Abstract
Electrophoretic deposition (EPD) has been used to combine multi-walled carbon nanotubes of diameter in the range 20–30 nm and commercially available TiO2 nanoparticles (23 nm particle size) in composite films. Laminate coatings with up to four layers were produced by sequential EPD, while composite coatings were obtained by electrophoretic co-deposition of carbon nanotubes and TiO2 nanoparticles, respectively. Scanning electron microscopy was used to characterize the resultant microstructures. The mechanism of EPD of carbon nanotube/TiO2 nanoparticle composites is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
New fields of research based on carbon nanotubes (CNTs) and CNT containing materials are the focus of considerable efforts worldwide (Kaneto et al. 1999). The high potential of CNTs for applications in functional and biomedical devices is based on their nanomorphology and outstanding mechanical and electrical properties (Kaneto et al. 1999; Berber et al. 2000; Krishnan et al. 1998; Deheer et al. 1995).
Titanium dioxide (TiO2) is a common biocompatible ceramic used for coatings of metal implants to achieve anti-bacterial effect or corrosion resistance, as well as high biocompatibility (Kokubo et al. 2003; Cui et al. 2005). Photocatalysis is another field where TiO2 is used, the crystalline phase anatase being a very effective photocatalyst (Hashimoto et al. 2005; Jitianu et al. 2004).
The combination of CNTs and TiO2 nanoparticles can lead to interesting novel composite materials and devices with relevant properties for a variety of functional and biomedical applications. It has been reported, for example, that the combination of CNTs with TiO2 can enhance the photocatalytic activity of TiO2 (Jitianu et al. 2004). CNTs are excellent carrier substrates for TiO2 nanoparticles, due to their high structural integrity and the high surface area they provide due to the possibility of building a mesoporous structure. A recombination of the e−/h+ pairs produced by irradiation of the TiO2 is less likely, as the 1-D structure of the CNTs leads to high conductivity and so to a high percolation of the electrons. This explains why CNTs may increase the quantum yield of the photocatalytic process. Yu et al. (2005) reported that functionalized CNTs on one hand increase the band gap leading to less recombination between the e−/h+ pairs and on the other hand increase the number of OH- radicals due to the large number of hydroxyl groups available.
Multilayer coatings of CNTs and TiO2 nanoparticles might find also applications in biomedical implant coatings and tissue engineering scaffolds. CNTs have been reported to be biocompatible (Zanello et al. 2006) and they support the proliferation of osteoblast cells. Other studies have demonstrated that porous structures containing CNTs can be used as scaffolds for tissue engineering (Corre-Duarte et al. 2004; Aoki et al. 2006).
Several processing methods for the production of ceramic nanocomposites and laminates are available. From the commercial and practical point of view, electrophoretic deposition (EPD) offers several advantages. EPD is a cost-effective and process efficient technique for fabrication of free-standing objects and coatings from particulate materials, which is applicable to complex component shapes and large dimensions (Boccaccini et al. 2006). EPD can be seen as two combined processes: first the migration of charged particles dispersed in a liquid medium (electrophoresis) under an applied electric field and secondly the coagulation process of the particles at the electrode (Van der Biest et al. 1999). In order to obtain a homogenous deposition it is essential that the suspension used is a well-dispersed and stable system. The development of EPD as a processing method for manipulating CNTs (Thomas et al. 2005; Du et al. 2002) and TiO2 nanoparticles (Kaya et al. 2005; Boccaccini et al. 2004) has started in recent years. There has been, however, no previous research done in the field of co-deposition of CNTs and TiO2 nanoparticles or on producing multilayer coatings consisting of CNTs and TiO2 nanoparticles by EPD.
