The main results obtained by metal scientists of the Chelyabinsk Polytechnic Institute (the South-Ural State University) under the guidance of M. M. Shteinberg in the field of the kinetics of martensitic transformations in iron alloys and its variation under the action of alloying, precipitation hardening and phase hardening are considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
M. M. Shteinberg has always declared his belonging to the Ural school of metal scientists famous for its pioneer studies of the laws of martensitic transformation. He devoted exceptional attention to the kinetics of martensitic transformations in iron alloys, its variation under the effects of alloying, precipitation hardening and phase hardening and to the mechanical properties of metastable austenitic alloys under various kinds of deformation. The main results of these studies are as follows.
A complex method has been developed for determining the type of the kinetics of martensitic transformation under cooling in the alloys with poorly manifested kinetic features. The method consists in joint investigation of the macro- and microkinetics of formation of martensite and of the action of a high-intensity pulsed magnetic field (about 330 kOe) on austenite. This method has made it possible to detect some special features of the kinetics of formation of martensite not observed earlier, which has lead to some novel conclusions and generalizations [1].
The transition from a “rapid isothermal” to an explosive transformation upon alloying occurs in a narrow concentration range within which the macrokinetics remains unchanged while the microkinetics exhibits a lot of jumps corresponding to formation of small groups of crystals of lenticular martensite. The reverse transition also develops gradually with disappearance of first macroscopic and then microscopic jumps [2].
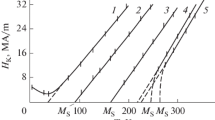
The effect of elements on the martensitic point depends on the composition of the matrix not only where its value is concerned but sometimes even its direction [3–6].
Only athermal martensite can form in a pulsed magnetic field (PMF) whatever the composition of the alloy and the kinetics of the transformation under cooling. In the alloys with isothermal type of the transformation martensite forms in a PMF only under the condition that the development of an athermal transformation is principally possible below M s isoth , and the temperature of the action of the field T puls is such that T puls – M s ath is lower than the shift δT 0 in the PMF. It has been shown that tests with PMF make it possible to predict the development of athermal transformation below M s isoth due to rapid cooling of alloys in which martensite is commonly formed by the isothermal mechanism [1, 7, 8].
The transition from one kinetic type to the other is most frequently observed upon a change in the chemical composition of the alloy. Growth in the strength of the austenite above some limiting value is followed by a change in the morphology of the martensite, which is required for lowering of the elastic energy, and by a corresponding change in the transformation kinetics from an isothermal one to an athermal one. This transition can occur not only upon alloying, but also due to other methods of hardening. i.e., upon phase hardening or precipitation hardening. In the latter case the transition to an explosive athermal transformation is observed after low-temperature aging (450 – 500°C) of some alloys susceptible to precipitation of intermetallics and develops despite the fact that the accompanying change in the chemical composition of the austenite should hinder the process. High-temperature aging (above 600°C) of “athermal” alloys transfers them into the category of “isothermal” ones. The initial isothermal kinetics of the martensitic transformation does not change as a result of high-temperature aging [7, 9, 10].
Phase hardening not aggravated by accompanying phenomena should lower M s of both isothermal and athermal transformations. In “explosive” alloys M s may lower, may remain unchanged, or may grow depending on the special features of the chemical composition, on the structure of the austenite, and on the degree of the development of the processes accompanying the phase hardening. The explosive formation of martensite is suppressed by phase hardening, and the martensite curve is smoothed. However, this does not remove the athermal mode of the transformation, i.e., large “explosions” are broken into a lot of small jumps detectable in a study of the microkinetics of the transformation [3, 11–13].
The appearance of an athermal transformation due to phase hardening in some alloys with isothermal kinetics of formation of martensite is a result of a shift of the T – τ C-curve and hence of a delay of the competing isothermal transformation [3, 11].
Thermal stabilization of austenite due to pinning of dislocations by interstitial impurities at a temperature exceeding M s (at 400 – 500°C) is possible not only in the alloys with isothermal transformation kinetics, as it has been assumed earlier, but also in the alloys with an athermal transformation. However, this requires a higher carbon content (about 0.05%) than in the alloys with isothermal transformation (0.001%). The formation of clusters, zones, or dispersed particles due to aging at 475°C intensifies considerably the thermal stabilization of the austenite, and the precipitation of carbides does not play a substantial role.
