Abstract
This work was based on the analysis of digital images of histochemical profile from subcutaneous lesions in sporotrichosis (ST) and chromoblastomycosis (CM) patients. An additional aim was the detection of carbohydrate expression using lectin histochemical analysis of the different carbohydrates in the fungal cell wall from four different species (Sporothrix schenckii, Fonsecaea pedrosoi, Phialophora verrucosa, and Cladophialophora carrionii) associated with diseases mentioned earlier. Slides from tissue biopsies from ST and CM positive patients (n = 10, each) were stained according to routine techniques. Slides were incubated with 25 μg/ml of Con A lectins and WGA conjugated to peroxidase. Digital image analysis was carried out in a workstation using OPTIMAS™ software system. Routine histochemistry results indicated that there is significantly higher collagen deposition and elastic fibers in ST characteristic lesions compared with that found in CM cases. The ST interstitial fibrosis area was larger than in CM lesions. Comparative lectin binding showed a positive and intense lectin staining pattern in the cell wall of S. schenckii, suggesting a higher expression of glucose/mannose and N-acetyl glucosamine in their cell surface as evidenced by Con A and WGA, respectively. However, these lectins were not effective to recognize some carbohydrates moieties in the F. pedrosoi, P. verrucosa, and C. carrionii. Such findings contribute to additional information about specific recognition processes between fungal parasites and their host cell targets may be mediated by the interaction of carbohydrate-binding proteins, such as lectins, on the surface of one type of cell that combine with complementary sugars on the surface of another cells into fibro-connective tissues associated with lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subcutaneous mycoses include a heterogeneous group of infection characterized by the development of lesions at the inoculation site. In some diseases, slow extension via lymphatic channels is a frequent occurrence (sporotrichosis); in others, hematogenous and lymphatic dissemination is rarely recorded (chromoblastomycosis) [1].
The diagnosis difficulty of the mycoses is related to several factors, such as subcutaneous lesions macroscopically similar, different etiologic agents, a wide variety of lesions, and their morphologic subtypes [2]. Based on that, immunohistochemical methods have been tested as a support tool for the differential diagnosis of mycoses [3, 4].
In fungus taxonomy, cell wall constitution may be an additional distinguishing criterion. Alongside techniques such as chemical extraction, enzymatic digestion followed by an analysis of hydrolysis products, comparison of antigen fractions using immune sera or by electron microscopy, lectin binding has proved to be a valuable method for the characterization of the cell wall sugar profile [5, 6]. Moreover, lectins interact with carbohydrates, favoring the understanding of pathobiochemistry alterations and progression of tumors originated by mycoses [7, 8].
Furthermore, specific recognition between fungal parasites and their host cell target may be mediated by the interaction of carbohydrate-binding proteins, as lectins on the surface of one type of cell, which combine with complementary sugars on the surface of another cell [9].
Recently, many studies were developed in our laboratories aiming the investigation of different pathologic process [10–12], indicating an important contribution of those macromolecules in this biomedical analysis.
In addition, image analysis system has been used as a support to the interpretation of results obtained from histochemistry techniques, which supplies qualitative and quantitative data related to histomorphology of lesions and anatomy of etiologic agents from skin infections [13–15]. Among advantages of the image analysis, one can include increased precision of measurement of pathological changes; evaluation of effects from different methods of histological processing; determination in the way; and standard sizes for teaching and diagnosis as well as maximization as research tool [11, 16, 17].
The present work aims to investigate the histochemical pattern of subcutaneous lesions in patients with sporotrichosis and chromoblastomycosis and the carbohydrate profile expressed in the cell wall of Sporothrix schenckii Hektoen and Perkins, Fonsecaea pedrosoi (Brumpt) Negroni, Phialophora verrucosa Medlar, and Cladophialophora carrionii (Trejos) using lectin histochemistry.
Materials and Methods
Case Selection
Biopsies from both sexes (mean age 40 years) diagnosed with sporotrichosis (ST; n = 10) and chromoblastomycosis (CM; n = 10), presenting granulomatous lesions, were selected from the Dermatology Service from the University Hospital located in Federal University of Pernambuco (UFPE) and from the Integrated Center of Anatomy and Pathology (CIAP) of the State University of Pernambuco (UPE). The study was approved by the Research Ethics Committee of UFPE, according to the Declaration of Helsinki.
