Abstract
Background
Corneal disease is a major cause of blindness. Transplantation of cadaver-derived corneas (keratoplasty) is still the current therapy of choice; however, the global shortage of donor corneas continues to drive a search for alternatives. To this end, biosynthetic corneal substitutes have recently begun to gain importance. Here, we present a novel method for the generation of a cornea-like tissue (CLT), using corneo-scleral rims discarded after keratoplasty.
Methods and results
Type I collagen was polymerized within the corneo-scleral rim, which functioned as a ‘host’ mould, directing the ‘guest’ collagen to polymerize into disc-shaped cornea-like material (CLM), displaying the shape, curvature, thickness, and transparency of normal cornea. This polymerization of collagen appears to derive from some morphogenetic influence exerted by the corneo-scleral rim. Once the CLM had formed naturally, we used collagen crosslinking to fortify it, and then introduced cells to generate a stratified epithelial layer to create cornea-like tissue (CLT) displaying characteristics of native cornea. Through the excision and reuse of rims, each rim turned out to be useful for the generation of multiple cornea-shaped CLTs.
Conclusions
The approach effectively helps to shorten the gap between demand and supply of CLMs/CLTs for transplantation. We are exploring the surgical transplantation of this CLT into animal eyes, as keratoprostheses, as a precursor to future applications involving human eyes. It is possible to use either the CLM or CLT, for patients with varying corneal blinding diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cornea comprises the outermost layer of any vertebrate eye. It is made up of transparent and multilayered avascular tissue. This transparent tissue plays a role in the initial (coarse) focusing of light that is incident upon the eye. Further (fine) focusing of light onto the retina, through the vitreous humor of the eye, is performed by another transparent and refractive tissue known as the eye lens. Blindness owing to the opacification of the cornea in both eyes affects about ~ 5.5 million individuals worldwide, while ~ 6.2 million are blind in one eye [1]. Keratoplasty, consisting of the surgery-aided replacement of an opaque cornea by a transparent cornea sourced from a cadaver-derived donor eye is the preferred mode of treatment of corneal blindness, but the demand for cadaver-derived transparent cornea is far larger than the supply. Recent advances in corneal surgery have made it possible for individual layers of the cornea to be transplanted, rather than ‘full-thickness’ corneas. After the transplantation of such a layer, natural healing mechanisms that involve the growth and proliferation of cells from the corneo-limbal region then facilitate a replacement of donor corneal layer-derived cells in the transplanted layers by keratocytes and endothelial cells that are derived from the recipient eye, leading to the post-surgical integration of the transplanted layers with the existing corneal layers [2]. This makes it possible for surgeons to use parts of donor corneas, especially when entire corneas prove to be unhealthy, or unsuitable for transplantation. Penetrating keratoplasty (PK) is the approach conventionally used to replace the entire thickness of the cornea, while deep anterior lamellar keratoplasty (DALK) is the approach used to replace just the thickness of the diseased stroma, using eyes supplied by eye banks [3]. Such surgical innovations have helped to create an ever-growing demand for cornea-derived material (CDM) that is suitable for transplantation. In turn, the successful use of CDMs has encouraged efforts to develop other cornea-like materials (CLM) and cornea-like tissue (CLT) that can be used for transplantation.
The need to transplant cadaver-derived corneas is associated with two shortcomings: (i) the occasional lack of achievement of the same corneal topography as that of the native (i.e., non-operated) cornea; (ii) the non-availability of donor corneas. The need to circumvent these two shortcomings has led to the development of biosynthetic corneal substitutes. Ideally, bio-engineered corneal substitutes are required to promote rapid host-mediated epithelialization and colonization by keratocytes and nerves, to restore corneal function. Corneal substitutes are generally based on natural or synthetic polymers, e.g., collagen or polyethylene glycol [4, 5], amniotic membrane [6], or hydrogels created using extracellular matrices from decellularized tissues [7]. Previous bio-engineered substitutes have used recombinant human collagen [8, 9], rat tail-derived collagen [10], singly-crosslinked porcine collagen [11,12,13], doubly-crosslinked porcine collagen [14], gelatin-based hydrogels [15, 16] and 3-D printed methacrylolyl bioink based on either extracellular matrix (ECM) materials, or gelatin [17, 18]. For a wide variety of reasons, none of the above have yet cleared Phase I clinical trials, possibly owing to complications involving astigmatism, altered steepness and surface irregularities.