Generalization of the experimental data resulted in the development of novel methods of hardening treatment of metastable austenitic alloys eliminating the appearance of martensite in them in the final condition [14–16].
It is expedient to classify the products of the transformation of austenite due to deformation as stress martensite, which resembles the cooling-induced martensite genetically and morphologically, and as fine dispersed strain martensite. As a rule, tensile testing makes it possible to observe only stress martensite; individual formation of these types of martensite is possible in torsion tests [7, 17].
In the case of formation of stress martensite in early stages of tensile testing the conventional yield strength σ0.2 may be attained long before the completion of the initial rectilinear segment of the stress–strain curve, when about 2.5% martensite forms on the average. The stress determined from the inflection of the stress–strain curve, which has earlier been treated as the conventional yield strength, has nothing to do with it and is much higher than the actual value of σ0.2.
To determine σ0.2 we should measure the residual elongation of the test pieces. A similar phenomenon has been observed in torsion tests [7, 17, 18].
Abnormal behavior of the temperature dependence of σ0.2 (its lowering near M s ) can be observed not only in the alloys with athermal martensitic transformation, as it has been assumed earlier, but also in the alloys with isothermal transformation kinetics upon cooling, especially if the latter have been strengthened by external or phase hardening, or alloying [18, 19].
The temperature dependence of the ductility of metastable austenitic alloys has a maximum whatever the kinetics of the martensitic transformation in cooling and the method of the deformation (uniaxial or biaxial tension, torsion). In tensile tests the ductility maximum is caused by formation of stress martensite and appears at a temperature 20 – 120°C above M s . The growth in the ductility under torsion is caused by the appearance of a high content of strain martensite and occurs at a temperature 120 – 250°C higher than the martensitic point [12, 18, 19].
The following results have practical importance for advancement of metastable austenitic alloys:
-
the data on the influence of the principal alloying elements on the martensitic point of a great number of ironnickel-,iron-nickel-carbon-, iron-chromium-nickel- and iron-nickel-manganese-base alloys make it possible to choose the chemical composition for alloys with specified values of M s appropriate from the standpoint of production [3–6];
-
the results of the study of the interrelation of the formation of martensite, precipitation hardening and phase hardening in alloys with carbide and intermetallic aging are demanded for choosing the composition and treatment processes of the alloys [7,9–11,20];
-
the determined special features of formation of tensile and torsional mechanical properties of metastable austenitic alloys should be allowed for in the development of materials either hardened by deformation accompanied by formation of martensite or subjected to cold deformation with the aim to provide the required shape [7, 8, 19, 21, 22].
The scientific results presented characterize M. M. Shteinberg as a prominent metal scientist.
References
V. D. Povolotskii, L. G. Zhuravlev, and M. M. Shteinberg, “Martensitic transformation in iron-nickel alloys in a pulsed magnetic field,” Fiz. Met. Metalloved., 35(3), 567 – 571 (1973).
L. G. Zhuravlev, M. M. Shteinberg, and Yu. B. Peisakhov, “Macro- and microkinetics of martensitic transformation,” Fiz. Met. Metalloved., 38(1), 191 – 197 (1974).
L. G. Zhuravlev, A. F. Shcherbakova, M. M. Shteinberg, et al., “Effect of alloying, external and internal phase hardening on “explosive” martensitic transformation in iron-nickel-carbonbase alloys,” Fiz. Met. Metalloved., 31(2), 431 – 433 (1971).
V. V. Golikova, V. N. Gonchar, L. G. Zhuravleva, M. M. Shteinberg, and G. I. Medvedeva, “A study of martensitic transformation in iron-nickel-base alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works No. 53, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1969), pp. 141 – 148.
A. F. Shcherbakova, L. G. Zhuravlev, M. M. Shteinberg, et al., “A study of martensitic transformation in iron-nickel-carbon- base alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works No. 78, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1970), pp. 1057 – 162.
V. V. Golikova, M. M. Shteinberg, L. G. Zhuravlev, et al., “Effect of alloying elements on martensitic transformation in iron-nickel-base alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works No. 66, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1970), pp. 136 – 146.
L. G. Zhuravlev, V. D. Povolotskii, and M. M. Shteinberg, “Influence of plastic deformation, phase hardening and pulsed magnetic field on martensitic transformation in alloys of various compositions,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 133, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1974), pp. 139 – 142.
V. D. Povolotskii, L. G. Zhuravlev, and M. M. Shteinberg, “Effect of magnetic field on martensitic transformation in Fe – Ni-base alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 118, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1973), pp. 132 – 245.