Microorganisms and Growth Conditions
Sporothrix schenckii (URM 2865), Fonsecaeapedrosoi (URM 3161), Phialophoraverrucosa (URM 2884), and Cladophialophoracarrionii (URM 2871) cultures were obtained from URM Culture Collection of Department of Mycology, Biological Sciences Center (UFPE) and have been stocked in mineral oil (Sherf, 1943). Viability test and taxonomic review were carried out by inoculating cultures in test tubes containing 5 ml of 2% dextrose broth [18], under non-stationary conditions, at 28°C, for 30 days. The organisms were indentified by observing macromorphologic (coloration, texture and colony diameter) and micromorphologic (somatic and reproductive structures) characteristics through incubation in potato dextrose agar broth (Difco) [19].
Histochemistry
Histological slices (4 μm) were submitted separately to the following staining techniques hematoxylin and eosin (HE), tricomic of Masson (TM), periodic acid of Shiff (PAS) and Van Gienson (VG) according to Spicer [20].
Lectin Histochemistry
Fungi studied were adhered to glass slides previously treated with 3-amine-propyltriethoxy-silane—APES, (Sigma Chemical Co, USA). Sporothrix schenckii, Fonsecaea pedrosoi, Phialophora verrucosa, and Cladophialophora carrionii, adhered to slides, were treated with a 2% trypsin solution for 1 h at 37°C and a methanol-H2O2 solution for 15 min, followed by incubation with Concanavalin A (Con A, 25 μg/ml) and wheat germ agglutinin (WGA, 25 μg/ml) lectins, both conjugated the peroxidase at 4°C for 3 h. Peroxidase was visualized using diaminobendizine–H2O2 solution for 5–8 min. Samples were counter stained with hematoxylin and evaluated through optical microscopy. Between each step, samples were washed twice (5 min each) with a solution of 0.01 M sodium phosphate buffer, pH 7.2, containing 0.15 m NaCl.
Digital Image Analysis
Digital image analysis (triplicates) was carried out using a digital video camera (Model KT2050-I, Sony, Japan) connected to a microscope (OLYMPUS, Model BH-2, Japan). Images were processed using OPTIMAS™ version 6.1 (Optimas Corporation, USA). All parameters were set according to manufacturer’s instruction. The morphometric parameters evaluated were total medium area (μm2) and number of particles for field captured in the histological slice (in pixels).
Staining control (tissues treated with sugar-inhibited lectins and normal skin samples) was obtained to minimize distortions in values due to the presence of non-marked cells, thus a correction factor (CF) was applied according to the equation CF = s/S, in which s means relative area of the surface and S, the total measured area [10, 12].
In cases which tissues presented total areas with similar and better histomorphologic conditions, the summary of lectin-binding cells per area was developed in three random areas of stained tissues (total area analyzed for each slice = 12,234 μm2).
Lectin-binding patterns were used to calculate (%) the mean area (weak stain = 15–20%; moderate stain = 25–55%; intense stain = 60–95%). Semiquantitative optical analysis was carried out taking into account the intensity of staining pattern indicated as weak (+), moderate (++), or intense (+++), according to Özer [21].
Statistical Analysis
Histochemistry staining patterns and morphometric parameters obtained from digital image analysis were evaluated using t-Student and Tukey analysis (P < 0.05) through the software PRISM™ 3.0.
Results
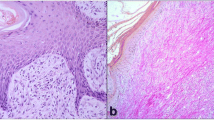
Results confirmed a large deposition of elastic fibers and collagen (Fig. 1) in the subcutaneous lesions found in sporotrichosis when compared with those observed in chromoblastomycosis. These data are also observed via the mean area of interstitial fibrosis (Table 1). The glycosaminoglycan expression, evidenced by periodic acid of Schiff, was not statistically different in the cellular deposits among studied cases.
a Subcutaneous chromoblastomycosis exhibiting few and short elastic fibers (arrow). b Skin lesion caused by sporotrichosis, exhibiting extensive elastic fibers (arrow). Van Gienson technique. c Interstitial collagen (arrow) in subcutaneous lesion of chromoblastomycosis. d Skin lesion caused by sporotrichosis, exhibiting intense deposits of collagen fibers (arrow). Trichromic of Masson technique. (magnification 400×)
Lectin histochemistry evidenced the carbohydrate profile expressed in glycoconjugates of the fungi cell surface, which presented an intense staining pattern in the Sporothrix schenckii cell wall, for both lectins used. This fact suggests that Sporothrix schenckii presents high expression of glucose/mannose and N-acetyl-glucosamine in those cell surface molecules. However, WGA and Con A did not provide evidences for carbohydrate residues in the cell surface of Fonsecaea pedrosoi, Phialophora verrucosa, and Cladophialophora carrionii (Table 2).
