Formation of structure and properties is analyzed in a number of stainless high-strength steels used in oil production for shafts of submerged centrifugal electric pumps. The factors elevating the cuttability and corrosion resistance of sparingly alloyed steel 14Cr17Ni2NV are determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Formation liquid, which consists of water, oil, and chemical and mechanical admixtures, is pumped-off from oil wells with the help of drowned motorized centrifugal pumps [1]. The most loaded part of such a pump unit is the shaft exposed to a pulsed action of torsional and bending moments in aggressive environments. The choice of the material for the shaft is determined considerably by the operating conditions of the pump and the reliability of the unit on the whole. Liquids with high corrosion activity are pumped by centrifugal pumps, the critical parts of which are produced from corrosion-resistant materials.
The corrosion resistance and the mechanical properties of stainless steels are determined by their chemical composition, structure and regime of heat treatment [2, 3]. Since a passive condition of the surface is created by a protective film of chromium oxides, the main alloying element of which is chromium, its minimum content for providing corrosion resistance is 12% [4, 5]. Nickel plays an important role in formation of properties. The presence of nickel in the chemical composition increases the ductility and toughness of the steel but also raises substantially its cost.
Imported chromium-nickel steel 03Cr14Ni7W has long been preferred for making shafts of drowned pumps, but today its operating properties to not meet the stringent requirements on the pipe equipment used in wells with hard operating conditions. This requires optimization of the chemical compositions of such steels for elevating their operating characteristics. Ferrite-forming (aluminum, molybdenum, vanadium, titanium) and austenite-forming (nitrogen, manganese, copper) elements may be introduced into the steels or this purpose. However, the choice of the alloying system is based not only on the operating conditions but also on the possibilities of the metallurgical production, i.e., availability of the alloying elements and of the appropriate facilities for melting, rolling, and heat treatment.
The efficiency of alloying of stainless chromium-nickel steels of the ferritic-martensitic class with vanadium becomes evident when we compare the mechanical characteristics of two steels, i.e., 14Cr17Ni2 and 13Cr14Ni3V. The steels have the same ductility but the strength characteristics of the vanadium-containing steel after quenching and high tempering are 1.5 times higher and the impact toughness is twice higher [6, 7]. Numerous studies of Russian and foreign researchers have shown that joint introduction of vanadium and nitrogen raises the strength level of structural steels both in hot-rolled and normalized conditions and after quenching and tempering [8, 9]. These facts have been allowed for in the development of a novel steel 14Cr17Ni2NV, which has been tested successfully in the production of shafts of drowned pumps.
The aim of the present work was to perform a comparative analysis of the structural state of steel 03Cr14Ni7W used traditionally for critical parts of pump facilities and of a novel steel 14Cr17Ni2NV sparingly alloyed with nickel and to determine the factors providing the elevated process and special properties of steel 14Cr17Ni2NV.
Methods of Study
The studied steels had chemical compositions presented in Table 1. Ingots with a mass of 5 tons were melted in arc electric furnaces. The metal was deoxidized with lump aluminum. During melting, steel 14Cr17Ni2NV was modified with calcium and barium.
The process of production of bars from these steels at the Serov Metallurgical Plant includes several operations. The rolling for long-length bars (up to 9 m) to a diameter of 17 mm is conducted with preliminary heating to a temperature of at least 1200°C for 0.5 h. The final shape is controlled, the bars are straightened and cut to length. The final heat treatment includes quenching and tempering. The modes of the treatment and the mechanical properties of the steels are presented in Table 2.
We studied the structure and the phase composition of the steels in heat treated condition. The metallographic structure was uncovered by etching in a mixture of hydrochloric and nitric acids in 3 : 1 proportion. To observe the structure, we used a “QUANTA-200” scanning electron microscope with an x-ray microanalyzer. The fine structure of the steels was studied by transmission electron microscopy using a JEM-200CX microscope for the electron diffraction analysis. The x-ray diffraction analysis was performed with the help of a DRON-UM1 diffractometer in copper radiation with monochromator at a voltage of 30 kV and a current of 20 mA. The diffraction patterns were obtained in a step mode with angle 2θ = 0.02° and 10-sec pulse duration.
The studies were performed at the collective use “Test Center for Nanotechnologies and Perspective Materials” of the M. N. Mikheev Institute for Metal Physics of the Ural Branch of the Russian Academy of Sciences.
