Abstract
Background
XIAP-associated factor 1 (XAF1) has been found to participate in the progression of multiple human cancers. Nevertheless, its role as well as the reaction mechanism in non-small cell lung cancer (NSCLC) still remains obscure.
Methods
In this study, the protein expression of XAF1 in NSCLC cell lines was evaluated using western blot. With the employment of CCK-8 assay, EdU staining, wound healing and transwell, capabilities of NSCLC cells to proliferate, migrate and invade were assessed. Cell apoptotic level and cell cycle were resolved utilizing flow cytometry. Western blot was applied for the estimation of apoptosis- and endoplasmic reticulum (ER) stress-related proteins.
Results
It was discovered that XAF1 expression was conspicuously reduced in NSCLC cell lines. XAF1 overexpression suppressed H1299 cell proliferative, invasive and migrative capabilities, but exhibited promotive effects on cell cycle arrest. Meanwhile, XAF1 overexpression inhibited cisplatin resistance in H1299 and H1299/DDP cells by promoting cell apoptosis and enhanced the expression levels of ER stress-related proteins CHOP, GRP78 and ATF4. What’s more, 4-PBA treatment reversed the impacts of XAF1 overexpression on the proliferative, invasive, migrative and apoptotic capabilities of H1299 cells, as well as cell cycle and cisplatin resistance.
Conclusion
In conclusion, XAF1 overexpression impeded the advancement of NSCLC and repressed cisplatin resistance of NSCLC cells through inducing ER stress, which indicated that XAF1 might be a novel targeted-therapy for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer, which is a predominant contributor to death resulting from malignant tumors, possesses the highest incidence and mortality worldwide [1]. Data released by the International Agency for Research on Cancer (IARC) demonstrated that lung cancer takes second place in the global incidence of malignant tumors in 2020 [2]. Non-small cell lung cancer (NSCLC), the most common histological type of lung cancer, makes up over 80% of lung cancer cases [3]. Due to the highly aggressive nature of NSCLC and the lack of effective early biomarkers, a plenty of patients suffering from NSCLC are already in the advanced stage at the time of diagnosis [4]. Despite the fact that tremendous progresses have been achieved in the treatment of lung cancer, the spread and metastasis of lung cancer cells still can’t be effectively controlled, resulting in poor prognosis of patients [5]. In recent years, the conventional chemotherapy regimen is mainly platinum combined with another chemotherapy drug (such as gemcitabine, pemetrexed), but the clinical treatment effect is not good enough, and some patients are susceptible to chemotherapy resistance [6]. The advent of targeted drugs has improved survival rates for patients with NSCLC [7]. However, drug resistance appears in the use of targeted drugs [8]. Some patients have various types of gene mutations after taking drugs, which causes drug resistance [9]. Therefore, it is of great urgency to develop new therapeutic targets for NSCLC patients.
As a XIAP-binding protein, X-linked inhibitor of apoptosis (XIAP)-associated factor 1 (XAF1) has been regarded as a tumor suppressor gene and was expressed lowly in a variety of malignant tumors including gastric, colon and pancreatic cancers [10,11,12,13]. XAF1 directly and preferentially binds XIAP BIR2 and antagonizes the anti-caspase activity of XIAP, thereby inducing apoptosis. In addition, XAF1 isolates XIAP after relocating it from the cytoplasm to the nucleus [14]. In human cancers, the loss of XAF1 has close association with tumor staging and cancer progression [15]. Existing study has shown that XAF1 expression was low in lung cancer tissues, and the regulation of XAF1 could repress lung cancer cell proliferative capability, induce apoptosis and impede tumor growth in nude mice [16]. However, the biological role of XAF1 in NSCLC and the potential mechanism are still incompletely understood. Therefore, this study aimed to investigate the role of XAF1 in NSCLC cells and the way in which XAF1 affects the progression of NSCLC.