Experimental
The optimization of suspension parameters for obtaining a stable and well-dispersed CNT suspension was carried out in a separate investigation (Chicatún et al. 2007). Multiwalled CNTs in dried powder form (Yorkpoint New Energy Sci. & Tech. Department Co. Ltd., Guangzhon, China) were used. Figure 1 shows a TEM image of the as-received CNTs, showing the presence of entangled multiwalled CNTs of diameter 20–30 nm. The suspension used for EPD was prepared using 100 ml distilled water, 0.6 g CNTs, 0.15 ml Triton X-100 and 6 ml iodine (99.999%, Aldrich). The mixture was ultrasonicated for at least 4 h and then centrifuged at 3,000 rpm at 20°C for 15 min, in order to eliminate possible CNT agglomerates. The recipe for the suspension of TiO2 nanoparticles in acetylacetone was taken from our previous studies (Boccaccini et al. 2004). The TiO2 nanopowder used was grade P25 (Degussa AG, Germany) which has a mean particle diameter of 23 nm. The theoretical densities of CNTs and TiO2 used in this investigation were 1.65 g/cm3 (Shaffer et al. 1998) and 3.96 g/cm3 (Fredel and Boccaccini 1996), respectively
For electrophoretic co-deposition, suspensions containing various volume ratios of CNT to TiO2 were prepared and ultrasonically mixed for at least 4 h. The following CNT:TiO2 volume ratios were investigated 1:4, 1:3, 1:2 1:1, 1:0.25 and 1:0.1. The electrodes were stainless steel (316L) foils with the following dimensions: 10 × 10 × 0.2 mm3. The stainless steel substrates were cleaned with acetone and distilled water in an ultrasonic bath before the deposition process. The deposition time for all experiments was set at 4 min and all EPD experiments were carried out at constant voltage conditions. The optimized electric field strength used for the single deposition of CNTs and TiO2 nanoparticles was 55 and 2.5 V/cm, respectively. The optimized electric field strength for the co-deposition varied between 20 and 55 V/cm. All samples were dried in horizontal position in air at room temperature. For the laminate coatings the deposited layers were dried for at least 20 h before deposition of the next layer.
The cross-sections of the deposits were observed by scanning electron microscopy (SEM) using a LEO Gemini Field Emission Gun-Scanning Electron Microscope (FEG-SEM, Carl Zeiss, Hertfordshire, UK). The coatings produced by co-deposition were further characterized by thermogravimetric analysis (TGA) (Perkin Elmer Pyris 1) in order to investigate the relative content of the deposited materials. The following TGA programme was used: heating rate of 10°C/min up to 1,000°C with an oxygen gas flow of 20 ml/min.
Results
EPD of CNTs and TiO2 layers
As mentioned previously, the optimal electric field strength to obtain successful CNT films was 55 V/cm. It was observed that increasing deposition time yielded a linear increase in thickness of the CNT films. A SEM image of the as-produced, randomly oriented homogeneous CNT films can be seen in Fig. 2. It was found that higher voltages produced more homogenous CNT films exhibiting uniform thickness. However, electric field strength above 55 V/cm resulted in excessive gas evolution at the depositing electrode (anode) leading to heterogeneous CNT film formation. On the other hand, CNT films produced from electric fields lower than 10 V/cm were non-uniform and they tended to detach from the electrode when the samples were removed from the suspension.
The adhesion of the CNT films to the substrate was qualitatively investigated by using adhesive tape as reported by Thomas et al (2005). In samples produced using the optimal EPD conditions, it was found after removal of the tape that the CNTs remained adhered to the substrates with minimal disruption of the CNT films. The result indicates good interfacial adhesion strength between the CNTs and the stainless steel substrate. The reasons for this qualitatively high adhesion using the current EPD technique may be related to the formation of metal hydroxides at the electrode surface during EPD (Van der Biest et al. 1999; Thomas et al. 2005). These hydroxides could then hydrogen bond to the surfaces of the CNTs.
Laminate composites by sequential EPD
Two and four layer CNT–TiO2 laminates were produced and their cross-sections were analysed. As the cross-sections for SEM examination were obtained by bending the substrate, the applied stress could induce delamination and cracking of the deposits. Therefore qualitative conclusions about the adhesion and structural behaviour can be drawn from observation of fracture surfaces. For example, the fracture surface of the two layer laminate showed that the upper layer, which consisted of TiO2 nanoparticles, delaminated but the CNT layer stayed adherent on the stainless steel substrate. This indicates that the bending stress induced was high enough for the delamination of the TiO2 layer and for crack development within that layer. On the other hand, a higher stress would be required for the delamination of the CNT layer.
SEM images of the four layer cross-section (Fig. 3) showed debonding of the first CNT layer from the stainless steel substrate. The bending stress induced seemed to increase due to the presence of the second CNT layer. However, higher stresses could be reached without debonding in the CNT–TiO2 nanoparticle interface region. As observed in Fig. 3, the CNT layer seems to be acting as a crack inhibiting barrier. Figure 4 shows the interface region between the third CNT layer and the adjacent TiO2 nanoparticle layers. The thickness of the CNT layers is about 1μm and that of the titania layers about 16 μm. Indeed, the effect of the CNT layer as possible reinforcement element in the sintered laminate composite remains to be investigated.
