The growth of austenite grains and the dissolution of carbonitrides under heating of a structural steel microalloyed with niobium and titanium are studied. The microstructure of the steel is investigated with the help of light and transmission electron microscopes. The content of elements in precipitated particles is determined using an energy dispersive spectrometer. The kinetic equation of grain growth is derived and the critical temperature above which the carbonitrides stop to hinder grain growth is determined. Recommendations are given on the temperature modes of hot rolling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and range of application of microalloyed steels is permanently widening. Microalloying with titanium, niobium and vanadium provides precipitation of the respective carbonitrides, which hinder migration of grain boundaries and thus promote the refinement of grains and improve the mechanical properties. High-strength steels with microadditives of Ti and Nb are widely used in machine building and, in particular, in the automobile industry. Growth of austenite grains has been studied in detail in [1 – 5]. Works [6 – 8] have been devoted to the joint action of titanium and niobium additives on the properties of steels. Some works present models of simultaneous growth of austenite grains and dissolution of precipitates [9, 10]. Refinement of austenite grains in the microstructure after rolling and cooling raises the mechanical properties. However, little attention has been devoted in published works to the effects of the temperature and duration of austenitizing on dissolution of carbonitrides and their precipitation in the process of rolling and subsequent cooling.
The aim of the present work was to study the growth of austenite grains and dissolution of precipitates in a microalloyed steel in order to choose the optimum temperature of austenitizing.
Methods of Study
We studied a steel containing (in wt.%) 0.083 C, 1.882 Mn, 0.068 Si, 0.135 Ti, 0.121 Mo, and 0.056 Nb. Specimens 15 × 15 × 15 mm in size were cut from the middle of a continuously cast slab to obtain a uniform distribution of precipitates. The specimens were heated in a furnace at a rate of 5 K/sec to 850, 1000, 1100, 1200, 1250, and 1300°C, held for 30, 60, and 120 min, and then quenched in water to room temperature. To uncover the boundaries of the initial austenite grains the specimens were cut in a spark cutting machine and etched at 70°C in a saturated aqueous solution of picric acid containing a low amount of a teepol solution. The structure and the sizes of the grains were studied using a ZEISS optical microscope and the Image-pro Plus 6.0 software. The microstructure of the steel was studied with the help of transmission electron microscopy on secondary carbon extraction replicas. The extraction replicas were obtained by depositing several drops of acetone onto the surface of the metallographic specimens. Then a sticking acetate fiber paper was glued onto the specimens. After a 20-min hold, the acetate paper was detached from the specimens, and carbon was sprayed over the surface of the paper on the side of the metallographic specimen. After this, the acetate paper with carbon was immersed into an acetone solution for several hours to be dissolved until formation of a thin carbon film. The extracted carbon replicas were rinsed in a mixture of water and ethyl alcohol, placed on a copper grid and dried. The replicas were studied under a JEM-2100F field emission transmission electron microscope at a voltage of 120 kV using the standard method of light-background images. The content of elements in the particles was determined with the help of an energy dispersive spectrometer.
Results And Discussion
Figure 1 presents micrographs of the steel after a 30-min hold at an austenitizing temperature of from 850 to 1300°C. It can be seen that the austenite grains grow in two temperature ranges. Up to 1200°C the grain size D is small and increases slowly with the temperature. After 1250°C the grain growth is accelerated and the grain size distribution becomes nonuniform. The dependences of the size of the austenite grains on the hold temperature are presented in Fig. 2 a. It can be seen that 1250°C is the temperature of abnormal grain growth, i.e., coarsening. The grains continue to grow at 1300°C, but the rate of the growth decreases.