Discussion
The fungi usually present intra-and/or extracellular host parasite interfaces, with the parasitism phenomenon dependent on complementary surface molecules, per example, presence of sugar-binding proteins (lectins) on the microconidia, suggesting a (glyco)protein nature of the binding sites [7].
The use of lectin histochemistry in mycological studies was first applied in France, in the 1970 decade, and turned out to be a good method for characterizing sugars from the fungal cell wall [22]. A previous study analyzed five species from three distinct genus (Neocallimastix frontalis, N. patriciarum, N. joyonii, Piramonas communis, and Sphaeromonas communis) and the eight different lectins used proved that such technique is an important auxiliary tool to distinguish different fungal species, besides characterizing the carbohydrate nature of the cell wall [5].
Moreover, lectin-binding analysis the cell surface glycoconjugates could be another approach for the identification and typing of medically important fungal species, and it is an indicative of a new and unrecognized mechanism by which the taxonomy may be readily performed in the majority of the laboratories [6].
Thus, Munõz et al. [23] describe lectin profiling for 93 Candida isolates of five different species, using 14 lectins to cover a wide range of sugar specificities, indicating the existence of various biotypes.
Furthermore, information over saccharide profile in fungal surface enables the use of lectins, besides promising cells probes, to be used in therapeutic treatment as already proposed for bacterial diseases [24]. These proteins might serve as carriers of antifungal agents, using as targets their specific carbohydrates found in the cell surface of microorganisms.
According to clinical and histological data [1, 25], subcutaneous lesions, in sporotrichosis and chromoblastomycosis, present quite similar morphologic characteristics (granulomatous lesions), which in most of the cases, it is necessary to isolate etiological agents and therefore to define sporotrichosis or chromoblastomycosis diagnosis. Studies aiming the elucidation of such questions are scarce.
In certain persistent or non-degraded antigens, commonly found in extracellular matrix associated with fungal cell wall, initial lymphocytic infiltration is substituted by macrophages. These mononuclear cells suffer modifications in the chronic disease. In chromoblastomycosis, it was also observed massive pseudoepitheliomatous hyperplasia with numerous small abscesses and brown sclerotic bodies of fungus within giant cell [26]. In addition, the histological lesions, caused by sporotrichosis, possess an absence of organisms in biopsy material, but present widespread fibrillar components deposition in the interstice, which demonstrates to be the main cause of the intense fibrosis [2].
Our study suggests a differentiated composition of carbohydrates in the cell wall of the fungi studied [6]. That would influence the distinct processes of interaction of the etiological agents of both mycosis with the host defense cells during the infection, since the hypersensitivity to etiological agent of fungal origin is type IV and so it is related to glycoproteins present in the cell wall [27–29].
Limongi et al. [30] showed previously that mannose and N-acetylglucosamine (GlcNAc) residues are involved in the process of adhesion of Fonsecaea pedrosoi, the causative agent of chromoblastomycosis, to epithelial cells. It was then suggested that lectin-like molecules would be involved in the interaction.
Expression of different glycoconjugates leads toward a differentiated immune response [28]. Our results indicated the absence or non-accessibility to residues of glucose and/or mannose and N-acetyl-glucosamine in glycoconjugates in the cell surface of Fonsecaea pedrosoi, Phialophora verrucosa, and Cladophialophora carrionii. Failure in recognizing such carbohydrates may be explained based on the fact that since Con A and WGA are not endolectins, lectins are able to bind internal saccharide residues of the glyco-structure of glycoconjugates, such sugars were not reached by both lectins. On the other hand, such molecules possess the ability to bond to glucose and/or mannose and N-acetyl-glucosamine, respectively, at a non-reducing terminal of the saccharide chain. Furthermore, several studies suggested that carbohydrate expression varies according to the age and environmental conditions of the fungus [31].