Since Collagen type I is the most abundant stromal protein in the cornea [19], its use as a mimetic of the extracellular matrix (ECM) is appropriate for the generation of tissue-engineered corneal scaffolds. Recent developments in corneal tissue engineering have employed plastically-compressed empty collagen scaffolds, or scaffolds compressed with keratocyte cellular components [20,21,22]. Numerous other alternatives are also being explored for the treatment of the large numbers of patients who need only the surgical introduction of a transparent cornea-derived cornea-like material (or CLM) that can support, and host, the growth and proliferation of limbal stem cells derived from the vascularized peripheral corneo-limbal region, to facilitate the integration of the introduced CLM with preexisting corneal tissue. Intensive research efforts are thus currently being directed towards developing better CLMs in the form of keratoprosthesis (KPros; or artificial cornea). Two keratoprosthesis, namely Boston® Keratoprosthesis, and AlphaCor™, have received approval for use from the Food and Drugs Administration of the USA; however, both are associated with prolonged wound healing time, and lack of satisfactory integration with the host tissue [23].
Given all of the above information and background, it is evident that scope still exists for the further development of CDMs, CLMs and CLTs, for use as keratoprostheses, since the search for a good corneal substitute has not yet concluded. In the present paper, we describe an innovative, simple, and cost-effective approach for creating CLMs that can then be converted into CLTs, through the use of scaffolds comprising corneo-scleral rims that are ordinarily discarded after keratoplasty surgery. The corneo-scleral rim is an annular ring constituted of mostly scleral, and some corneal tissue that remains over as residue, after the surgeon initially (i) makes a circular incision in the white of the donor cadaver-derived eyeball, to obtain a transparent and circular cornea ringed by opaque (white) scleral tissue, and then (ii) makes a subsequent (second) circular incision to excise the transparent circular cornea out from the combined corneal-scleral tissue, in the vicinity of the corneo-limbal region of disc of tissue that is originally excised from the donor eyeball. The making of these two serial incisions leaves an annular ring of white scleral tissue with a smaller, concentric ring of corneal tissue. This annular ring, which is usually subsequently discarded, is called the corneo-scleral rim (Fig. 1).
The corneo-scleral rim. The corneo-scleral rim is an annular ring of white scleral tissue (normally discarded by the surgeon), that is shown here. In panel A, the corneo-scleral rim is shown with the epithelial surface facing upwards, whereas in Panel B, the rim is shown with the epithelial surface facing downwards. The central hollow cavity (originally occupied by a cornea that has been removed and transplanted), measures ~ 8 mm
We have used corneo-scleral rims as receptacles, or scaffolds, to support repeated (cyclic) rounds of creation of cornea-like material (CLM). Inside these scaffolds, a poorly-understood differential polymerization of collagen was observed in a manner that is somewhat akin to a phase-separation of the polymerized collagen into at least two phases; (i) a collagen-poor phase consisting of the collagen solution that lies outside of the hollow space or cavity (originally occupied by the cornea) that happens to gellate with characteristics that are very different from that of (ii) the collagen that lies within the hollow space, which polymerizes into a collagen-rich phase (making up the CLM). This poorly-understood, but entirely reproducible, differential polymerization of collagen causes the collagen polymerized within the scleral rim (where a cornea was originally located, prior to surgical excision for keratoplasty) to be very different from the collagen polymerized outside the scleral rim, i.e., on its side, or above or below it, in terms of rigidity and fluidity. This allows the corneo-scleral rim, with its cavity now occupied by a newly-formed CLM, to be simply picked up through the use of forceps, and removed from its state of submergence within the otherwise loosely-polymerized collagen surrounding it.
It appears to us that the different polymerization of collagen within the corneo-scleral rim in the shape of a cornea owes to some poorly-understood morphogenetic influence exerted by the rim that somehow causes only the collagen polymerizing inside the scleral rim to form a transparent, non-fluid layer of considerable rigidity, as opposed to the fluid collagen polymerized outside the rim, to its sides, or above, or below. This process invariably leads, however, to an intact, transparent, ‘acellular’, collagen-based, CLM that occupies the region or cavity originally occupied by the cornea.