Isothermal grain growth is commonly described by the equation
where D is the mean diameter of the grains, D 0 is the initial diameter, n is the time exponent, K is the rate constant, and t is the duration of the hold. Exponent n is determined by the mechanism of grain growth [11]. In a pure alloy n = 2, which corresponds to the absence of defects and precipitates. In the presence of impurities n > 2 and increases with decrease of the temperature [12]. Constants n and K depend on the material and on the temperature and can be determined experimentally. We used the Origin software and the method of regression analysis to obtain the values of these constants for the austenitizing temperatures of 1250 and 1300°C, i.e.,
For 1250 and 1300°C the initial grain size turned out to be 10 μm. The curves obtained from Eq. (1) for the two austenitizing temperatures are presented in Fig. 2 b. It can be seen that the experimental points match the approximating curves well. The rate of the grain growth decreases after a hold for 60 min.
With allowance for the temperature dependence of the grain size the kinetics of the growth grains is describable by the equation [13]
where A is a constant for the given material, Q is the apparent activation energy of grain growth, R = 8.3 J/(mole · K) is the gas constant, and T is the absolute temperature.
We obtained the following values for the constants of Eq. (2) for the steel studied: A = 5.93 × 1016 μm/sec, Q = 469363.03 J/mole, and n = 2.40.
The data of Fig. 2 a show that the convergence of the computational and experimental kinetics of grain growth is better at lower holding temperatures. This is connected with dissolution of precipitates at the high temperature, when the grain growth is abnormal. These results agree with the data of [14], where the curves of growth of austenite grains do not exhibit a tendency to abnormal growth due to the absence of particles of second phase. In addition, in accordance with the results of [15, 16] above the temperature Ac 3 austenite forms by a diffusion-free process, i.e., its growth is not limited by the time of carbon diffusion. This implies that the abnormal growth of austenite grains at about 1250°C (Fig. 2 a ) may be a result of the diffusion-free nature of the transformation.
The electron microscopy of the precipitates and the energy dispersive spectra are presented in Fig. 3. It can be seen that after the quenching from 1200°C a great number of precipitates is distributed uniformly. Most precipitates have a size of 60 – 200 nm; there is also a group of precipitates with a size of 200 – 340 nm.
After the quenching from 1250°C the content of precipitates is much lower than after the quenching at 1200°C, which indicates dissolution of a part of precipitates at the higher temperature. The distribution of the precipitates remains uniform; most of them have a size of 70 – 200 nm, and the other group has a size ranging within 200 – 320 nm.
At 1300°C the content of precipitates continues to decrease, and the size distribution becomes less uniform. Most of the grains have a size of 70 – 200 nm; the first coarse particles with a size of 200 – 500 nm appear. It should be noted that some particles with an irregular shape are carbides (indicated by the arrow in Fig. 3 e ). The energy spectra in Fig. 3 show that the precipitated particles contain titanium and niobium and most likely are carbonitrides (Nb, Ti)(C, N).
The steel contains some nitrogen (0.008 wt.%). Titanium nitride TiN forms at a high temperature and cannot dissolve during the austenitizing. Therefore, we will consider in subsequent computations and discussions only carbides of titanium and niobium. Works [17, 18] contain formulas for the product of the solubilities of Ti, Nb and C in austenite, i.e.,
where Ti, Nb and C denote the content of these elements in wt.%. The solubility of titanium and niobium carbides at different temperatures of austenitizing was calculated using Eqs. (3) and (4) under the assumption that titanium and carbon have been fully bound in the carbide. It followed from Eq. (3) that the temperature of dissolution of TiC was 1216°C. At a higher temperature the TiC particles start to dissolve. The dissolution temperature of the NbC carbide computed from Eq. (4) is 1146°C. Thus, to provide total dissolution of TiC and NbC the temperature of the hold should be higher than 1216°C.
