open sash were minor compared to operating at low sash height.
It appears that when handling nanopowders, the optimum range for hood face velocity is 0.4–0.6 m/s (80–120 ft/min), which is consistent with the velocity range recommended by the American Conference of Governmental Industrial Hygienists (ACGIH 2007) and ANSI 2003. Values below this range are subject to room air currents and rapid operator movements that can pull nanoparticles out of the hood, while values above this range create excessive turbulence in the wake of the worker that can also pull nanoparticles out of the hood.
A recent study showed that complicated turbulent flow patterns were induced around the bottom edge of the sash, side rails and doorsill, where the containment leakage was highest in a conventional fume hood (Tseng, et al. 2006). Poorly designed hoods, such as the conventional hood tested in this study, can have large vertical eddies that can carry nanoparticles released by the handling tasks up the back of the hood, across the top and then down toward the sash opening, where they can interact with room air currents and/or turbulent wake eddies.
The turbulent wake eddies are created between the worker and the hood face by exhaust air drawn into the hood past the worker. These eddies, also called a bluff-body wake, (Tseng, et al. 2006) can draw contaminants from eddy regions in the hood near the sash opening where nanoparticles can accumulate due to the interior hood eddy described above. When considering both the exterior eddy—which is a strong function of the hood face velocity, and the interior eddy—which depends on hood design and the position of the sash, it is apparent that particle release must be a complicated function of the interaction between the hood vertical eddy, the worker wake eddy, external crossdrafts, the position of the sash, hood face velocity, and worker activities, such as hand and arm motions, that may be occurring at the interface between the two eddies. In these tests, measurements showed that the conventional fume hood had the highest particle release to the breathing zone among the three hood types (Fig. 12). A large vortex region circulating downward and reaching the sash bottom was observed in the conventional hood using a fog test; the vortex (Pathanjali and Rahman 1996) carried many particles into the wake region which then flowed out of the hood and toward the worker’s breathing zone.
As a further complication, external air motion such as crossdrafts can interact with the two eddy patterns, causing further air escape from the hood interior; this effect should predominate at low face velocities and/or high sash positions. ANSI 2003 contends that excessive crossdraft velocities above 50% of the average face velocity significantly affect hood containment; ANSI further states that ideally, crossdraft velocities should be less than 30% of the face velocity. In our experiments, the crossdraft velocity of 0.2 m/s at the by-pass hood exceeded the 50% of face velocity of 0.3 m/s at full open sash. This excessive crossdraft velocity may have affected the air flow toward hood and caused additional particle release whereas the crossdraft velocity would be less likely to affect air flow at half-open sash face velocity of 0.5 m/s.
The above discussion highlights many potential problems that can be caused by adverse airflow patterns in and around some hoods, such as the conventional and by-pass hoods studied in this research. On the other hand, a well-designed hood, operated at an optimum face velocity, such as the constant velocity hood tested here, can offer excellent protection to the operator. Very few nanoparticles were found to escape from this hood under any of the test conditions.
Effect of handling method on hood containment
Both transferring and pouring methods were performed in the three hoods, and the measurements taken inside and outside hoods are summarized in Table 3. The Table shows that for the 100 g experiments, pouring consistently produced more airborne particles inside the hood than transferring. In addition, pouring or handling 100 g produced significantly more airborne particles than pouring or handling 15 g. For the 15 g experiments, airborne concentrations inside the hood were about the same for both handling methods, and breathing zone concentration showed no correlation with handling method.
Pouring is a more energetic handling method that results in significant higher total particle concentrations measured at both downstream and upstream sides compared to the transferring method. The exposure level at the worker’s breathing zone, however, was affected by the interaction of several factors. The worker’s hand and arm motions associated with the handling methods and the vortex area inside the hood interacted with the airflow in the wake region in front of worker resulting in a range of exposures to the worker. In the conventional hood the key factor contributing to particle release was that the hood recirculation zone extended to the sash opening. Therefore, since the pouring method operating in this conventional hood generated more nanoparticles in the hood air than the transferring method, it resulted in higher concentrations measured at the breathing zone.