One manner of describing the formation of this CLM in the language of chemistry is to use the ‘guest-host concept’ commonly used to describe chemical cages and other receptacles. One could say that the scleral rim somehow functions as a ‘host’ that facilitates the differential polymerization of a substantially-curved ‘guest’ layer of collagen of the same thickness as the scleral rim, within the rim, through some morphogenetic influence exerted upon collagen lying in direct contact with the region of the rim that was earlier in intimate contact with living corneal tissue. When we combined this natural differential polymerization of collagen with riboflavin-assisted crosslinking of such polymerized collagen, this caused the CLM that had assembled within the rim to display viscoelastic characteristics comparable to those of natural cornea. We then found that a scleral rim hosting such a CLM (containing crosslinked collagen) could be easily transferred using forceps, from its submerged state in loosely-polymerized collagen to a well of a cell culture plate containing growth medium. We further found that the addition of a seeding population of human corneal limbal epithelial (HCLE) cells facilitated ‘cellularization’ of the CLM through the proliferation and stratification of HCLE cells in culture. This resulted in the creation of cellularized CLM or cornea-like tissue (CLT) possessing the naturally curved shape of the cornea, and exhibiting optical and biomechanical properties similar to the cornea, as well as a natural cornea-like integration with the surrounding scleral rim. This CLT was then excised and studied through optical coherence tomography (OCT) and various other methods, in comparison with native corneal tissue. We believe that surgeons could be interested in the surgical transplantation of this CLT.
Materials and methods
Polymerization and crosslinking of collagen into cornea like material (CLM)
The collagen used to make CLM was rat tail collagen type I (A1048301; GIBCO). A collagen gel was prepared based on the manufacturer’s protocol. Briefly, to make a 1 ml collagen gel solution, 500 µl collagen (3.0 mg/ml) was added to 100 µl of 10X phosphate buffered saline (PBS) and 385 µl of DMEM HG media (Dulbecco’s modified eagle medium with high glucose). The pH of the collagen gel was set to 7.0 through addition of 15 µl of 1 N NaOH. Corneo-scleral rims derived through surgical incision of donor cadaver eyeballs were obtained from the eye bank of PGIMER. The collagen solution was added to a corneo-scleral rim placed in a well of a 6-well culture plate, with the epithelium facing the base of the plate. After one hour, 5 ml of 0.1% riboflavin (Sigma- R9504) in PBS was added to the well, which was then exposed to UVA (from a CUV 40 A transilluminator) for 30 min, to facilitate cross-linking of the polymerized collagen. After this, the rim was washed thrice with DMEM HG, and then left in DMEM HG overnight. Following this, the rim was taken out of the loosely-held collagen using forceps, with the circular cavity in the center of the rim now occupied by the CLM constituted of collagen that had polymerized into a cornea-like shape, fortified through oxyradical-mediated crosslinking, facilitated by riboflavin and UV light.
Assessment of the transmittance of visible and UV light by the CLM
The cornea like material (CLM) was placed between two rectangular quartz plates of 1 × 4 cm comprising a demountable circular dichroism cuvette, and transmission was measured using a Cary-50Bio (Varian) UV-Visible spectrophotometer in the range of 800 − 200 nm.
Formation of an epithelial cell layer to convert CLM into cornea-like tissue (CLT)
A telomerase-immortalized human corneal limbal epithelial cell line (HCLE) was obtained as a gift from Dr. Shiva Swamynathan (University of Pittsburgh, USA). HCLE cells (1 × 105) were allowed to grow over the surface of the crosslinked collagen constituting the CLM in KSFM (keratinocyte serum free medium) for two days. Cells were then allowed to differentiate in growth medium consisting of KSFM:DMEM/F12 (in a ratio of 1:1) for one day. HCLE cells were then allowed to stratify over a period of six days in the presence of stratification medium consisting of DMEM/F12, 10 ng/ml EGF (epidermal growth factor) and 10% FBS, at 37ºC and 5% CO2 [24]. The timeline of formation of this stratified/cellularized CLT is shown in Fig. 2.