Materials and methods
Cell culture
Human NSCLC cell lines H1703, H441, A549, H1299 and human normal bronchial epithelial cell line HBE were obtained from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). All cells were cultivated in DMEM (Hyclone, Logan, UT, USA), which was equipped with 10% FBS, 1% penicillin-streptomycin, in the presence of 5% CO2 at 37 °C. Cisplatin (DDP; Sigma-aldrich, USA) was dissolved in double-distilled water. The NSCLC cisplatin-resistant cell line H1299/DDP was developed by exposure of its parental cell to progressively increasing doses of DDP in cell culture medium. The drug-resistant phenotypes were maintained in drug-free medium for two weeks before the experiments. Then, 30 µM cisplatin was added into H1299/DDP cells. In addition, 4-PBA (500µM) was exposed to H1299 cells or H1299/DPP cells for 72 h to function as an endoplasmic reticulum (ER) stress inhibitor. Thapsigargin(THA; 0.5µM)was added into H1299 cells for 24 h to induce ER stress.
Cell transfection
To overexpress XAF1, the pc-DNA3.1 vector containing the whole length of XAF1 (OV-XAF1) and the empty vector (OV-NC) were synthesized by Gene Pharma. By virtue of Lipofectamine 2000 reagent (Invitrogen, USA), the transfection of 100 nM recombinants into H1299 cells was implemented in light of standard protocol. 48 h pos-transfection, cells were harvested for follow-up studies.
Cell counting kit-8 (CCK-8) assay
H1299 cells were seeded into 96-well plates and transfected with OV-XAF1 for 48 h in the presence or absence of 4-PBA for 72 h. Subsequently, cells were cultivated in DMEM medium with 10% FBS for 24 h, 48 and 72 h. After that, each well was added with 10 µL of WST-8 (Beyotime, Haimen, China) to cultivate cells for 2 h. The absorbance value was evaluated applying a microplate reader (Bio-Rad, USA) at 450 nm.
5-Ethynyl-2’-deoxyuridine (EdU) cell proliferation assay
The inoculation of H1299 cells in six-well plates (4 × 105 cells/well) was conducted, following which was the overnight cultivation. Subsequently, H1299 cells subjected to 4% polyformaldehyde fixation at room temperature for 1 h and 0.5% Triton X-100 cultivation for 15 min. Finally, cells were stained by Cell-Light™ EdU Cell Proliferation Detection Assay (Life, USA) and DAPI was employed for counterstaining for 10 min. The positive cells were totaled by means of a fluorescent microscope.
Wound healing assay
The inoculation of transfected H1299 cells into six-well plates (4 × 105 cells/well) was initially implemented, following which was the incubation till 90% cell confluency was achieved. The cell monolayers were then wounded with white pipette tips and washed four times with phosphate-buffered saline. After 24 h incubation, the migration rate was calculated based on the formula: (wound width at 0 h - wound width at 24 h)/wound width at 0 h × 100%. Five randomly chosen fields were analyzed in each well.
Transwell assay
The suspension of transfected H1299 cells was conducted in serum-free DMEM at a final concentration of 2 × 105 cells/mL. The transwell chambers were pre-coated with 0.1mL of matrigel (Becton Dickinson, MA) at 37 °C for 1 h. Cell suspensions were then loaded into the upper compartment, while medium with 10% FBS was placed on the lower compartment. 24 h post-incubation, the bottom of the chamber insert was subjected to 100% methanol fixation as well as 0.1% crystal violet staining. The number of invaded cells was totaled utilizing a microscope. Five randomly chosen fields were counted for each group.
Flow cytometry analysis
After indicated treatment, the transfected H1299 cells were subjected to 70% ethanol fixation, following which was the staining with a Cell Cycle Detection Kit (KeyGene, Holland) in light of recommended specifications. H1299 cells were analyzed at the Flow Cytometry Core Facility of University of Colorado Denver (UCD) with a FACScan flow cytometer (BD Biosciences, San Jose, CA).
Cell apoptosis analysis
Cell apoptosis was detected by the FITC Annexin V/PI Apoptosis Detection Kit I (Ribobio, Guangzhou, China) according to the manufacturer’s protocol. In short, the transfected H1299 cells were rinsed by precooled PBS and then re-suspended in binding buffer. After that, cells were probed with Annexin V-FITC for 15 min and PI (10 mg/ml) for 5 min at room temperature away from light. Flowjo software (Tree Star, Ashland, OR, USA) was employed for the analysis of apoptosis.