However, the energetic pouring method could cause less exposure when the vortex was minimized; in our experiments, the transferring method caused a higher concentration at the breathing zone (Table 3) in the by-pass hood. In this case the number of particles escaping the hood was affected primarily by the hand motion and the reverse airflow in the wake region, since the hood vortex did not reach the wake region. Transferring 100 g nanoalumina took about 4 min to complete the task while pouring 100 g nanoalumina took less than 1 min; more opportunities thus were created to carry particles out through frequent dynamic motion of the worker’s hands and arms when continuously transferring particles between beakers by spatula. However, there were no measurable difference between handling methods in the number of particles released when handling 15 g nanoalumina particles in the conventional and constant velocity hoods (Table 3); the exposure level was controlled by the low number of particles initially released during handling (see the following section). Although the difference in particle number concentrations between handling methods was not significant, the results of the Pearson correlation test on total number concentration between handling method gave a low correlation coefficient of less than 0.25 (Table 3). In other words, transferring and pouring methods yield different patterns of particle release and the correlation of exposure magnitude to handling method is not clear. These results illustrate the importance of training laboratory personnel in the use of procedures that minimize the potential for particle release inside the fume hood.
Effect of material quantity and type on particle release
These tests confirm the logical finding that handling larger quantities of nanopowders increased the airborne release of nanoparticles, leading to a greater number escaping the hood and reaching the worker’s breathing zone. The larger quantity handled (100 g) is more than six times greater than the smaller amount (15 g), but the ratio of the number of particles released is much greater than a factor of six. However, since only two quantities were used in these pilot studies, it is not possible to correlate particle release with quantity handled with any degree of certainty.
Considering the type of nanoparticle handled, the reason more nanoalumina particles than nanosilver were detected inside the hood while pouring (Figs. 10, 11) is likely to be different flow patterns of nanoalumina and nanosilver when dumping bulk nanoparticle agglomerates into the beaker (Hemeon 1963). In addition, the volume of nanoalumina used is about three times the volume of nanosilver at the 15 g handling task, yielding a larger number of nanoalumina particles being handled than nanosilver. The low density nanoalumina agglomerates fall more slowly in bulk flow and are less affected by gravity than the bulk silver particles. The displaced air stream inside the beaker flowing upward while dumping alumina particles behaved differently than when dumping nanosilver. Upward-flowing air easily penetrated the plug flow of the falling nanoalumina nanoparticles, whereas the denser nanosilver particles fell as one mass, making air penetration more difficult. This resulted in many more nanoalumina particles being entrained into the displaced air stream compared to the number of nanosilver particles. Due to this, compared to nanosilver, nanoalumina particles were carried out in dramatically larger numbers into the air inside the hood and re-circulated in the vortex. The frequent dynamic motions of handling nanoparticles in the wake region carried nanoparticles out to the worker’s breathing zone, and this effect was much more pronounced for nanoalumina than for nanosilver.
Laboratory background concentrations
Nanoparticles released and carried out of the hoods accumulated in the laboratory air; for the laboratory with the conventional hood, one-half to 2 h for 15–100 g handling passed before the particle number concentration gradually returned to the baseline concentrations present before the experiments. The rate of particle clearance is a function of the general exhaust airflow in the laboratory; in this case, inadequate general exhaust caused the particles to clear slowly and increased the potential nanoparticle exposure of laboratory personnel.
Effect of humidity on particle release
When handling nanoalumina, low humidity caused more small agglomerate nanoparticles to become airborne during handling. This result is in a good agreement to the Tsai et al. (2008) study of the effect of humidity on the agglomeration of releasing nanoalumina particles during compounding of alumina nanocomposites. These results are to be expected for highly hygroscopic materials such as nanoalumina particles.