Timeline of formation of corneal like tissue (CLT) with layers of corneal epithelial cells. The process of creation of CLT using corneo-scleral rim includes steps of collagen polymerization and crosslinking, followed by culturing of epithelial cells to form stratified epithelial layer over the surface of CLM
Characterization of the cells infiltrating into the CLM, and the epithelial cell layer formed upon the CLM to generate CLT
To check infiltration of keratocytes in the CLM, the expression of keratocan was assessed in the cells. The formation of epithelial cell layer over the surface of the CLM to generate CLT, was examined by (i) Hematoxylin and Eosin (H&E) staining as well as by (ii) Immunofluorescence staining of the epithelial marker, CK12 (cytokeratin 12) [25]. Briefly, blocks were prepared and sections of 4 to 5 μm thickness were cut and placed upon 0.01% poly-L-lysine-coated slides. Tissue sections were dewaxed using xylene, and rehydrated in decreasing concentrations of ethanol solution (100–50%). Antigen retrieval was done by the heat retrieval method, using citrate antigen retrieval solution (pH 6.0). Tissue sections were permeabilized and non-specific binding was blocked through addition of bovine serum albumin. The primary antibody, CK12 (1:100; Santacruz; catalog no sc-515,882) for epithelial layer and keratocan (1:100; Bioss; bs-11054R) for keratocytes, was applied overnight in a moist chamber at 4 °C, followed by incubation with the secondary antibody, goat anti-mouse IgG, Alexa fluor 594 conjugated (1:100; A11032, Invitrogen) and goat anti-rabbit IgG, Alexa Fluor 568 conjugated (1:100; A11011, Invitrogen) respectively, for 1 h at 4 °C. DAPI (1:10,000) was used to stain cell nuclei for 15 min. Slides were washed and mounted with glycerol. Marker expression was confirmed by capturing images at 40X magnification using a confocal microscope (Olympus Fluoview FV3000).
Transparency of the CLT
The transparency of the CLT was assessed through photographic capturing of the image of the letter “A” placed below the CLT.
Optical coherence tomography (OCT) of the CLT
OCT was performed by placing the CLT on a contact lens or by holding it with a forceps in front of the OCT instrument (CASIA2).
Scanning electron microscopy (SEM) of the CLT
For SEM analysis, fixation of the CLT was done in a solution of 3% paraformaldehyde, 3% glutaraldehyde and 2.5% dimethylsulfoxide (DMSO) in 0.1 M sodium cacodylate buffer (pH 7.4) at 4 °C for 24 h. Thereafter, the CLT was washed twice with 0.1 M sodium cacodylate buffer, dehydrated through placement for 10 min each in progressively-increasing concentrations of ethanol (50%, 70%, 90%, 95% and 100%), and dried using the Critical Point Drying (CPD) method before being coated with platinum. Images were visualized at different magnifications (250x, 500x, 1000x, 5000x and 10,000x) using a JSM-IT300 SEM (Jeol, Japan).
Thermogravimetric (TGA) analysis of the CLT
Thermogravimetric analysis of the CLT was carried out using a TA Shimadzu instrument. Around 8 mg of CLT was heated from 26 ºC to 500 ºC under a dinitrogen atmosphere maintaining a heating rate of 10 ºC/min using an Al pan. The weight (%) was plotted against temperature (ºC) to determine the water content in the sample.
Rheometry involving the CLT
Rheological measurements of the CLT were done using the MCR 302, Anton Paar rheometer (Austria) and the analysis was performed using RheoCompass software using a 12.5 mm diameter parallel plate (PP) with a measuring distance of 0.1 mm at 25 oC. In the case of the corneal tissue, a gap of 1 mm was maintained. Oscillatory amplitude sweep studies were performed at 1 rad/s to estimate the linear viscoelastic region (LVR). The storage modulus (G′) and loss modulus (G″) were obtained using frequency sweep oscillatory rheology at an angular frequency (ω) of 0.1 to 200 rad s− 1 and 0.1% strain in the linear viscoelastic region [26].
Results
Use of corneoscleral rims as scaffolds for the generation of collagen-based cornea-like material (CLM)
Annular corneo-scleral rims with a central circular hollow region or cavity (Fig. 1) were used as ‘host’ scaffolds for polymerization of ‘guest’ collagen. In every instance, this led to the formation of collagen-based cornea-like material (CLM) within the central cavities of corneo-scleral rims submerged in collagen subjected to conditions of polymerization (as described in the Methods section), with the epithelial surface facing the bottom of the well of a cell-culture plate. Upon removal of the rim from the loosely-held collagen, the central hollow circular cavity of the rim was invariably found to be occupied by CLM that had formed to seamlessly integrate with the limbal sections of the rim, over more than ten independent sets of experiments (representative experiments shown in Fig. 3).