Western blot analysis
The total proteins were extracted from H1299 or H1299/DPP cells using RIPA buffer (Auragene, Changsha, China). BCA Protein Assay Kit (Dingguo, Beijing, China) was performed to detect the protein concentration in light of standard protocol. Following The separation with 10% SDS-PAGE, equal amount of protein was transferred onto PVDF membranes. Sealed with 5% BSA at room temperature for 1 h, the sample proteins were cultivated with primary antibodies (XAF1, Bcl-2, Bax, cleaved caspase 3, CHOP, GRP78, ATF4, MMP2, MMP9 and GAPDH) overnight at 4 °C, after which was the subjection to secondary antibodies for 2 h at 37 °C. The visualization of protein bands was implemented utilizing ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in light of standard protocol and Image J software was applied for the analysis of protein density.
Statistical analysis
All data that displayed in the format of mean ± SD got analyzed with SPSS 23.0 software. To demonstrate differences among multiple groups, one-way ANOVA with a post hoc Bonferroni multiple comparison test was adopted. P less than 0.05 meant that all experimental data indicate statistical significance.
Results
XAF1 expression is downregulated in NSCLC cell lines
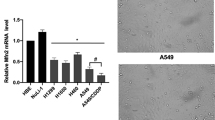
With the purpose of investigating the role of XAF1 in NSCLC, XAF1 expression in NSCLC cells was appraised. As shown in Fig. 1, western blot assay results revealed a significant decrease in protein expression of XAF1 in NSCLC cell lines when compared with the control HBE cells. It was noted that XAF1 had the lowest expression in H1299 cells, in this way, H1299 cells were adopted for follow-up studies.
Overexpression of XAF1 inhibits the proliferation and migration of H1299 cells and induces cell cycle arrest
To investigated the biological role of XAF1 in NSCLC cells, we overexpressed XAF1 in H1299 cells and the transfection efficiency was evaluated by western blot assay (Fig. 2A). Results obtained from CCK-8 assay demonstrated that XAF1 overexpression conspicuously weakened the proliferation of H1299 cells compared with the OV-NC group (Fig. 2B). In addition, EdU staining results showed an obvious reduction in the number of positive-green cells after the transfection with OV-XAF1 (Fig. 2C). Moreover, OV-XAF1 reduced the rate of cell migration when compared with the OV-NC group (Fig. 2D). Transwell assay results revealed that the invasive ability of H1299 cells was restrained by XAF1 overexpression (Fig. 2E). Western blot assay also revealed that OV-XAF1 inhibited the levels of MMP2 and MMP9 (Fig. 2F). Furthermore, flow cytometry analysis showed that the percentage of cells in the G1 phase was remarkably increased while that in S phase was decreased by XAF1 overexpression (Fig. 2G).
Overexpression of XAF1 inhibits the proliferation and migration of H1299 cells and induces cell cycle arrest. A, Protein level of XAF1 in H1299 cells transfected with OV-XAF1 were detected by western blotting. Cell proliferation was evaluated by CCK-8 assay (B) and Edu assay (C). D, Wound healing assay was used to detect cell migration. E, Transwell assay was used to measure cell invasion. F, Western blot assay was performed to assess the levels of MMP2 and MMP9. G, Cell cycle was detected by flow cytometry analysis. Results are the mean ± SD. **P < 0.01, ***P < 0.001
Overexpression of XAF1 restrains cisplatin resistance in NSCLC cells
To observe the effects of XAF1 overexpression on cisplatin resistance in H1299 cells, cisplatin resistant cell line H1299/DDP was constructed by increasing cisplatin concentration. As shown in Fig. 3A-B, flow cytometry analysis showed that the apoptosis rates of H1299 and H1299/DDP cells were obviously increased compared with the negative control, which was in line with western blot results that Bcl-2 level was decreased but the contents of Bax and cleaved caspase 3 were increased by XAF1 overexpression (Fig. 3C-D).