Relevance of hood performance tests to nanoparticle release
The standard test for fume hood performance, ANSI/ASHRAE 110-1995, “Method of Testing Performance of Laboratory Fume Hoods,” (ANSI/ASHRAE 1995) uses a tracer gas such as sulfur hexafluoride (SF6) to measure hood containment. ANSI/AIHA Standard Z9.5-2003, “American National Standard for Laboratory Ventilation,” (ANSI/AIHA 2003) requires that a hood tested using 110-1995 produce an SF6 concentration measured at the breathing zone of less than 0.1 ppm; this corresponds to approximately 1012 SF6 molecules/cm3. In order to produce an SF6 concentration approaching this value, a considerable amount of air must leak from the hood. The breathing zone particle number concentrations measured in this study of greater than 10,000 particles/cm3, when converted to a volume fraction, is eight orders of magnitude lower than the SF6 molecular concentration. Thus, a hood that has passed the ASHRAE test could easily be releasing many thousands of nano-sized particles into the researcher’s breathing zone. Due to the current lack of adequate toxicity data, it is not known whether nanoparticle exposures at the level measured in this study present an actual risk to human health. On the other hand, the documentation of significant exposures to fume hood operators found here is cause for concern, at least until health effects are better understood and exposure standards are established.
Adequacy of current hood designs
These tests also raise the possibility that standard fume hood designs may prove to be inadequate to protect researchers while working with dry nanopowders. It may be necessary to use more sophisticated designs, such as the air-curtain fume hood recently developed by Huang et al. (2007) that is specifically designed to reduce or eliminate all turbulent air penetrations from the fume hood.
Conclusions and recommendations
The tests performed here demonstrate conclusively that the handling of dry powders consisting of nano-sized particles inside laboratory fume hoods can result in a significant release of airborne nanoparticles from the fume hood into the laboratory environment and the researcher’s breathing zone. It appears that many variables affect the extent of particle release including hood design, hood operation (sash height, face velocity), work practices (e.g., pouring versus transferring by spatula, fast movement versus slow movement), type and quantity of the material being handled, room conditions (e.g., magnitude and direction of cross-drafts, humidity level), and the adequacy of the room general exhaust.
Although the pilot tests indicate that many variables can adversely affect hood performance when handling nanoparticles, it appears that several generalizations should prove to be true in most cases:
-
Fume hoods should be operated with a face velocity between 0.4 and 0.6 m/s (80–120 ft/min);
-
Sash heights should remain as low as possible when manipulating nanomaterials, within this velocity range;
-
Constant velocity hoods are a better design than compensating hoods, which in turn are better than standard fume hoods;
-
Efforts should be made to reduce/eliminate room air currents in the vicinity of the hood; and
-
Nanoparticle operations inside fume hoods should be performed using the smallest possible quantity of nanoparticles and the least energetic handling methods.
The work discussed here was a pilot study meant to develop basic data on the performance of laboratory fume hoods when handling nanoparticles. The results are of concern, and indicate the need for a systematic study of the variables that affect fume hood performance when working with nanoparticles. The results also suggest that more sophisticated hood designs, such as the air curtain fume hood, may be required when working with dry nanoparticles.
Notes
CHN is a collaboration among the University of Massachusetts Lowell, Northeastern University and the University of New Hampshire.
Materials were retrieved from manufacturer’s website, http://www.nanophase.com/technology/capabilities.asp (2007).
Materials were retrieved from manufacturer’s website, http://www.ndmaterials.com/silver.php (2007).
References
American Conference of Governmental Industrial Hygienists (ACGIH) (2007) Industrial ventilation, a manual of recommended practice, 26th edn. ACGIH, Cincinnati, pp 13–40
American National Standards Institute (ANSI) and American Industrial Hygiene Association (AIHA) 2003. ANSI/AIHA Laboratory ventilation standard Z9.5. American Industrial Hygiene Association, American National Standards Institute, Inc.