Generation of corneal like material (CLM). After polymerization of collagen, the corneo-scleral rim was pulled out of the loosely-held collagen (panel A); collagen was found to have polymerized firmly in the central cavity of the rim (panel B); scanning electron microscopy (SEM) of the CLM showed it to be integrated with the corneo-limbal region of the rim (panel C); and the transmission characteristics of CLM were measured in the range of 800 nm to 200 nm (panel D)
Transmittance of light by the CLM
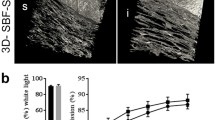
The transmission characteristics of the CLM were measured in the range of 800 nm to 200 nm. Representative data is shown in Fig. 3D for a single experiment. The transmission can be seen to be above ~ 70% for visible light above 450 nm. In terms of further detail, the transmission can be seen to vary from 82 to 94% between 600 and 800 nm; from 70 to 82% between 450 and 600 nm; and reduce to 20% in the far-ultraviolet, at ~ 200 nm. These characteristics are entirely comparable to the known transmission characteristics of cornea [27].
Lack of birefringence in the CLM
We also examined the CLM for birefringence using a polarizing microscope. No birefringence was seen and no light could be seen to be transmitted under cross polarizers (data not shown), with this indicating a consistent and uniformly-random orientation of collagen fibers throughout the CLM.
Inconsistent cell infiltration into the CLM from the rim
Since riboflavin crosslinking is performed using ultraviolet light, this could be expected to kill any cells that infiltrate the CLM from the rim. Therefore, in order to assess the infiltration of cells from the rim, we made the CLM without crosslinking. We observed that cells from the rim tissue naturally infiltrated into the CLM; on an average, we found that cells would begin to ingress into the CLM from day 3 onwards, and by day 12, large numbers of cells were seen to have made their way into the CLM (Supplementary Figure S1), providing evidence that the CLM can support the growth of cells that could be anticipated to migrate from the recipient corneo-scleral junction, this being something that also ordinarily occurs after keratoplasty, leading to the replacement of donor cells by cells from the recipient eye [2, 28]. Three types of cells can potentially enter from the scleral rim into the CLM, including epithelial cells, keratocytes and endothelial cells, although the relative proportion of surviving donor cells as well as the migration of the recipient cells into the graft, is subject to several factors, such as the quality of the graft [2]. Immunostaining with the keratocyte marker (keratocan) established the presence of at least one of these cell types (Supplementary Figure S2). This ingress of cells from the rim was neither consistent, nor always reproducible, being dependent on the quality and nature of the donor rim.
Crosslinking and cellularization of the CLM to create CLT
In order to assess the biocompatibility of the CLM after collagen crosslinking, we populated the CLM with exogenously added epithelial cells. The riboflavin- and UV-crosslinked CLM was transferred to a well containing growth medium, with the rim’s epithelial surface now facing upwards, to allow an overlaying of the CLM by human corneal limbal epithelial (HCLE) cells intended to cellularize and stratify the CLM. The addition of HCLE cells led to the formation of stratified epithelium that effectively turned the CLM into what we refer to as cornea-like tissue (CLT). The conversion of the CLM into cellularized CLT proves that riboflavin- and UV-mediated crosslinking does not affect the biocompatibility of the CLM after crosslinking. The conversion of CLM into CLT also did not appear to alter the transmission characteristics (data not shown), with these remaining similar to the characteristics of CLM shown in Fig. 3. Our cellularization of the CLM was conducted with the understanding that this CLM would anyway be expected to be remodelled, following transplantation, by cells from the recipient eye. Our reproducible method of cellularizing CLM to create CLT, through the use of human corneal limbal epithelial cells to form a stratified layer over the CLM, was confirmed as follows. H&E staining-based imaging, together with immunofluorescence imaging involving the epithelial cell marker CK12, confirmed the formation of such a stratified epithelial cell layer over the CLM to form CLT (Fig. 4).
