The effect of iron in an amount ranging from 0.7 to 17.7% and of carbon on the high-temperature strength, corrosion-resistance, and mechanical characteristics of an alloy of type KhN78T is studied. It is shown that the admissible content of iron in the alloy may be raised to 10% at an inconsiderable decrease in the high-temperature strength and without changes in the other operating properties and processing behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel alloys with 20% chromium (KhN78T, Nimonic 75, Nicrofer 7520, etc.) are used in various branches of engineering as heat-resistant and corrosion-resistant materials. Alloy KhN78T (ÉI 435) possesses good stability in oxidizing environments at high temperatures. The recommended maximum temperature of long-term (for up to 10,000 h) operation is 1100°C. The temperature of the start of intense formation of scale in air is above 1150°C. The ultimate long-term strength (σ1000 ) at 1000 and 1100°C is 8 and 4 N/mm2 respectively [1].
Due to its expensiveness and scarceness alloy KhN78T is used for making little loaded critical parts in the aircraft, space and other important industries, where the requirements on reliability are especially strict.
In addition to the high high-temperature stability alloys of the Ni – 20% Cr system have a high corrosion resistance in such aggressive environments as chlorine hydride, fluorine hydride (up to 560°C), etc. [2].
When an alloy is used as a corrosion-resistant material, it should also possess resistance to intercrystalline corrosion in welded joints. This kind of local fracture is determined in the first turn by the carbon content in the alloy.
In accordance to the GOST 5632–72 Standard [3] nickel-base alloy KhN78T (ÉI 435) has the following chemical composition (in wt.%): ≤ 0.12 C, ≤ 0.8 Si, ≤ 0.7 Mn, 19.0 – 22.0 Cr, 0.15 – 0.3 Ti, ≤ 0.15 Al, ≤ 1.0 Fe, ≤ 0.010 S, ≤ 0.015 P [3].
Since the price of nickel has increased markedly in the recent years, it is expedient to lower its content in the alloy, which may be attained, in particular, by raising the content of iron, which is limited to 1% in the Standard. In alloy KhN78T iron is an admixture not introduced into the metal on purpose but getting into it from the blend. Should its admissible content be increased, the blend material will become less expensive.
The aim of the present work was to determine the laws of the influence of iron (0.7 – 17.7%) and carbon (0.023 –0.072%) on the high-temperature strength, corrosion resistance including that to intercrystalline corrosion, and other characteristics of an alloy of type KhN78T in order to determine the possibility of saving nickel by raising the iron content in the alloy without lowering its operating and production properties. This would lower the cost of the alloy and widen the range of its application.
Methods of Study
We studied specimens of laboratory heats of alloy 20% Cr – (0.16 – 0.45)% Ti – Ni (base) with different contents of carbon and iron (Table 1).
The chosen compositions of the heats allowed us to estimate the effect of iron in an amount of 0.7 – 17.7% on the properties of the chromium-nickel base with 20% Cr. We could also compare the properties of heats with different carbon contents.
The specimens were prepared from hot-rolled sheets with a thickness of 5 mm in quenched condition (1080°C, water cooling).
The GOST 5632–72 Classification Standard [3] recommends the maximum temperature of 1100°C for long-term (for up to 10,000 h) operation of the alloy. To estimate the dependence of the high-temperature strength of the chromium-nickel base on the content of iron and carbon we tested specimens 3.0 × 15 × 40 mm in size by the weighing method in laboratory furnaces in an atmosphere of still air at a temperature of 1100 and 1200°C for 500 h with a cycle of 100 h. We tested 3 specimens of each heat at each temperature (GOST 6130–71).
After each cycle of testing the specimens were cooled and weighed together with the detached scale and then without the scale. This allowed us not only to determine the growth in the mass of the specimens during the long-term test and the oxidation rate but also the content of the scale detached from the specimens.
The scale formed on alloy KhN78T as a result of cyclic high-temperature testing is represented by spinel of type NiO · Cr2O3 [5]. In the specimens with elevated iron content the formula has the form (Fe, Ni)O · (Fe, Cr)2O3. The proportion of the mass of the metal to the mass of oxygen in the scale is about 2.6.