Results and Discussion
Quantitative proportion of chromium and nickel places steel 03Kh14N7W into the two-phase martensite-austenite range [10]. By the data of the x-ray diffraction analysis, the content of retained austenite in the structure of the steel is 13%. The matrix of the structure of the steel after annealing and tempering is represented by tempered martensite (Fig. 1 a ). The “former” austenite grains with a size of 30 – 40 μm contain several packs of martensite. The martensite crystals have a lath morphology (Fig. 2 a ). Layers of retained austenite are observed between martensite laths; in the dark-background images obtained in the reflection of the γ-phase such regions are in a reflecting position (Fig. 2 b ).
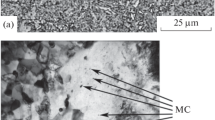
Steel 03Kh14N7W contains precipitates of chromium carbide with a size fluctuating in a wide range. The largest particles (0.2 – 0.5 μm) are observed in the images obtained under the scanning electron microscope (Fig. 1 a ). Transmission electron microscopy of thin foils has made it possible to detect precipitates of fine dispersed carbides of type Cr23C6 with a maximum size of 20 – 40 nm (Fig. 2 c and d ).
The content of chromium in the chemical composition of steel 14Cr17Ni2NV has been increased from 14.0 to 17.28 wt.%; the content of nickel was reduced from 7.0 to 2.24 wt.%. This places the steel into the class of ferritic steels. The diffraction pattern contains reflections from only one modification of iron with bcc crystal lattice. The structure has no retained austenite. The size of the ferrite grains is 10 – 20 μm. The fine structure of the ferrite has a developed subgrain pattern (Fig. 3 a ). The dislocation density in individual ferrite subgrains differs. In addition to the regions free of dislocations, we observe net and cellular dislocation substructures (Fig. 3 b and c).
The chemical composition of steel 14Cr17Ni2NV implies the presence of several excess phases in the structure. The electron diffraction analysis has shown that in addition to the matrix reflections from the crystal lattice of bcc iron (ferrite) the electron diffraction patterns bear reflections from a fcc lattice of vanadium carbonitride V(C, N) and a complex fcc lattice of chromium carbide of type Cr23C6. In the electron diffraction pattern presented in Fig. 3 c we can observe reflections (222) and (200) from vanadium carbonitride, as well as reflection (113) from chromium carbide and reflection (110) from bcc iron, which coincides partially with (200) V(C, N). The particles of these phases differ in size and morphology.
Vanadium carbonitrides are nanosize precipitates; they manifest themselves through decoration of the dislocation substructure; at the places of their location the dislocation net exhibits a kind of compaction from 5 to 10 nm in size (Fig. 3 b and c). These compactions are characterized by a high dislocation density. The presence of nanosize particles of vanadium carbonitride affects the condition of the bcc matrix of the steel studied. It has been shown in [11] that tetragonal and monoclinic distortions appear in the crystal lattice of the V(C, N) particles less than 10 nm in size, and the structure becomes susceptible to atomic ordering with respect to the interstitial atoms. Formation of such particles is accompanied by considerable growth of the atomic volume and gives rise to considerable local stresses.
Chromium carbides in the structure of steel 14Cr17Ni2NV form a complex hierarchy of precipitates. In the images obtained by transmission electron microscopy chromium carbides are represented by individual oval particles 50 – 120 nm in size. These particles seem to have formed during tempering. The images obtained by scanning electron microscopy exhibit coarser particles up to 1 – 2 μm in size, which form discontinuous chains and border some grain boundaries (Fig. 1 b ). The streak arrangement of the particles seems to be inherited from the production process and indicates that the crystal structure defects that have appeared under rolling become substrates for precipitation of chromium carbides and are partially preserved in the structure under heating. The relatively large size of particles in steel 14Cr17Ni2NV has made it possible to identify them by the method of diffraction analysis. Scanning over the line containing such particles gives maximums of the characteristic radiation for chromium at the places of their location (Fig. 1 b ).
Combination of quenching with high tempering is a traditional heat treatment for stainless chromium-nickel steels. Let us estimate how the changes in the chemical composition of the steels affect the efficiency of each stage of the treatment with allowance for the data of the structural studies.
The temperature of heating for quenching is chosen so as to provide maximum possible dissolution of carbides at a relatively small grain size. Since steel 14Cr17Ni2NV contains hardly soluble vanadium carbonitrides and the content of chromium and hence of chromium carbides in it is higher than in steel 03Kh14N7W, its quenching should be performed at a considerably higher temperature (1050 and 800°C, respectively). The grain size in steel 14Cr17Ni2NV is virtually twice smaller (10 – 20 μm instead of 30 – 40 μm). The refinement of the grains is a result of the presence of vanadium in the composition of 14Cr17Ni2NV, which restricts the growth of grains under heating. It should also be noted that the presence of subgrain boundaries in ferrite, which contribute into the growth of the yield strength in accordance with the Hall – Petch relation, is also important for formation of the mechanical properties of the steel.