Composite coating by electrophoretic co-deposition
Suspensions for the co-deposition of CNTs and TiO2 nanoparticles were prepared containing different volume concentrations of CNTs and TiO2 nanoparticles. A stable dispersion was achieved by sonication for at least 4 h. No sedimentation was observed within the first hour for low titania concentrations and within minutes for higher amounts of titania.
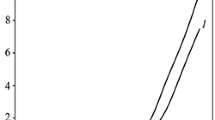
SEM observation of the deposited cross-sections reveals that the quality of the coatings varies strongly. The coatings for the volume ratios 1:4 (Fig. 5a), 1:3 and 1:1/10 (Fig. 5b) exhibit a homogenous distribution of CNTs and TiO2 throughout the whole thickness with a high amount of porosity. The coatings for the concentration ratios (CNT:TiO2) 1:2, 1:1 and 1:1/4 did not exhibit a good distribution of the nanoparticles. The coatings seemed to be very dense and have excessive surfactant incorporated, as the titania nanoparticles were almost not visible under SEM. This could be due to the stability problems of the suspension during the actual EPD process. TGA was performed to investigate if the coatings contained the same CNT–TiO2 volume concentration ratio as the starting suspensions. The experimentally determined values were compared to the ideal ones, which were taken to be those of the fresh suspensions, as shown in Fig. 6. In all cases the experimental values were found to be higher than those of the starting suspensions, e.g. more titania nanoparticles than expected had been deposited. The increasing relative concentration of titania in the suspension is thought to be responsible for this behaviour, even if the value for the suspension with volume ratio 1:4 is in fairly good agreement with the expected ratio.
By further analysis of the SEM images (e.g. in Fig. 5) it was possible to observe that titania nanoparticles were individually infiltrated into the porous CNT films rather than adsorbed onto individual CNTs. The mechanisms involved in electrophoretic co-deposition can be explained with a schematic diagram as shown in Fig. 7 describing the motion of the nanoparticles in suspension. The first region, namely the “approaching trajectory” (Stoll et al. 2006), occurs in the suspension at a given distance away from the electrodes, where both CNTs and titania particles move towards the deposition electrode only under the influence of the externally applied electric field. The second region, the “infiltration trajectory” (Stoll et al. 2006), occurs close to the deposition electrode where the charge of the deposited CNT films influences the motion of the charged particles. Knowing that both CNTs and particles possess the same charge, there are repulsive forces acting between particles and CNTs as schematically shown in Fig. 7. Due to the applied external electric field, each negatively charged particle is attracted to the previously deposited CNT film because this is fixed (adhered) to the depositing electrode. However, the particles are repelled before they can reach the CNT surfaces due to the charges on the CNTs. It can be hypothesized that under the effect of the repulsive forces due to the surrounding CNTs, the particles will follow the path with the fewest possible obstacles until reaching the next interstice between adjacent CNTs. A similar mechanism has been proposed for the infiltration of aluminium fibre mats by Al2O3 particles (both positively charged) in a recent investigation (Stoll et al. 2006). Thus, when the particles reach the electrode or the surface of previously deposited particles, they have no further possibility to move and so the electrophoretic ceramic deposit grows with certain extent of porosity.
This mechanism of infiltration could be equally applied to the sequential EPD process described above, where the titania nanoparticles initially infiltrate the porous CNT layer and then build up to form a second layer. Schematic illustrations summarizing the two experimental approaches investigated in this work, e.g. sequential EPD and electrophoretic co-deposition, are shown in Fig. 8.
Conclusions
The objective of this work was to develop composites of multiwalled CNTs and TiO2 nanoparticles by sequential EPD and electrophoretic co-deposition. The development of a novel stable aqueous suspension containing both components for EPD was presented and the electrophoretic co-deposition of CNTs and titania nanoparticles was demonstrated for the first time. It was shown that it is possible to produce CNT–TiO2 laminate coatings using sequential EPD on stainless steel substrates. The adhesion strength of the CNT layer to the stainless steel substrate was shown to be qualitatively higher than the strength of the interface between the CNT and TiO2 layers, as demonstrated for the two-layer composite coating. Investigation of four-layer coatings indicated that in the non-sintered material the CNT layer may reinforce the ceramic coating by providing a crack deflection and delamination path. Further work should be done on sintered laminates and on the quantitative investigation of the mechanical properties of the coatings. Experiments evaluating the electrical conductivity and the photocatalytic properties of these coatings are of interest too.