Overexpression of XAF1 restrains cisplatin resistance in H1299 cells. Cell apoptosis was detected by flow cytometry analysis in H1299 cells (A) and H1299/DDP cells (B). Western bolt assay was used to assess apoptotic-related protein levels in H1299 cells (C) and H1299/DDP cells (D). Results are the mean ± SD. ***P < 0.001
XAF1 overexpression enhances the expression levels of ER stress-related proteins in H1299 cells
Next, we discussed the effects of XAF1 overexpression on ER stress in H1299 cells. As shown in Fig. 4, western blot assay demonstrated that the protein expressions of CHOP, GRP78 and ATF4 in H1299 cells treated with ER stress agonist Thapsigargin were markedly elevated when compared with those in negative group, and these protein levels were further exacerbated by the overexpression of XAF1.
Upregulation of XAF1 suppresses the proliferation, metastasis, cell cycle and cisplatin resistance in NSCLC cells by enhancing ER stress
As presented in Fig. 5A, the treatment of ER stress inhibitor 4-PBA significantly revived the decreased cell proliferative ability mediated by XAF1 overexpression. Consistently, Edu staining assay showed that the number of positive cells was increased after 4-PBA treatment (Fig. 5B). Moreover, it was observed by wound healing and transwell assays that 4-PBA increased the migrative and invasive capacities of XAF1-overexpressed cells (Fig. 5C-D). The data from western blot assay revealed that 4-PBA elevated the levels of MMP2 and MMP9 (Fig. 5E). Besides, 4-PBA reduced the cell population in G1 phase and increased the fraction of cells in S phase in OXAF1-overexpressed H1299 cells (Fig. 5F). In addition, an obvious decrease in cell apoptosis rate was observed following 4-PBA treatment in H1299/DDP cells when compared with the negative control cells (Fig. 6A-B). Meanwhile, the addition of 4-PBA led to increased Bcl-2 level and increased levels of Bax and cleaved caspase 3 in H1299/DDP cells (Fig. 6C-D).
Upregulation of XAF1 suppresses the proliferation, metastasis and cell cycle of NSCLC cells by enhancing ER stress. Cell proliferation was evaluated by CCK-8 assay (A) and Edu assay (B). C, Wound healing assay was used to detect cell migration. D, Transwell assay was used to measure cell invasion. E, Western blot assay was performed to assess the levels of MMP2 and MMP9. F, Cell cycle was detected by flow cytometry analysis. Results are the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
Upregulation of XAF1 inhibits cisplatin resistance in NSCLC cells by enhancing ER stress. Cell apoptosis was detected by flow cytometry analysis in H1299 cells (A) and H1299/DDP cells (B). Western bolt assay was used to assess apoptotic-related protein levels in H1299 cells (C) and H1299/DDP cells (D). Results are the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Lung cancer is a predominant contributor to cancer-related mortality globally, and NSCLC represents the major histological subtype of the disease [17]. It was estimated that nearly two-thirds of NSCLC patients have oncogenic driver mutations, and half of them have therapeutically targetable lesions [18]. Relative to conventional chemotherapy, this expands treatment options and improves survival rate and prognosis [19]. However, responses to these agents are generally incomplete and temporary although targeted therapies improve outcomes in NSCLC patients [20]. Therefore, research on drug resistance to targeted therapies for NSCLC is necessary. In the present study, we demonstrated that XAF1 expression was reduced in several NSCLC cell lines. XAF1 overexpression imparted suppressive impacts on H1299 cell proliferation and migration but facilitated cell apoptosis and cycle arrest. XAF1 overexpression restrained cisplatin resistance and induced ER stress response. In addition, the addition of ER stress inhibitor 4-PBA reversed the impacts of XAF1 overexpression on H1299 cell proliferation, migration, apoptosis as well as cycle arrest, which indicated that the upregulation of XAF1 may regulate NSCLC cells by enhancing ER stress.