To determine the loss in mass of the metal to oxidation (i.e., mass of the metal that has gone to scale) the growth in the mass (i.e., the mass of the oxygen) should be multiplied by 2.6. The mass the whole of the formed scale is equal to the sum of the masses of oxygen and metal in the formed scale, i.e., to the sum of the increase and decrease in the mass as a result of oxidation.
To evaluate the depth of oxidation of the metal in millimeters per year we should divide the value of the decrease in the mass (in g/m2· h) by the density of the alloy, which is equal to 8.4 g/cm3, or multiply the value of the increase in the mass by 2.7.
If the mass of the oxidized metal is 2.6 times higher than the mass of the oxygen that has participated in the oxidation reaction, the mass of the scale is 3.6 times higher than the mass of the oxygen, i.e., than the growth in the mass due to oxidation of the metal.
The mass of the scale retained on the metal and not detached under cooling is determined as the difference in the mass of the formed scale and the mass of the detached scale.
The corrosion resistance and the susceptibility to intercrystalline corrosion (ICC) of the rolled sheets was evaluated after quenching (1080°C, 10 min, in water) and subsequent provoking heating at 600, 700, and 800°C for from 10 to 60 min. The tests were performed in a control solution of H2SO4 (335 cm3 ) + HNO3 (268 cm3) + H2O (1000 cm3 ) at a temperature of 80°C for 96 h. The corrosion rate was evaluated by the method of gravimetry; the susceptibility to ICC was estimated by bending of the specimens and by metallography according to the GOST 6032–2003 Standard [6].
The microstructure of the alloy after quenching and tempering was studied by the method of optical metallography. We determined the mechanical properties of the alloy by tensile tests at 20°C [7] and in the range of hot plastic deformation (1100 – 1200°C) [8].
Results and Discussion
High-Temperature Strength
The results of the study of the high-temperature strength are presented in Table 2 and in Figs. 1 and 2.
Kinetic curves of oxidation of an alloy of type KhN78T with variable content of iron (∆m is the growth in the mass, v ox is the oxidation rate): a, c, e) t test = 1100°C; b, d, f ) t test = 1200°C; a – d ) growth in the mass as a function of the duration of testing; e, f ) oxidation rate as a function of the duration of testing; 1 ) 0.7% Fe; 2 ) 4.5% Fe; 3 ) 8.9% Fe; 4 ) 10.4% Fe; 6 ) 17.7% Fe.
High-temperature strength of alloys of type KhN78T (_m is the growth in the mass) as a function of the iron content (a, b ) and of the duration of the test (c – f ): a, c, e) t test = 1100°C; b, d, f ) t test = 1200°C; a, b ) duration of testing: 1 ) 100 h, 2 ) 200 h, 3 ) 300 h, 4 ) 400 h, 5 ) 500 h; c, d ) content of scale detached from the specimens during testing; e, f ) content of scale retained on the specimens after testing; iron content in the alloys: 1 ) 0.7%, 2 ) 4.5%, 3 ) 8.9%, 4 ) 10.4%, 6 ) 17.7%.
Comparing the rates of oxidation of the metal of the heats with compositions close to the base one but differing in the carbon content (heats 3 and 4 in Table 1) we detected a substantial negative action of carbon on the resistance of the metal to oxidation only at 1200°C and maximum holds (Table 2). At 1100°C the carbon produced no effect. Should it affect the metal by bonding into chromium carbides, which makes the system refractory, the effect would have manifested itself at 1100°C too. It is possible that at the higher temperature the oxidation of carbon promotes loosening and detachment of a thicker scale layer.
Comparing the results of the study of heats 1 and 3 with minimum and high contents of carbon (0.7 and 8.9% respectively) and virtually equal contents of carbon we established that the effect of iron at 1100°C was not high and decreased with time. At 1200°C the effect of carbon was much stronger.
Since the carbon content did not influence the resistance to oxidation at 1100°C, we plotted the results obtained at this temperature for all the heats containing from 0.023 to 0.072% C. The results of the tests of the heat with 0.023% C at 1200°C were not plotted.