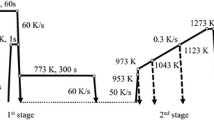
It is well known that the fine particles of second phase may hinder migration of grain boundaries. In the steel studied the microalloying elements Ti and Nb provide precipitation of fine carbide particles in the matrix below 1216°C. These particles fix the grain boundaries and prevent their coarsening. When the temperature is increased from 1200 to 1250°C, most of the particles of TiC and NbC precipitated in the casting process dissolve in the austenite grains; the effect of fixation of grain boundaries weakens, and the austenite grains grow. Analysis of the precipitates agrees with the results presented in Fig. 2 a. However, it should be noted that the abnormal growth of austenite grains may also be a result of the diffusion-free nature of the transformation at high temperatures [15, 16]. Therefore, for complete dissolution of the carbides of the microalloying elements the hold temperature should exceed 1216°C. However, by the data of the analysis given above, the hold temperature should be below 1216°C in order to prevent coarsening of the austenite grains. It is assumed that the austenite grains should be fine for obtaining a fine final microstructure. On the other hand, the precipitates of titanium and niobium carbides should be dissolved completely during the austenitizing for the refinement of the grains to develop due to dynamic and static recrystallization in the rolling process so that the subsequent cooling could provide precipitation hardening. The coarse austenite grains should be refined under multiple recrystallization in the rolling process. To prove this hypothesis, we performed a contrasting test. Figure 4 a and b present the microstructure of specimens after 60-min annealing at 1200 and 1300°C under laboratory conditions. The grain size D of the austenite in these specimens is 14 and 74 μm respectively. Figure 4 c and d present the structure of specimens from commercial hot-rolled strips after hot rolling, cooling, and holding at 1190 and 1300°C for 150 min.
The chemical composition of the specimens is the same, the temperatures of the end of the treatment and of coiling are 860 and 570°C respectively. The final grain size is 3.30 and 3.34 μm; the yield strength is 645 and 712 MPa respectively. The difference in the strength characteristics of the specimens is explainable by the difference in the distribution of precipitates in them, because the microstructure is about similar, but the higher heating temperature promotes precipitation hardening.
The size of the austenite grains in the commercial specimens heat treated at 1300°C should be equivalent or higher than in the specimen treated in the laboratory at the same temperature due to the longer hold time. In addition, the size of the austenite grains in the specimen of commercial steel after austenitizing at 1300°C should be much larger than after austenitizing at 1190°C. However, the final size of the austenite grains in the two industrial specimens after multipass rolling, laminar cooling and holding at different temperatures is about equal and the grains are strongly refined, though the size of the austenite grains after the hold at 1300°C is 74 μm. The industrial test allows us to assume that the size of the austenite grains does not influence much the final microstructure of the steel after multipass rolling and cooling. Consequently, the dissolution of precipitates from the compounds of microalloying elements is the most important factor to be considered in austenitizing. Only when the microalloying elements are dissolved, they can precipice in the form of carbonitrides during subsequent rolling, hinder the growth of austenite grains and thus refine the final grains. At the same time, cooling may yield an additional number of nanoscale precipitates intensifying the precipitation hardening and raising the final mechanical properties. The analysis made allows us to conclude that the temperature of austenitizing for the steel studied should exceed 1216°C.
Conclusions
We have studied the structure of a high-strength steel alloyed with titanium and niobium and obtained an approximating kinetic equation for growth of the austenite grains. There is a critical austenitizing temperature below which the austenite grains grow slowly due to fixation of the grain boundaries by precipitates. Above this temperature the grains grow rapidly due to dissolution of the carbonitrides. For a refining process to develop in the course of dynamic and static recrystallization of the austenite under rolling and for precipitation hardening under cooling, the carbonitrides of titanium and niobium should be dissolved completely during the austenitizing. For this reason, the heating temperature should be higher than the critical temperature of coarsening of the austenite grains.
References
A. Danon, C. Servant, A. Alamo, and J. C. Brachet, “Heterogeneous austenite grain growth in 9Cr martensitic steels: influence of the heating rate and the austenitization temperature,” Mater. Sci. Eng. A, 348, 122 – 132 (2003).