American National Standards Institute (ANSI) and American Society of Heating Refrigeration and Air Conditioning Engineers (ASHRAE) (1995) Method of testing performance of laboratory fume hoods (ANSI/ASHRAE Standard 110–1995). ASHRAE, Atlanta
British Standards Institution (BSI) (1994) Laboratory fume cupboards. Part 4. Method for determination of the containment value of a laboratory fume cupboard (BS 7258: Part 4: 1994). BSI, London
Department of Energy (DOE) Nanoscale Science Research Centers, Approach to Nanomaterial ES&H, Revision2, June 2007, http://www.sc.doe.gov/bes/DOE_NSRC_Approach_to_Nanomaterial_ESH.pdf
Ellenbecker MJ (2007) Interim best practices for working with nanoparticles. Center for High-rate Nanomanufacturing, University of Massachusetts Lowell
European Committee for Standardization (CEN) (2003) Fume upboards-Part 3: type test methods (prEN 14175–3). CEN, Brussels
Flynn MR, Ljungqvist B (1995) A review of wake effects on worker exposure. Ann Occup Hyg 39:211–221
Gerritzen G, Huang L, Killpack K, Mircheva M, Conti J (2006) A review of current practices in the nanotechnology industry, phase two report: survey of current practices in the nanotechnology workplace. International Council on Nanotechnology. http://cohesion.rice.edu/CenterAndInst/ICON/emplibrary/ICONNanotechSurveyFullReduced.pdf
Greenley PL, Billings CE, DiBerardinis LJ, Edwards RW, Barkley WE (2000) Containment testing of laboratory hoods in the as-used condition. Appl Occup Environ Hyg 15:209–216. doi:10.1080/104732200301719
Hemeon W (1963) Plant and process ventilation, 2nd edn. The industrial Press, New York
Hinds WC (1999) Aerosol technology, 2nd edn. Wiley, New York
Huang RF, Wu YD, Chen HD, Chen CC, Chen CW, Chang CP et al (2007) Development and evaluation of an air-curtain fume cabinet with considerations of its aerodynamics. Ann Occup Hyg 51:189–206. doi:10.1093/annhyg/mel051
Kim TH, Flynn MR (1991a) Airflow pattern around a worker in a uniform freestream. Am Ind Hyg Assoc J 52:287–296. doi:10.1080/15298669191364749
Kim TH, Flynn MR (1991b) Modeling a worker’s exposure from a hand-held source in a uniform freestream. Am Ind Hyg Assoc J 52:458–463. doi :10.1202/0002-8894(1991)052≤0458:MAWEFA≥2.0.CO;2
Kim TH, Flynn MR (1992) The effect of contaminant source momentum on a worker’s breathing zone concentration in a uniform freestream. Am Ind Hyg Assoc J 53:757–766. doi :10.1202/0002-8894(1992)053≤0757:TEOCSM≥2.0.CO;2
Pathanjali C, Rahman MM (1996). Study of flow patterns in fume hood enclosures. Proceedings of the 31st intersociety energy conversion engineering conference
Tsai S, Ashter A, Ada E, Mead JL, Barry CF, Ellenbecker MJ (2008) Control of airborne nanoparticles released during compounding of polymer nanocomposites. In press, NANO
Tseng LC, Huang RF, Chen CC, Chang CP (2007) Aerodynamics and performance verifications of test methods for laboratory fume cupboards. Ann Occup Hyg 51:173–187. doi:10.1093/annhyg/mel057
Tseng L, Huang RF, Chen C, Chang C (2006) Correlation between airflow patterns and performance of a laboratory fume hood. J Occup Environ Hyg 3:694–706. doi:10.1080/15459620601015695
Acknowledgment
Authors would like to acknowledge the financial support from the Nanoscale Science and Engineering Centers for High-rate Nanomanufacturing funded by the National Science Foundation (Award No. NSF-0425826).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, SJ.(., Ada, E., Isaacs, J.A. et al. Airborne nanoparticle exposures associated with the manual handling of nanoalumina and nanosilver in fume hoods. J Nanopart Res 11, 147–161 (2009). https://doi.org/10.1007/s11051-008-9459-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9459-z