At 1100°C (Fig. 1a and c ) the oxidation process obeyed a parabolic law for all the heats, and the intensity of the process lowered with time. This is confirmed by the form of the curves describing the dependence of the oxidation rate on the time (Fig. 1e ). Note that the differences in the characteristics of the high-temperature strength of all the alloys studied are not high; with time, the values of the oxidation rates for different heats get closer, and range from 0.08 to 0.13 g/(m2 · h), i.e., are less than 0.5 mm/year in the case of the 500-h test. After testing, the surface of the specimens is smooth and the content of the detached scale is low.
Since the recommended maximum temperature of operation of alloy KhN78T in an oxidizing environment is 1100°C, the results obtained allow us to conclude that from the standpoint of preservation of high heat resistance the admissible content of iron in KhN78T should be increased considerably as compared to the 1% limit standardized by GOST 5632.
At a temperature of 1200°C the rate of oxidation of all the alloys increases markedly (Fig. 1b, d, and f ). In addition, the process of oxidation of some alloys acquires a linear form at a hold of 200 – 300 h, i.e., the intensity of the process does not decrease with time, and the true oxidation rate remains constant. The differences in the alloys increase too. The alloy with 0.7% Fe exhibits the best heat resistance.
The oxidation rate of the alloy with 17.7% Fe increases especially strongly (Fig. 1b, d, and f ) and amounts to 0.42 g/(m2 · h). Recalculation shows that the depth of the oxidation in this case is over 1 mm/year. The surface of the specimens after the tests is uneven; the content of the detached scale is high.
Figure 2a and b present dependences of the heat resistance on the iron content. The heat resistance is affected the most when iron is introduced in an amount of 4.5%.
The scale formed as a result of oxidation of the standard alloy KhN78T containing less than 1% Fe in cyclic tests consists of NiO · CrO3 spinel [5]. Upon the introduction of 4.5% Fe into the alloy the scale is rearranged, and the oxidation is accelerated. Iron ions may be present in the structure of the scale in bivalent and trivalent variants, and the spinel formula acquires the form (Fe, Ni)O · (Fe, Cr)2O3.
Increase in the content of iron to 8.9% decreases somewhat the oxidation rate, which remains virtually constant until 17.7% Fe. This may be connected with stabilization of the structure of the scale.
Growth of the temperature to 1200°C (Fig. 2b ) intensifies the influence of iron on the heat resistance of the chromium-nickel base and changes the nature of the influence. At short holds the effect of iron is not considerable and resembles that at 1100°C. When the hold time is prolonged to 500 h, the effect is multiplied strongly: the oxidation rate does not decrease at 8.9% Fe. At a hold of 100 h the oxidation rate of the alloy with 17.7% Fe is 22.2% higher than that of the alloy with 0.7% Fe; in the case of 500-h hold it is higher by a factor of 2.4.
Here we should pay attention to the carbon content. Firstly, the heat with 17.7% Fe contains less carbon than the heat with 0.7% Fe (0.037 and 0.070% respectively), i.e., the differences at the same carbon content should be even higher. Secondly, Fig. 2b (the large badges) presents the results of the tests for heat 4 with 0.023% C and 10.4% Fe. At long holds (300 – 500 h) the badges are arranged much below the curves plotted for the heats with higher carbon content.
The data obtained by weighing of specimens together with the detached scale and without the scale present interest. The content of scale fallen from the specimens tested for 100 – 400 h is presented in Fig. 2c and d . The mass of the scale fallen from all the alloys increases monotonically, and the increase is more considerable at 1200°C.
We have mentioned already that the scale formed on alloy KhN78T due to cyclic high-temperature tests is represented by NiO · Cr2O3 spinel [5]. In the alloys studied the ratio of the mass of the metal to that of oxygen in the scale in the presence of iron is about 2.6. The proportion of the total mass of the scale formed to the mass of the oxygen participating in its formation is 3.6 : 1.
Having the data on growth in the mass of the specimens as a result of oxidation, which corresponds to the mass of the oxygen spent for the formation of scale, we can calculate the total mass of the scale formed and the mass of the scale retained on the metal.