R. Stas’ko, H. Adrian, and A. Adrian, “Effect of nitrogen and vanadium on austenite grain growth kinetics of a low alloy steel,” Mater. Charact., 56, 340 – 347 (2006).
J. Maity and D. K. Mandal, “Isothermal grain growth of austenite in hypoeutectoid and hypereutectoid plain carbon steels,” J. Iron Steel Res. Int., 17, 38 – 43 (2010).
S. S. Zhang, M. Q. Li, Y. G. Liu, et al., “The growth behavior of austenite grain in the heating process of 300M steel,” Mater. Sci. Eng. A, 28, 4967 – 4972 (2011).
C. G. Andre’s, M. J. Bartolome, C. Capdevila, et al., “Metallographic techniques for the determination of the austenite grain size in medium-carbon microalloyed steels,” Mater. Charact., 46, 389 – 398 (2001).
L. N. Duan, J. M. Wang, Q. Y. Liu, et al., “Austenite grain growth behavior of X80 pipeline steel in heating process,” J. Iron Steel Res. Int., 17, 62 – 66 (2010).
L. Zheng, Z. X. Yuan, S. H. Song, et al., “Austenite grain growth in heat affected zone of Zr – Ti bearing microalloyed steel,” J. Iron Steel Res. Int., 19, 73 – 78 (2010).
S. Illescas, J. Fernandez, and J. M. Guilemany, “Kinetic analysis of the austenitic grain growth in HSLA steel with low carbon content,” Mater. Lett., 62, 3478 – 3480 (2008).
M. Maalekian, R. Radis, M. Militzer, et al., “In situ measurement and modelling of austenite grain growth in a Ti/Nb microalloyed steel,” Acta Mater., 60, 1015 – 1026 (2012).
K. Banerjee, M. Militzer, M. Perez, and X. Wang, “Nonisothermal austenite grain growth kinetics in a microalloyed X80 linepipe steel,” Metall. Mater. Trans. A, 41, 3161 – 3172 (2010).
C. X. Yue, L.W. Zhang, S. L. Liao, and H. J. Gao, “Kinetic analysis of the austenite grain growth in GCr15 steel,” J. Mater. Eng. Perform., 19, 112 – 115 (2010).
Y. Xu, D. Tang, Y. Song, and X. G. Pan, “Prediction model for the austenite grain growth in a hot rolled dual phase steel,” Mater. Des., 36, 275 – 278 (2012).
C. M. Sellars and J. A. Whiteman, “Recrystallization and grain growth in hot rolling,” Metall. Sci., 13, 187 – 194 (1979).
P. A. Manohar, D. P. Dunne, T. Chandra, and C. R. Killmore, “Grain growth predictions in microalloyed steels,” ISIJ Int., 36, 194 – 200 (1996).
V. Sadovsky, Metal Science and Heat Treatment of Steel, Vol. 2 (1983).
V. D. Sadovsky, Inheritance of Structure in Steel [in Russian], Metallurgiya, Moscow (1973).
K. J. Irvine, F. B. Pickering, and T. Gladman, “Grain refined C – Mn steels,” J. JISI, 205, 161 – 182 (1967).
H. Nordberg and B. Aronsson, “Solubility of niobium carbide in austenite,” J. JISI, 12, 1263 – 1266 (1968).
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (NSFC) (No. 51274154). The National High Technology Research and Development Program of China (No. 2012AA03A504), the State Key Laboratory of Development and Application Technology of Automotive Steels (the Baosteel Group), and the Hubei Education Committee (key project, No. 2121101).
Author information
Authors and Affiliations
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 1, pp. 7 – 12, January, 2017.
Rights and permissions
About this article
Cite this article
Yang, H.L., Xu, G., Wang, L. et al. A Study of Growth of Austenite Grains in a Steel Microalloyed with Ti and Nb. Met Sci Heat Treat 59, 8–13 (2017). https://doi.org/10.1007/s11041-017-0093-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0093-1