As a recognized cancer suppressor, XAF1 has low expression in many types of cancers. Sun et al. reported that the downregulation of XAF1 reduced the apoptosis of paclitaxel-induced triple-negative breast cancer cells with PGK1 silence [21]. In addition, overexpression of XAF1 in ovarian cancer can regulate invasion, cell cycle, apoptosis as well as elevate cisplatin sensitivity [22]. Moreover, XAF1 upregulation has been shown to induce cell apoptosis in gastric and colorectal cancers and strengthen the apoptotic effects of chemotherapeutic agents and TNF-related apoptosis inducing ligand (TRAIL), suggesting that XAF1 can influence the malignant process and chemical resistance of cancer [23]. In this study, we used western blot assay to detect the expression of XAF1 and found that XAF1 expression was downregulated in NSCLC cells compared with that in HBE cells. Moreover, we transfected OV-XAF1 into H1299 cells to overexpress XAF1 and it was discovered that XAF1 overexpression imparted inhibitory impacts on cell proliferative and migrative capabilities, as well as cell cycle. Furthermore, XAF1 overexpression reduced the cisplatin resistance in both H1299 and H1299/DDP cells by promoting cell apoptosis. These data indicated that XAF1 played a suppressive role in NSCLC cell growth and cisplatin resistance.
Under certain cytotoxic conditions, such as hypoxia and nutritional deficiency, protein misfolding occurs via the disruption of proper ER function, leading to ER stress characterized by unfolded proteins accumulation and aggregation in the ER [24]. ER stress is involved in anticancer drug resistance via unfolded protein response [25]. Lee et al. revealed that XAF1 drives ER stress by disrupting the stability of GRP78 and CHIP, thus affecting the process of apoptosis [26]. Additionally, Park et al. found that ampelopsin-induced reactive oxygen species enhanced the apoptosis of colon cancer cells by activating ER stress-mediated AMPK/MAPK/XAF1 signaling [27]. In the current study, XAF1 overexpression increased the production of CHOP, GRP78 and ATF4 in H1299 cells. In addition, ER stress inhibitor 4-PBA was applied for the administration of H1299 and H1299/DDP cells. It was found that 4-PBA reversed the suppressive effects of XAF1 overexpression on cell proliferative and migrative capabilities as well as cell cycle. Moreover, 4-PBA reduced cell apoptosis in H1299 and H1299/DDP cells transfected with OV-XAF1. These data indicated that the induction of ER stress repressed the malignant progression and chemotherapy resistance of NSCLC cells by XAF1 overexpression. However, there are several limitations in our study. Due to the constraints of financial and time, only H1299 cell line was used for biological validation in vitro. Secondly, the endoplasmic reticulum stress-related pathway underlying the regulation of XAF1 in NSCLC will be explored in further study. Moreover, further experiments using more cell lines, animals, as well as clinical studies are required to confirm our findings.
Conclusion
In summary, the study uncovered the inhibitory impacts of XAF1 overexpression on NSCLC cells and identified that XAF1 could induce ER stress, which revealed the mechanism by which XAF1 impede the advancement of NSCLC, implying that XAF1 could be a prospective therapeutic target for NSCLC.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
30 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11033-024-09591-6
References
Bade BC, Dela Cruz CS (2020) Lung Cancer 2020: epidemiology, etiology, and Prevention. Clin Chest Med 41:1–24
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Jonna S, Subramaniam DS (2019) Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med 27:167–170
Wang M, Herbst RS, Boshoff C (2021) Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 27:1345–1356
Pennell NA, Arcila ME, Gandara DR, West H (2019) Biomarker testing for patients with Advanced Non-small Cell Lung Cancer: real-world issues and tough choices. Am Soc Clin Oncol Educ Book 39:531–542
Blakely CM, Riess JW (2019) Interpretation of ceritinib clinical trial results and future combination therapy strategies for ALK-rearranged NSCLC. Expert Rev Anticancer Ther 19:1061–1075
Imyanitov EN, Iyevleva AG, Levchenko EV (2021) Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol 157:103194
Tan AC, Tan DSW (2022) Targeted therapies for Lung Cancer patients with oncogenic driver molecular alterations. J Clin Oncol 40:611–625