The results of the calculation (Fig. 2e and f ) show that at both test temperatures the lowest content of scale, the thickness of which depends little on the duration of the test, is retained on the metal with the least iron content, which possesses the best heat resistance. The thickness of the scale retained on the alloys bearing 4.5 – 10.4% Fe is somewhat higher; it is virtually the same in the three alloys and increases somewhat with time (Fig. 2e ).
Note that when the temperature is increased from 1100 to 1200°C, the oxidation of all the alloys studied is accelerated, and the acceleration is manifested chiefly by growth in the mass of the scale fallen from the metal (Fig. 2c and d ). The thickness of the scale retained on the specimens after the tests changes little (Fig. 2e and f ).
Our study has shown that alloy KhN78T possesses high heat resistance at a temperature of 1100°C, which does not depend on the carbon content in the range studied (0.023 –0.072%) and decreases insignificantly upon the introduction of iron (instead of nickel) in the range studied (0.7 – 17.7%).
Corrosion Resistance
Table 3 and Fig. 3 present the results of testing of specimens of an alloy of type KhN78T in a control solution of (H2SO4 + HNO3 ) for total corrosion strength and susceptibility to ICC after quenching and subsequent provoking heating at 600, 700 and 800°C for from 10 to 60 min.
When the iron content in the alloy of the Ni – 20% Cr system was increased from 0.7 to 17.7% (independently of the carbon content), the total corrosion resistance was not affected either in the quenched condition or after the provoking heating at 800°C for 30 min. The corrosion rate in both cases did not exceed 0.020 g/(m2 · h). At the same time, in the case of 30-min heating at 700°C the corrosion rate increased with growth in the carbon content (from 0.023 to 0.072%) and in the iron content (from 0.7 to 17.7%) (Table 3).
The rate of corrosion of the alloy with 0.7% Fe and high carbon content (0.07% C at Ti/C = 2.3, heat 1 ) did not exceed 0.030 g/(m2 · h), whereas the alloy with 17.7% Fe and reduced carbon content (0.037% C at Ti/C = 7.8, heat 6 ) had a 3.6 times higher rate of corrosion, i.e., 0.110 g/(m2 · h). The increase in the corrosion rate was caused by intercrystalline corrosion, the degree of the development of which was determined by the iron content in the alloy.
The “temperature – time – ICC” curves in Fig. 3 reflect the fact that growth in the iron content accelerates the development of ICC in the alloy. For example, in the alloy bearing 0.07% carbon and 8.9 and 0.7% iron the minimum hold (τmin ) at 700°C at which the ICC arises is 10 min and 30 min respectively (Fig. 3a ). A similar law is observed in the alloy with 0.037 – 0.05% C upon decrease in the iron content from 17.7 to 4.5% (Fig. 3b ). In both cases the decrease in the iron content is accompanied by decrease in the Ti/C ratio. At a higher iron content the negative effect of carbon is manifested more strongly and this requires increase of Ti/C.
In the alloys with 8.9% Fe and 0.072% C (Ti/C = 4.6) and with 10.4% Fe and 0.023% C (Ti/C = 11.7) τmin at 700°C is equal to 10 and 60 min respectively (Fig. 3c ).
The development of ICC in alloys of the Ni – 20% Cr system is caused by depletion of the near-boundary regions of chromium due to precipitation of M23C6 carbides in the form of interconnected chains over grain boundaries.
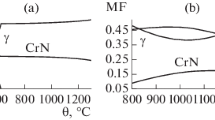
Studying the microstructure of the alloys after quenching and tempering at 700 and 800°C for 30 min we established (Fig. 4) that increase in the carbon content (from 0.023 to 0.072%) and in the iron content (from 0.7 to 17.7%) in the alloy with 20% chromium after tempering at 700°C was accompanied by growth of the content of grain-boundary precipitates of M23C6 carbides, which affected negatively the resistance of the alloys to ICC.
