A study of sigma phase formation, its characterization, and its effect on the properties of duplex stainless steel 2205 are presented. The steel has been heat treated according to the mode: heating to a temperature of 850°C, holding during 6 h and cooling in various media, i.e., furnace, air, and water. The optical metallographic methods reveal sigma phases within the ferrite region of the steel microstructure after all the heat treatments; a slightly elevated amount of the phase is detected after cooling with the furnace. Some fine carbides and nitrides are encountered along with the sigma phase. The SEM and EDS characterization confirms that the chemistry of the sigma phase is Fe – 12.2Cr – 2.1Mo. The hardness of the water-quenched steel is 336 HB, which is 47% higher than its hardness before the heat treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Stainless steels containing over 11 wt.% Cr and other alloying elements possess a favorable combination of mechanical and corrosion properties, good weldability and other parameters [1,2,3]. Duplex stainless steels are superior to single-phase steels [4, 5]. They are highly resistant tostress corrosion cracking and pitting corrosion even in chloride environments. According to the pitting resistance equivalent number (PREN), duplex stainless steels are classified into three families, i.e., lean, standard and super ones. The higher the value of PREN the more resistant the steel is to local pitting corrosion stimulated by chlorides. The lean steels have the lowest PREN, the super duplex steels have the highest PREN. Despite the wide application of duplex stainless steels in various articles serving in various environments, they have a restricting factor, i.e., thermal embrittlement at 280 – 525°C. Embrittlement of this type is often referred to as “embrittlement at 475°C,” because its rate is the highest near this temperature [6]. The steels are embrittled due to formation of a chromium-rich sigma-phase, and their corrosion resistance degrades simultaneously because the matrix is depleted of chromium [7].
Sigma-phase commonly forms in the structure of the steel under elevated temperatures, for example, in rolling, welding, casting, aging, or forging [8,9,10]. If the duplex stainless steel is heated to about 550°C or a higher temperature for a long time, its structure acquires a chi-phase, a sigma-phase etc., and M23C6 carbides form in ferrite grains and on their boundaries. All these factors lower the mechanical properties, and therefore it is important to detect the sigma-phase in the structure of the steel [11, 12]. The sigma-phase can be identified and studied in duplex steels by electron microscopy (images in secondary and back-scattered electrons), electron probe microanalysis, energy dispersive x-ray spectroscopy, and wavelength dispersive x-ray spectroscopy [13, 14].
The aim of the present work was to study the effects of heat treatment modes on the structure and of the sigma-phase on the hardness of duplex stainless steel 2205 (03Kh22N6M2).
METHODS OF STUDY
We studied duplex stainless steel 2205 (03Kh22N6M2) supplied by the Aadhya Engineering Services in annealed condition. By the data of the spectrum analysis, the steel had the following chemical composition (in wt.%): 0.0289 C, 22.219 Cr, 4.530 Ni, 3.0163 Mo, 0.399 Si, 0.160 Cu, 0.1100 Co, 0.1026 V, 0.0551 W, 0.0180 Nb, 0.0181 P, 0.0018 S, the remainder Fe.
Three groups of samples were hold for 6 h in a muffle furnace at 850°C and then cooled in different environments, i.e., with the furnace, in air, and in water. The temperature fluctuation is the furnace did not exceed 10°C.
We measured the hardness of the samples using a Brinell hardness tester. The surface of the samples was ground, polished, and subjected to electrochemical etching in a solution of 40% NaOH. The metallographic studies were carried out using an optical microscope and a scanning electron microscope. The compositions of the sigma-phase, ferrite and austenite were determined using energy dispersive spectroscopy (EDS).
RESULTS AND DISCUSSION
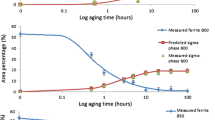
Figure 1a and b present the microstructure of the steel in the initial (annealed) condition. Figures 1c – h present the microstructure after the heat treatment and cooling in different environments. In all the cases, the structure contains austenite (white) islands incorporated into a gray matrix of etched ferrite. The delta (brown) and chi phases occur on the δ/γ interfaces of grains and then grow into the region of ferrite. Nucleation of the sigma-phase starts on the ferrite/austenite interfaces, and then it grows only in the region of delta-ferrite. The eutectoid decomposition of the delta-ferrite causes a δ → (σ + γ) transformation, i.e., the delta-ferrite is substituted by a sigma-phase and secondary austenite with a laminar-cellular structure. Optical microscopy has not revealed changes in the sizes of the primary (initial) γ-grains. The high rate of the phase transformation is a result of the presence of ferrite in the structure [15]. The content of the sigma-phase increases with decrease of the cooling rate [16] and is the highest after the furnace cooling. Precipitation of sigma-phase is well observable only in the ferrite phase. The sigma-phase is accompanied by precipitates of very fine carbides and nitrides (colored dark), which should raise the strength of the steel. Different phases form in different times. The sigma-phase precipitates after carbides, nitrides, and the chi-phase. After a longer hold, the sigma-phase absorbs the chi-phase, but the nitrides remain steady [17]. It should be noted that we have not detected a chi-phase in the structure of the steel after the slow cooling with the furnace (Fig. 1c and d ).
Figure 2 presents the values of the microhardness of the steel heat treated by different variants. It can be seen that the hardness grows with increase of the cooling rate. This is connected with the fact that growth of the cooling rate promotes increase of the content of delta-ferrite and formation of acicular martensite in the structure, which elevates the hardness [18].
The results of the diffraction analysis and of the determination of the chemical composition of different phases in the steel are presented in Fig. 3. The austenite is enriched with nickel and the ferrite is enriched with chromium, which agrees with the data of [17]. The intermetallic sigma-phase contains primarily Fe, Cr and Mo, which explains the depletion of the matrix of chromium and molybdenum.