The mode of tempering for the steels is chosen with allowance for the combination of mechanical properties and corrosion resistance. Chromium carbides of different type can precipitate in chromium steels. With growth of the tempering temperature from 400 to 700°C the stoichiometric composition of the carbides varies in the following succession: (Fe, Cr)3C, (Fe, Cr)7C3, (Fe, Cr)23C6, Cr23C6 [12]. At relatively low tempering temperatures fine carbides (Fe, Cr)3C and (Fe, Cr)7C3 precipitate and provide the best set of strength and ductility parameters. However, the limited diffusion processes result in inhomogeneity of the solid solution of the bcc iron; the regions directly adjoining the carbide particles are depleted of chromium and, therefore, the corrosion resistance after such treatment worsens. At high tempering temperatures the precipitation of Cr23C6 carbides is accompanied by their coagulation; due to the active development of diffusion the chromium concentration in the body of the grains levels and the corrosion resistance of the steel increases.
The results of the study of the structures show that tempering of the steels at 650 – 670°C causes precipitation of carbides of type Cr23C6 at a relatively uniform distribution of chromium in the matrix metal. The effect of precipitation hardening of steel 03Kh14N7W is connected with precipitation of chromium carbides with a maximum size not exceeding 0.2 – 0.5 μm. We have established experimentally that the structure of steel 14Cr17Ni2NV contains chromium carbides up to 1 – 2 μm in size and nanosize vanadium carbonitrides. Reported results allow us to expect the presence of fine copper particles in the structure, which should also contribute into elevation of the corrosion resistance [13].
The structural state of the steels after the heat treatment provides about the same level of the yield strength, i.e., σ0.2 = 880 – 900 N/mm2. However, the difference in the natures, sizes and volume fractions of the phases precipitated under tempering determines different margins of ductility of the steels under subsequent loading in mechanical tests. The elongation of 17% for steel 03Kh14N7W and of 14% for steel 14Cr17Ni2NV indicates that the latter is less susceptible to strain hardening under uniaxial tension. The energy capacity of fracture under impact loading of the steels also differs. The elevated impact toughness of steel 03Kh14N7W (KCU = 1.18 MJ/m2 ) is a result of the ductile fracture behavior, which develops by the mechanism of formation and merging of micropores (Fig. 4 a ). For steel 14Cr17Ni2NV typical elements of the fracture surface are facets connected by detachment ridges and shallow dimples (Fig. 4 b ). The quasi-brittle pattern of fracture limits substantially the impact toughness of the steel (to KCU = 0.81 MJ/m2 ).
A very important operating characteristic of steels serving in aggressive environments is their corrosion resistance, which depends on a number of factors. The capacity to resist to hydrogen sulfide corrosion is very important. In accordance with ISO 15156-3, the effect of chemical composition on the susceptibility of steels to pitting corrosion in such environments may be evaluated in terms of parameter PREN, which characterizes quantitatively the comparative efficiency of alloying, i.e., PREN = (% Cr) + (3.3% Mo) + (16% N). For steels 03Kh14N7W and 14r17Ni2NV PREN = 14 and 18% respectively; the difference in these value amounts to 28% and reflects the higher corrosion resistance of steel 14Cr17Ni2NV. In the structural aspect steel 14Cr17Ni2NV is also preferable, because in contrast to the double-phase martensitic-austenitic steel 03Kh14N7W it does not acquire local galvanic pairs. In addition, the protective oxide film providing a passive condition of the metal is stronger because the quality of its surface after cutting is higher.
The special features of straining create process advantages for steel 14Cr17Ni2NV under mechanical treatment in the stage of fabrication of parts as compared to steel 03Kh14N7W. The cutting process is implemented due to plastic deformation of a thin surface layer of the metal; in the final stages of the deformation it breaks and forms chips [14]. In the more brittle steel 14Cr17Ni2NV the margin of plasticity is exhausted faster, which makes the formation of chips easier, improves the cuttability, and provides lower surface roughness. The practice of production of shafts from this steel has shown that the cuttability of the metal has improved by 15%. The susceptibility of steel 03Kh14N7W to strain hardening creates difficulties with cutting and is responsible for intense wear of the cutting tools.