L. G. Zhuravlev, M. M. Shteinberg, and Yu. B. Peisakhov, “Stabilization of austenite in alloys with isothermal and athermal kinetics of martensitic transformation,” Izv. Vysh. Uchebn. Zaved., Chern. Metall., No. 4, 138 – 140 (1976).
V. Yu. Kuznetsov and M. M. Steinberg, “Effect of phase and external hardening on stability of supercooled austenite of structural steels in the intermediate range,” Izv. Vysh. Uchebn. Zaved., Chern. Metall., No. 2, 64 – 67 (1982).
L. G. Zhuravlev, V. D. Povolotskii, and M. M. Shteinberg, “Effect of phase hardening on isothermal and athermal martensitic transformation,” Fiz. Met. Metalloved., 36(1), 109 – 114 (1973).
L. G. Zhurvalev, Yu. B. Peisakhov,M.M. Shteinberg, et al., “Effect of phase hardening and plastic deformation on martensitic transformation in precipitation-hardening alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 147, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1974), pp. 121 – 126.
L. G. Zhuravlev, M. M. Shteinberg, Yu. B. Peisakhov, and A. N. Emelyushin, “Martensitic transformation in precipitation- hardening alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 177, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1976), pp. 117 – 122.
Yu. B. Peisakhov, L. G. Zhuravlev, and M. M. Shteinberg, “A method for treating metastable austenitic iron-nickel alloys, USSR Inv. Certif. No. 855017, MKI C 21 D 1/78, No. 2845461/22-02,” Appl. 03.12.79; Publ. 15.08.81, Byull. Izobr. Polezn. Modeli, No. 30.
Yu. B. Peisakhov, L. G. Zhuravlev, and M. M. Shteinberg, “A method for heat treatment of articles from metastable austenitic steels with intermetallic hardening, USSR Inv. Certif. No. 876744, MKI C 21 D1/78, No. 2858150/22-02,” Appl. 26.12.79; Publ. 30.10.81, Byull. Izobr. Polezn. Modeli, No. 40.
Yu. B. Peisakhov, L. G. Zhuravlev, and M. M. Shteinberg, “A method for heat treatment of articles from austenitic iron-nickel alloys with athermal kinetics of martensitic transformation, USSR Inv. Certif. No. 985084, MKI C 21 D 6/04, No. 3001081, Appl. 03.10.80; Publ. 30.12.82, Byull. Izobr. Polezn. Modeli, No. 48.
M. M. Shteinberg, A. F. Shcherbakova, L. G. Zhuravlev, et al., “Effect of plastic deformation, phase hardening and aging on martensitic transformation in alloyed alloys based on Fe – Ni – C,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 107, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1972), pp. 146 – 150.
L. G. Zhuravlev, M. M. Shteinberg, O. P. Chernogorova, and V. V. Zhuravleva, “Formation of martensite under various kinds of deformation,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 299, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1979), pp. 66 – 72.
M. M. Shteinberg, L. G. Zhuravlev, Yu. B. Peisakhov, and O. P. Chernogorova, “Abnormal lowering of the yield strength near M s in metastable austenitic alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 177, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1976), pp. 123 – 125.
M. M. Shteinberg, D. A. Mirzaev, and L. G. Zhuravlev, “Special features of martensitic transformation in iron and its alloys” Metalloved. Term. Obrab. Met., No. 9, 21 – 29 (1972).
V. V. Golikova, L. G. Zhuravlev, M. M. Shteinberg, et al., “Formation of strain martensite in iron-nickel-base alloys,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 66, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1970), pp. 151 – 158.
L. G. Zhuravlev, M. M. Shteinberg, and O. P. Chernogorova, “Laws of formation of martensite in metastable austenitic alloys under various methods of deformation,” in: Aspects of Production and Treatment of Steel, Coll. Works, No. 202, Chelyabinsk Polytechnic Inst. [in Russian], Chelyabinsk (1978), pp. 89 – 98.
Author information
Authors and Affiliations
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 9, pp. 4 – 6, September, 2014.
Rights and permissions
About this article
Cite this article
Zhuravlev, L.G. Scientific Achievements of M. M. Shteinberg in the Field of Martensitic Transformations. Met Sci Heat Treat 56, 459–461 (2015). https://doi.org/10.1007/s11041-015-9782-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-015-9782-9