CONCLUSIONS
We have studied the effect of a 6-h hold at 850°C followed by cooling with the furnace, in air, and in water on formation of sigma-phase in the structure and on the properties of a duplex stainless steel 2205 (03Kh22N6M2). During the holding at 850°C, the structure of the steel acquires inclusions of a sigma-phase, of a chi-phase, carbides and nitrides. The sigma-phase precipitates on the δ/γ interfaces and grows only into the region of ferrite. Carbides and nitrides have the form of fine particles on grain boundaries and inside grains. Since the sigma-phase forms by a diffusion process, the lowering of the cooling rate (water → air → furnace) raises its content in the structure of the steel. The formation of intermetallics and the transformations of the γ-matrix raise the hardness of the annealed steel from 228 HB (as-received condition) to 309, 311, and 336 HB after the cooling with the furnace, in air, and in water respectively. In the case of furnace cooling, the hardness of the steel is mainly affected by the fine dispersed inclusions of the intermetallics; in water cooling it is chiefly affected by the austenite-to-martensite transformation. The hardness of the steel after the water quenching increases by 47% as compared to the hardness in the annealed condition. Formation of the sigma-phase with composition Fe – 12.8 at.% Cr – 2.17 at.% Mo results in depletion of the matrix of chromium and molybdenum, which can reduce the corrosion resistance of the steel.
We are grateful to Prince Kansagara, Jaydip Maru and Raj Savaliya for helping to carry out this experiment. We are also grateful to Adhya Engg. Services and Met-heat pvt ltd for granting us permission for hardness, chemical and microscopy testing. Hearty thanks to Dr. Vandana J. Rao for SEM-EDS testing.
References
J. C. Lippold and D. J. Kotecki, Welding Metallurgy and Weldability of Stainless Steels, Wiley India Pvt. Limited (2005), 376 p.
J. K. L Lai, C. H. Shek, and K. H. Lo, Stainless Steels: An Introduction and Their Recent Developments, Bentham Science Publishers Ltd. (2012), 168 p.
J. R. Davis, Stainless Steels, ASM International (1994), 576 p.
J. Kazior, M. Nykiel, T. Pieczonka, et al., “Activated sintering of P/M duplex stainless steel powders,” J. Mater. Process. Technol., 157(1), 712 – 717 (2004).
I. Alvarez-Armas and S. Degallaix-Moreuil, Duplex Stainless Steels, ISTE Ltd and John Wiley & Sons Inc. (2009), 464 p.
H. Chung, “Aging and life prediction of cast duplex stainless steel components,” Int. J. Press. Vessel. Piping, 50(1 – 3), 179 – 213 (1992).
T. Chen, K. Weng, and J. Yang, “The effect of high-temperature exposure on the microstructural stability and toughness property in a 2205 duplex stainless steel,” Mater. Sci. Eng. A, 338(1 – 2), 259 – 270 (2002).
C.-C. Hsieh and W. Wu, “Overview of intermetallic sigma,” ISRN Metallurgy, 2012(1), Art. ID 732471 (2012).
V. I. Gorynin, S. Yu. Kondrat’ev, and M. I. Olenin, “Raising the resistance of pearlitic and martensitic steels to brittle fracture under thermal action on the morphology of the carbide phase,” Met. Sci. Heat Treat., 55(9 – 10), 533 – 539 (2014).
V. I. Gorynin, S. Yu. Kondrat’ev, M. I. Olenin, and V. V. Rogozhkin, “A Concept of carbide design of steels with improved cold resistance,” Met. Sci. Heat Treat., 56(9 – 10), 548 – 554 (2015).
F. Kurosawa, I. Taguchi, M. Tahino, et al., “Observation and analysis of precipitates formed in steel at elevated temperatures using a non-aqueous electrolyte-potentiostatic etching method,” Nihon Kinzoku Gakkai Shi., 45(1), 72 – 81 (1981).
A. Chino, M. Ihida, and H. Iwata, “Determination and precipitation behavior of σ-phase, carbides and nitrides in duplex stainless steels,” Tetsu-to-Hagané, 75(10), 1936 – 1942 (1989).
K. Kumada, “About σ phase,” Bull. Japan Inst. Metal., 2, 261 – 271 (1963).
I. Calliari, K. Brunelli, M. Dabala, and E. Ramouet, “Measuring secondary phases in duplex stainless steels,” JOM, 61(1), 80 – 83 (2009).
R.Wang, “Precipitation of sigma phase in duplex stainless steel and recent development on its detection by electrochemical potentiokinetic reactivation: A review,” Corr. Commun., 2, 41 – 54 (2021).
T. Chen and J. Yang, “Effects of solution treatment and continuous cooling on σ-phase precipitation in a 2205 duplex stainless steel,” Mater. Sci. Eng. A, 311(1 – 2), 28 – 41 (2001).
N. Llorca-Isern, H. López-Luque, I. López-Jimenéz, and M. V. Biezma, “Identification of sigma and chi phases in duplex stainless steels,” Mater. Charact., 112, 20 – 29 (2016).
D. S. Petroviè, M. Pirnat, G. Klanènik, et al., “The effect of cooling rate on the solidification and microstructure evolution in duplex stainless steel,” J. Therm. Anal. Calorim., 109(3), 1185 – 1191 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 9, pp. 34 – 38, September, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagar, R., Patel, K.K. & Parmar, A. Study and Characterization of Sigma Phase in Duplex Stainless Steel 2205 (03Kh22N6M2). Met Sci Heat Treat 65, 558–562 (2024). https://doi.org/10.1007/s11041-024-00969-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-024-00969-8