Conclusions
We have performed a comparative analysis of the structure and phase composition of high-strength steels 03Kh14N7W and 14Cr17Ni2NV. It has been shown that in the heat treated condition the structure of the former consists of tempered martensite, while the structure of the latter is represented by ferrite containing precipitates of chromium carbides and vanadium carbonitrides.
At the same level of strength, the fracture behavior of steel 14Cr17Ni2NV is quasi-brittle, which creates process advantages in the stage of mechanical treatment of the surface. Optimization of the chemical and phase compositions and the high quality of the surface provided by mechanical treatment promote elevation of the corrosion resistance of steel 14Cr17Ni2NV.
The data of laboratory studies and the results of full-scale tests of steel 14Cr17Ni2NV sparingly alloyed with nickel allow us to recommend it as a stainless high-strength material for the production of critical highly loaded parts operating under the conditions of aggressive environment in oil extraction facilities.
A pilot batch of precision polished rolled products has been produced from steel 14Cr17Ni2NV at a domestic plant. The field tests of the pumps in oil wells located in different regions (Tataria, Bashkiria, Tyumen) have proved advantages of the operation of shafts from the novel steel, i.e., 30% prolongation of the service life and 20% growth of the resistance to pitting corrosion.
References
V. N. Ivanovskii, Equipment for Oil and Gas Extraction [in Russian], Heft i Gaz, Moscow (2002), 769 p.
A. A. Babakov and M. V. Pridantsev, Corrosion-Resistant Steels and Alloys [in Russian], Metallurgiya, Moscow (1971), 318 p.
V. S. Malov and V. A. Vasil’ev, “Effect of reduction ratio and heat treatment on mechanical properties of a forging from steel 14Kh17N2,” Metalloved. Term. Obrab. Met., No. 4, 33 – 36 (2014).
S. Yu. Kondrat’ev, G. P. Anastasiadi, and A. I. Rudskoy, “Nanostructure mechanism of formation of oxide film in refractory alloys based on Fe – 25Cr – 35Ni,” Metalloved. Term. Obrab. Met., No. 10, 15 – 20 (2014).
I. V. Semenova, G. M. Florianovich, and A. V. Khoroshilov, Corrosion and Protection from Corrosion [in Russian], Fizmatlit, Moscow (2002), 336 p.
A. S. Zubchenko (ed.), List of Grades of Steels and Alloys [in Russian], Mashinostroenie, Moscow (2001), 672 p.
L. A. Smirnov, V. I. Syreishchikova, B. Z. Belen’kii, et al., Vanadium-Containing Steels and Alloys [in Russian], UrO RAN, Ekaterinburg (2003), 309 p.
L. A. Smirnov and P. C. Mitchell (eds.), Application of Vanadium in Steel [in Russian], UrO RAN, Ekaterinburg (2002), 384 p.
L. M. Panfilova and L. A. Smirnov, “Unique properties of steels of the new generation microalloyed with vanadium and nitrogen,” Stal’, No. 5, 116 – 121 (2010).
E. Gudremon, Special Steels, Vol. 1 [in Russian translation], Gos. Nauch. Tekh. Izd. Lit. po Chern. Tsvet. Metall., Moscow (1959), 705 p.
N. A. Polekhina, I. Yu. Litovchenko, A. N. Tyumentsev, et al., “Effect of tempering temperature on phase transformations in ferritic-martensitic 12% chromium steel ÉK-181,” VANT, Ser. Termoyad. Sintez, 37(1), 34 – 40 (2014).
K. A. Lanskaya, High-Chromium Refractory Steels [in Russian], Metallurgiya, Moscow (1967), 216 p.
V. N. Urtsev, D. A. Mirzaev, I. L. Yakovleva, et al., “On the problem of the mechanism of nucleation of copper in aging of Fe – Cu alloys,” Fiz. Met. Metalloved., 4(4), 364 – 373 (2010).
V. S. Muratov and V. V. Sakharov, “Formation of structure and properties of corrosion-resistant steels for improving the cuttability,” Zagot. Proizvod. Mashinostr., No. 6, 53 – 55 (2005).
The work has been performed within state assignment on topic “Structure” (No. 01201463331) with support of Project No. 15-15-2-16 of the Ural Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 1, pp. 17 – 22, January, 2017.
Rights and permissions
About this article
Cite this article
Yakovleva, I.L., Tereshchenko, N.A., Smirnov, L.A. et al. Stainless High-Strength Sparingly Alloyed Steel for Operation in Aggressive Environments. Met Sci Heat Treat 59, 18–23 (2017). https://doi.org/10.1007/s11041-017-0095-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0095-z