Formation of corneal epithelial layer on the surface of CLT: H&E staining showed formation of stratified epithelial layer over the CLT; 40X magnification; scale bar 100 μm (Panel A); epithelial marker (CK12) expression was confirmed by confocal microscopy (Panel B) (DAPI: nuclear staining, Alexa fluor 594: CK12 and merged; 40X magnification, scale bar: 100 μm)
Visual transparency of the CLT
The CLT was assessed to be transparent to visible light, as judged through the viewing of the letter “A” through the CLT. Figure 5A shows the CLT as seen on day 0 prior to cellularization, and Fig. 5B shows the CLT on day 12 upon completion of cellularization, according to the time-line shown in Fig. 2.
Characterization of the CLT: The CLT maintained transparency at day 0 A and at day 12 B, following collagen polymerization, crosslinking and cellularization of the CLM. The thickness of CLT was 477 μm as determined by optical coherence tomography C. The water content of CLT was determined by thermogravimetric analysis. A three-step water loss from CLT was observed in the TG profile D
Shape and thickness of the CLT
En-face and cross-section images of CLT were obtained through optical coherence tomography as shown in Fig. 5C. This allowed assessment of the thickness and shape of the CLT. The thickness was determined to be ~ 477 μm; in comparison, the thickness of native human cornea ranges from 500 to 625 μm [29].
Water content in the CLT
To estimate water content, CLT was subjected to thermogravimetric analysis (TGA), as shown in Fig. 5D. The TG analysis indicated a three-step water loss on heating from 26 to 500 ºC. An initial loss of 75% was observed between 26 ºC and 80 ºC. Following this, approximately 19% further weight loss was observed between 80 ºC and 140 ºC. A final weight loss of 3% was encountered between 233 ºC and 370 ºC. The final and penultimate weight losses were presumably due to water molecules tightly-bound to the collagen. Therefore, it may be estimated that approximately 97% of the CLT, by weight, is water, with the remainder consisting mainly of collagen (discounting for the dried weight of the few layers of cells). This suggests that the relatively collagen-poor solution used for polymerization (0.15% w/v; or 1.5 mg/ml) had given rise to a collagen-rich (3% w/w) CLM through a differential phase-separation of collagen within the cavity of the rim that was not observed outside the rim. In comparison, the TG analysis of the cornea also shows a three-step weight loss (55%, 33% and 5%, respectively) over the same ranges of temperature (Fig. 5D). This corroborates well with the TG analysis of bovine cornea, which has been reported to have a water content of 96% [30], in comparison with human cornea reported to have a water content of ~ 80% [31]. In this context, it is worth noting that previously bioengineered corneas used as implants have been reported to have water contents as different as 88%, for porcine constructs used to reshape native corneal stroma successfully in keratoconus patients [14], and 91.5%, for crosslinked recombinant human collagen hydrogels [9].
Viscoelastic properties of the CLT
We conducted rheological measurements of the CLT and compared these with rheological measurements of human corneal tissue. Three independently made CLT specimens were subjected to amplitude sweep, as shown in Supplementary Figure S3, to determine the linear viscoelastic region (LVR). From this region, a value of 0.1% strain was chosen for conducting a frequency sweep in the range of 0.1–200 rad/second. As seen from Fig. 6, the G′ values of the CLT were consistently higher than the loss modulus (G′′) of the corresponding tissue. This indicates that the CLT remains stable and maintains a gel-like state. At 100 rad/s, the CLT showed an average storage modulus (G’) of 2575 Pa and loss modulus (G’’) of 348 Pa, comparable to the corresponding values of 2510 Pa and 0.125 Pa respectively, measured for human corneal tissue (as determined by us; Supplementary Figure S4). Overall, the data is indicative of the presence of viscoelastic properties in the CLT that are cornea-like and, therefore, likely to lend themselves to surgical handling and stitching.
Scanning electron microscopic imaging of the CLT
Scanning electron microscopy was performed to analyze the microstructure of collagen within the CLT. It was observed that the collagen fibrils were well organized, as shown in Fig. 7.