In addition, Sidrim and Rocha [25] cited that in pathogenic fungi, a few reports suggest the occurrence of sialic acids in C. neoformans, Sporothrix schenckii, Fonsecaea pedrosoi, and Paracoccidioides brasiliensis. On the other hand, both N-acetyl and N-glycolyl derivatives were reported in S. schenckii and F. pedrosoi. In S. schenckii, sialic acids protect yeast forms against phagocytosis, and in F. pedrosoi, neuraminic acid derivatives were associated with morphogenesis and cellular integrity.
Our results are in agreement with those few studies that used lectin histochemistry in order to investigate the correlation between cell surface glycoconjugates and tissue inflammatory response to the fungi as Sporothrix schenckii [9, 32–34].
According to Esquenazi and coworkers [7], lectin–carbohydrate interaction was shown to play a crucial role in the infectious process. Many investigations recognized that all cells are coated with sugars and that many also express surface lectins. Since epithelial cells may express these carbohydrates, the next step will be to investigate the possible role of these carbohydrates on the cellular interaction using skin tissues from dermatophytosis.
It is possible to conclude that even if subcutaneous lesions related to sporotrichosis and chromoblastomycosis present similar morphologic characteristics, both conditions present different histopathologic profiles regarding to deposit of collagen, elastic fibers, and level of interstitial fibrosis. The expression of carbohydrate profile in the cell surface was observed to be different and characteristic to the species evaluated using lectin histochemistry.
References
Baron S. Subcutaneous mycoses. In: Clanton DR, editor. Medical microbiology, 4th ed. Texas, USA: University of Texas (Medical school); 1996. p. 234–260.
Queiroz-Telles F, McGinnis MR, Salkin I, Graybill JR. Subcutaneous mycoses. Infec Dis Clin North Am. 2003;17:59–85.
Duquia RP, Souza PRM, Gervini RL, Schwartz J, Prochnau A, Almeida HL Jr. Micose fungóide hipopigmentar com 20 anos de evolução. An Bras Dermatol. 2005;80:189–91.
Koga T, Duan H, Furue M. Immunohistochemical detection of interferon-γ-producing cells in granuloma formation of sporotrichosis. Med Micol. 2002;40:111–4.
Guillot J, Breton A, Damez M, Dusser M, Gaillard-Martinie B, Millet L. Use of lectins for a comparative study of cell wall composition of different anaerobic rumen fungal strains. FEMS Microbiol Lett. 1990;67:151–6.
Lima-Neto RG, Beltrão EIC, Oliveira PC, Neves RP. Adherence of Candida albicans and Candida parapsilosis to epithelial cells correlates with fungal cell surface carbohydrates. Mycoses. 2009;54:23–9.
Esquenazi D, Souza W, Alviano CS, Rozental S. The role of surface carbohydrates on the interaction of microconidia of Tricophyton mentagrophytes with epithelial cells. FEMS Immunol Med Microbiol. 2003;35:113–23.
Melo-Júnior MR, Telles AMF, Albuquerque FEB, Pontes-filho NT, Carvalho LB Jr, Beltrão EIC. Altered lectin-binding sites in normal colon and ulcerative colitis. J Bras Patol Med Lab. 2004;40:123–5.
Mendes-Giannini MJS, Taylor ML, Bouchara JB. Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi. Med Micol. 2000;38:113–23.
Araújo-Filho JLS, Melo-Júnior MR, Carvalho LB Jr, Pontes-Filho NT. Galectina-3 em tumores de próstata: imuno-histoquímica e análise digital de imagens. J Bras Patol Med Lab. 2006;42:469–75.
Melo-Júnior MR, Araújo-Filho JLS, Machado MCFP, Patú VJRM. Análise digital de imagens em patologia a interface com a engenharia biomédica. Rev Bras Eng Biomed. 2006;22:203–6.
Melo-Júnior MR, Araújo-Filho JLS, Patú VJRM, Beltrão EIC, Carvalho LB Jr. Digital image analysis of skin neoplasms evaluated by lectin histochemistry: potential marker to biochemical alterations and tumour differential diagnosis. J Bras Patol Med Lab. 2006;42:455–60.
Demirkaya O, Cothren RM, Vince DG, Cornhill JF. Automated identification of stained cells in tissue sections using digital image analysis. Anal Quant Cytol Histol. 1999;21:93–102.