Leonetti A, Assaraf YG, Veltsista PD, El Hassouni B, Tiseo M, Giovannetti E (2019) MicroRNAs as a drug resistance mechanism to targeted therapies in EGFR-mutated NSCLC: current implications and future directions. Drug Resist Updat 42:1–11
Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ, Kim HR, Chi SG (2003) Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res 63:7068–7075
Ma TL, Ni PH, Zhong J, Tan JH, Qiao MM, Jiang SH (2005) Low expression of XIAP-associated factor 1 in human colorectal cancers. Chin J Dig Dis 6:10–14
Huang J, Yao WY, Zhu Q, Tu SP, Yuan F, Wang HF, Zhang YP, Yuan YZ (2010) XAF1 as a prognostic biomarker and therapeutic target in pancreatic cancer. Cancer Sci 101:559–567
Fong WG, Liston P, Rajcan-Separovic E, St Jean M, Craig C, Korneluk RG (2000) Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics 70:113–122
Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW et al (2001) Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat Cell Biol 3:128–133
Wang Y, Mao H, Hao Q, Wang Y, Yang Y, Shen L, Huang S, Liu P (2012) Association of expression of XIAP-associated factor 1 (XAF1) with clinicopathologic factors, overall survival, microvessel density and cisplatin-resistance in ovarian cancer. Regul Pept 178:36–42
Yang WT, Chen DL, Zhang FQ, Xia YC, Zhu RY, Zhou DS, Chen YB (2014) Experimental study on inhibition effects of the XAF1 gene against lung cancer cell proliferation. Asian Pac J Cancer Prev 15:7825–7829
de Sousa VML, Carvalho L (2018) Heterogeneity in Lung Cancer. Pathobiology 85:96–107
Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K et al (2021) Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-Mutant NSCLC. J Thorac Oncol 16:205–215
da Cunha Santos G, Shepherd FA, Tsao MS (2011) EGFR mutations and lung cancer. Annu Rev Pathol 6:49–69
Girard N, Basse C (2022) EGFR-mutant NSCLC: monitoring the molecular evolution of tumors in 2022. Expert Rev Anticancer Ther 22:1115–1125
Sun S, Wu H, Wu X, You Z, Jiang Y, Liang X, Chen Z, Zhang Y, Wei W et al (2021) Silencing of PGK1 promotes sensitivity to Paclitaxel Treatment by Upregulating XAF1-Mediated apoptosis in Triple-negative breast Cancer. Front Oncol 11:535230
Zhao WJ, Deng BY, Wang XM, Miao Y, Wang JN (2015) XIAP associated factor 1 (XAF1) represses expression of X-linked inhibitor of apoptosis protein (XIAP) and regulates invasion, cell cycle, apoptosis, and cisplatin sensitivity of ovarian carcinoma cells. Asian Pac J Cancer Prev 16:2453–2458
Tu SP, Sun YW, Cui JT, Zou B, Lin MC, Gu Q, Jiang SH, Kung HF, Korneluk RG et al (2010) Tumor suppressor XIAP-Associated factor 1 (XAF1) cooperates with tumor necrosis factor-related apoptosis-inducing ligand to suppress colon cancer growth and trigger tumor regression. Cancer 116:1252–1263
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Avril T, Vauléon E, Chevet E (2017) Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 6:e373
Lee KW, Hong HR, Lim JS, Ko KP, Lee MG, Chi SG (2022) XAF1 drives apoptotic switch of endoplasmic reticulum stress response through destabilization of GRP78 and CHIP. Cell Death Dis 13:655
Park GB, Jeong JY, Kim D (2017) Ampelopsin-induced reactive oxygen species enhance the apoptosis of colon cancer cells by activating endoplasmic reticulum stress-mediated AMPK/MAPK/XAF1 signaling. Oncol Lett 14:7947–7956
Funding
This work was supported by Key research and Development Program of Anhui Province in 2021 (202104j07020055) and Beijing Science and Technology Innovation Medical Development Foundation (KC2021-JX-0186-46).
Author information
Authors and Affiliations
Contributions
Bin Chen, Yuanjun Cheng and Jie Yao designed and performed the experiments. Bin Chen and Yuanjun Cheng wrote the main manuscript text and Hanqing Wu prepared all figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The missing Ethical Approval, Funding and Competing interests are included. The affiliation ‘People’s Hospital of Chizhou, No. 3 Baiya Road, Guichi District, Chizhou 247000, China’ is removed.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, B., Cheng, Y., Wu, H. et al. XAF1 overexpression inhibits the malignant progression and cisplatin resistance of NSCLC by activating endoplasmic reticulum stress. Mol Biol Rep 51, 435 (2024). https://doi.org/10.1007/s11033-024-09347-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09347-2