Microstructure of rolled sheets from the metal of the tested heats of the Ni – 20% Cr system with 0.7% Fe and 0.07% C (a), with 4.5% Fe and 0.050% C (b ), with 8.9% Fe and 0.072% C (c), and with 10.4% Fe and 0.023% C (d ) after a heat treatment involving quenching from 1080°C in water and tempering at 700°C for 30 min (×100).
Mechanical Properties
The mechanical properties of rolled 5-mm-thick sheets from alloys with different contents of iron and carbon, which were tested at 20°C according to the GOST 1497–84 Standard [7] (after water quenching from 1080°C) and at 1100 and 1200°C according to the GOST 9651–84 [8] (in the state after hot rolling) are presented in Table 4.
Independently of the content of carbon and iron in the alloy the metal of all the heats studied in quenched condition exhibits a high level of strength and ductility properties at a temperature of 20°C. At the same time, whatever the iron content in the alloy, decrease in the carbon content is accompanied by decrease in the strength properties. When the carbon content decreases from 0.07% (heats 1 and 3 ) to 0.023% (heat 4 ), the rupture strength decreases from 700 to 635 MPa and the yield strength decreases from 380 to 300 MPa.
It follows from the data presented in Table 4 that independently of the content of iron and carbon the alloys differ little in the level of the strength and ductility properties in the temperature range of hot plastic deformation (1000 – 1200°C).
Conclusions
-
1.
As a result of a complex study of the structure and properties (the heat and corrosion resistances including the resistance to intercrystalline corrosion, the mechanical properties at 20°C and in the range of hot plastic deformation) of alloys of type KhN78T with varied content iron and carbon we have shown the possibility of widening the admissible content of iron to 8 – 10% at insignificant lowering of the high-temperature strength and without worsening of the other operating and production properties.
-
2.
Alloys of the Ni – 20% Cr – 0.3% Ti system containing up to 10% Fe should have a Ti/C ratio no lower than 6 in order to provide resistance to intercrystalline corrosion.
-
3.
When the carbon content is increased within 0.023 –0.072%, the heat resistance of the matrix with 10% Fe at a temperature of 1100°C remains unchanged; at 1200°C it decreases.
-
4.
When the test temperature is raised from 1100 to 1200°C, the oxidation process is accelerated, which is manifested chiefly in a considerable increase in the content of fallen scale and affects little the thickness of the scale retained on the metal.
References
A. P. Shlyamnev, T. V. Svistunova, O. B. Lapshina, et al., Corrosion-Resistant, Refractory and High-Strength Steels and Alloys, a Reference Book [in Russian], Intermet Engineering, Moscow (2000), pp. 172 – 174.
E. A. Ul’yanin, T. V. Svistunova, and F. L. Levin, Corrosion-Resistant Alloys Based on Iron and Nickel [in Russian], Metallurgiya, Moscow (1986), 254 p.
GOST 6532–72. High-Alloy Steels and Corrosion-Resistant Refractory Alloys. Grades and Performance Specification [in Russian].
GOST 6130–71. Metals. Methods of Determination of Heat Resistance [in Russian].
A. R. Krylova, N. N. Kozlova, and D. N. Zharkova, “Long-term oxidation of standard heat-resistant steels and alloys KhN78T, KhN70Yu, KhN60Yu, Kh25N20S2 at 1150 and 1200°C,” Sbornik Trudov TsNIIChERMET, No. 52, 113 – 119 (1968).
GOST 6032–2003. Corrosion-Resistant Steels and Alloys. Methods of Determination of Resistance to Intercrystalline Corrosion [in Russian]
GOST 1497–84. Metals. Methods of Testing of Metals for Tensile Strength [in Russian].
GOST 9651–84. Metals. Methods of Testing for Tensile Strength at Elevated Temperatures [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 7, pp. 12 – 19, July, 2015.
Rights and permissions
About this article
Cite this article
Svistunova, T.V., Kozlova, N.N., Shevakin, A.F. et al. Effect of Iron and Carbon on the Structure and Properties of Chromium-Nickel Alloy. Met Sci Heat Treat 57, 379–385 (2015). https://doi.org/10.1007/s11041-015-9893-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-015-9893-3