Erler BS, Marchevsky AM. Microphotometry in pathology. In: Marchevsky AM, Bartels PH, editors. Image analysis. A primer for pathologists. New York: Raven Press Ltd; 1994. p. 181–206.
Lima-Neto RG, Araújo-Filho JLS, Melo-Junior MR. Avaliação dos micronúcleos de células inflamatórias em pacientes com esporotricose e cromomicose. Rev Cienc Med Biol. 2008;7:175–81.
Melo-Júnior MR, Araújo-Filho JLS, Patú VJRM, Mello LA, Carvalho LB Jr. Langerhans cells in cutaneous tumours: immunohistochemistry study using a computer image analysis system. J Mol Histol. 2006;37:321–5.
True LD. Morphometry applications in anatomic pathology. Hum Pathol. 1996;27:450–67.
Fennell DI. Conservation of fungus in cultures. Bot Rev. 1960;26:79–141.
Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of clinical fungi. 2nd ed. Utrecht: Centraal Bureau voor Schimmel Cultures/Universitat Rovira i Virgili; 2000.
Spicer SS. Histochemistry in pathologic diagnosis. 1st ed. New York: Dekker; 1987.
Özer E. Effects of prenatal exposure on neuronal migration, neurogenesis and brain myelinization in the mice brain. Clin Neuropathol. 2000;19:21–5.
Jetry K, Schottelius J, Dollet M. Differentiation of Phytomonas sp. and lower trypanosomatids (Herpetomonas, Crithidia) by agglutination tests with lectins. Parasitol Res. 1987;74:1–4.
Muñoz A, Alonso B, Alvarez O, Llovo J. Lectin typing of five medically important Candida species. Mycoses. 2003;46:85–9.
Freire MGM, Gomes VM, Corsine RE, Machado OLT, Simone SG, Novello JC, Machado NLR. Isolation and partial characterization of a novel lectin from Talisia esculenta seeds that interferes with fungal growth. Plant Physiol Biochem. 2002;40:61–88.
Sidrim JJC, Rocha MGF. Micologia médica à luz de autores contemporâneos. Guanabara Koogan, Rio de Janeiro. 1ª Ed. 2004.
Vardar-Ünlü G, McSharry C, Julia-Douglas L. Fucose-specific adhesins on germ tubes of Candida albicans. FEMS Immunol Med Microbiol. 1998;20:55–67.
Carlos ZI, Sgarbi DBG, Placeres MCP. Host organism defense by a peptide-polysaccharide extracted from the fungus Sporothrix schenckii. Mycopathol. 1999;144:9–14.
Restrepo A, Mcewen JG, Castañeda E. The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med Mycol. 2001;39:233–41.
Soares RMA, Alviano DS, Angluste J, Alviano CS, Travassos LR. Identification of sialic acids on the cell surface of Candida albicans. Biochim Biophys Acta. 2000;1474:262–8.
Limongi CL, Alviano CS, De Souza W, Rozental S. Isolation and partial characterization of an adhesin from Fonsecaea pedrosoi. Med Mycol. 2001;39:429–37.
Alviano DS, Rodrigues ML, Almeida CA. Differential expression of sialylglycoconjugates and sialidase activity in distinct morphological stages of Fonsecaea pedrosoi. Arch Microbiol. 2004;181:278–86.
Fernandes KS, Mathews HL, Lopes Bezerra LM. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J Med Microbiol. 1999;48:195–203.
Lopez-Ribot JL, Martínez JP, Monteagudo C, Alloush HM, Mattioli NV, Chaffin WL. Evidence for the presence of complex carbohydrates in Candida albicans cell wall glycoproteins. Rev Iberoa. Micol. 1999;16:23–6.
Rodrigues ML, Rozental S, Couceiro JN. Identification of N-acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans: influence on fungal phagocytosis. Infect Immun. 1997;65:4937–42.
Acknowledgments
Authors thank Dr. Rejane Pereira Neves and Dr. Oliane Maria Corrêia Magalhães for their scientific assistance and Dr. William Ledingham for his critical review.
Conflict of Interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Melo-Júnior, M.R., de Lima-Neto, R.G., Lacerda, A.M. et al. Comparative Analysis of Extracellular Matrix and Cellular Carbohydrate Expression in the Sporotrichosis and Chromoblastomycosis. Mycopathologia 171, 403–409 (2011). https://doi.org/10.1007/s11046-011-9399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-011-9399-5