References
Aoki N, Yokoyama A, Nodasaka Y, Akasaka T, Uo M, Sato Y et al (2006) Strikingly extended morphology of cells grown on carbon nanotubes. Chem Lett 25:5–7
Berber S, Kwon YK, Tomanek D (2000) Unusually high thermal conductivity of carbon nanotubes. Phys Rev Lett 84(20):4613–4616
Boccaccini AR, Karapappas P, Marijuan JM, Kaya C (2004) TiO2 coatings on silicon carbide and carbon fibre substrates by electrophoretic deposition. J Mater Sci 39:851–859
Boccaccini AR, Roether JA, Thomas B, Shaffer MSP, Chavez E, Stoll E (2006) The electrophoretic deposition of inorganic nanoscaled materials. J Ceram Soc Jpn 114:1–14
Chicatún F, Cho J, Schaab S, Brusatin G, Colombo P, Roether JA, Boccaccini AR (2007) Carbon nanotube (CNT) deposits and CNT/SiO2 composite coatings by electrophoretic deposition. Adv Appl Ceram in press
Correa-Duarte MA, Wagner N, Rojas-Chapana J, Morsczeck C, Thie M, Giersig M (2004) Carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett 4:11–15
Cui C, Liu H, Li Y, Sun J, Wang R, Liu S, Greer L (2005) Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater Lett 59:3144–3148
Deheer WA, Chatelain A, Ugarte D (1995) Carbon nanotube field-emission electron source. Science 270(5239):1179–1180
Du C, Heldbrandt D, Pan N (2002) Preparation and preliminary property study of carbon nanotubes films by electrophoretic deposition. Mater Lett 57:434–438
Fredel MC, Boccaccini AR(1996) Processing and mechanical properties of biocompatible Al2O3 platelet reinforced TiO2. J Mater Sci 31:4375–4380
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: A historical overview and future prospects. Jpn J Appl Phys Part 1–Regul Pap Brief Commun Rev Pap 44:8269–8285
Jitianu A, Cacciaguerra T, Benoit S, Delpeux S, Béguin F, Bonnamy S (2004) Synthesis and characterization of carbon nanotubes-TiO2 nanocomposites. Carbon 42:1147–1151
Kaneto K, Tsuruta M, Sakai G, Cho WH, Ando Y (1999) Electrical conductivities of multi-wall carbon nano tubes. Synth Met 103(1–3):2543–2546
Kaya C, Kaya F, Su B, Thomas BJC, Boccaccini AR (2005) Structural and functional thick ceramic coatings by electrophoretic deposition. Surf Coatings Technol 191:303–310
Kokubo T, Kim HM, Kawashita M (2003) Novel bioactive materials with different mechanical properties. Biomaterials 24(3):2161–2175
Krishnan A, Dujardin E, Ebbesen TW, Yianilos PN, Treacy MM (1998) Young’s modulus of single-walled nanotubes. J Phys Rev B 58(20):14013–14019
Shaffer MSP, Fan X, Windle AH (1998) Dispersion and packing of carbon nanotubes. Carbon 36:1603–1612
Stoll E, Mahr P, Krüger H-G, Kern H, Thomas BJC, Boccaccini AR (2006) Fabrication technologies for oxide–oxide ceramic matrix composites based on electrophoretic deposition. J Euro Ceram Soc 26:1567–1576
Thomas BJC, Shaffer MS, Boccaccini AR (2005) Multi-walled carbon nanotube coatings using electrophoretic deposition (EPD). J Am Ceram Soc 88:980–986
Van der Biest O, Vandeperre LJ (1999) Electrophoretic deposition of materials. Annu Rev Mater Sci 29:327–356
Yu Y, Yu JY, Yu J-G, Kwok Y-C, Che Y-K, Zhao J-C et al (2005) Enhancement of photocatalytic activity of mesoporous TiO2 by using carbon nanotubes. Appl Catal A 289:186–196
Zanello LP, Zhao B, Hu H, Haddon RC (2006) Bone cell proliferation on carbon nanotubes. Nano Lett 6:562–567
Acknowledgements
The authors would like to thank financial support from the European Commission via Network of Excellence “Knowledge-based Multicomponent Materials for Durable and Safe Performance” (KMM-NoE, NMP3-CT-2004-502243).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, J., Schaab, S., Roether, J.A. et al. Nanostructured carbon nanotube/TiO2 composite coatings using electrophoretic deposition (EPD). J Nanopart Res 10, 99–105 (2008). https://doi.org/10.1007/s11051-007-9230-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-007-9230-x