Discussion
The current treatment of corneal blindness involves the transplantation of transparent cadaver-derived cornea into recipient eyes; however, ~ 50% of cadaver-derived corneas turn out to be unsuitable for surgical transplantation. Thus, only about 1-in-70 individuals requiring corneal transplants actually receive such transplants. With high-risk recipients (i.e., those with inflammation, severe pathologies, autoimmune conditions, alkali burns, or recurrent graft failures), tissue-engineered grafts are preferred to donor cornea. Therefore, across the world, attempts are being made to generate artificial cornea-like materials (CLMs) that can be turned into cornea-like tissue (CLT) that can be used in surgery, with prospects for acceptance and remodeling of such CLT implants/grafts by recipient eye(s). Further, regardless of whether any surgically-introduced transparent object happens to be a cadaver-derived cornea, a CLM infiltrated by recipient cells, or a CLM that has been turned into a CLT through cellularization, it is anticipated that keratocytes, epithelial and endothelial cells from recipient eyes would eventually replace all preexisting cells to fully integrate the surgically-transplanted cornea/CLM/CLT with the recipient eye. There is evidence to show that decellularized corneal matrices are remodelled after being implanted [32]. Further, it is remarkable that remodeling of fibrotic tissue (along with regeneration of stromal tissue) follows keratectomy surgery, which can even restore corneal transparency through a remodelling process involving post-surgical cell and extracellular matrix (ECM) interactions, collagen deposition, organization and alignment [33]. Therefore, what is of utmost relevance is not the identicality of the CDM, CLM or CLT with natural cornea. Rather, the biocompatibility and structural characteristics of the CDM, CLM or CLT are relevant. We believe that the remodeling process, together with the special milieu provided by corneo-scleral rims (used as moulds), could provide the necessary organization and strength to the CLT, despite the use of very low percentages of collagen (0.15% as opposed to 10–18% collagen used by others [14, 34]) to generate CLMs or CLTs in this work.
We would also like to point out that the biomechanical properties of human cornea differ substantially from report to report. Differences are largely determined by the method used for making rheological measurements, making it critical for the cornea and the CDM, CLM or CLT to be compared under nearly identical conditions, since rheological measurements depend upon plate diameters, probe-sample spacing and also upon whether parallel (or other) plate arrangements are used. It has been noted that modulus values may vary over several orders of magnitude amongst studies, which can be attributed to variations in the instruments used for measurement, the conditions of measurement (mentioned above), and biological variations amongst samples, as well as to variations in hydration levels of ex vivo samples, and variations owing to the high non-linearity of corneal elasticity [35,36,37]. Remarkably, using an Instron apparatus, it has been reported that the tensile strength of porcine and human corneas is similar (~ 3.8 MPa) [38]. The values of storage and loss modulus of the CLT reported by us (2.5 kPa and 0.3 kPa respectively), using an Anton Paar instrument, are in accordance with those reported for porcine cornea (2 kPa and 0.3 kPa respectively; [39]), or rabbit cornea (4 kPa and 1 kPa respectively; [40]), using rheometers manufactured by TA Instruments. Therefore, the key to assessing rheometry-based measurements lies in the comparison of values obtained on cornea and CDMs, CLMs or CLTs, using similar instruments, and similar modes/methods of measurement. It is notable that others working with other types of CLMs have proposed that it may be therapeutically advantageous for bioengineered corneal tissue to be softer than native cornea to begin with, to avoid scar formation associated with tough donor tissue post-transplantation, since a remodelling of the graft is expected to occur [14].
The method that we describe in this paper for the development of CLM and CLT is novel, and also relevant. It makes use of corneo-scleral rims, and allows (what we discover to be) the natural tendency of collagen to polymerize into curved cornea-shaped discs integrated with the corneo-scleral rim. This offers several advantages. Firstly, the corneo-scleral rim serves a self-defining boundary that determines the diameter of the CLM that is generated (8–12 mm). Secondly, for reasons that we do not fully understand, this polymerization generates an object with a cornea-like thickness of ~ 500–800 μm, and a curvature which is close to that of native cornea, ostensibly due to some morphogenetic influence of the rim, since the rim’s thickness appears to dictate the thickness of the CLM that is generated. Thirdly, the generated CLM clearly demonstrates an ability to become integrated with host sclero-limbal tissue. Presumably, this provides the milieu to encourage the migration, growth and survival and/or differentiation of limbal epithelial cells, resulting in stratified epithelium akin to that seen in native human cornea. We have seen evidence of migration and ingress of cells from the rim into the CLM, and presented this evidence. However, since this did not occur consistently, we also reinforced the CLM through collagen crosslinking by methods that kill any ingressing cells, and introduced HCLE cells to create stratified layers of epithelial cells while converting CLM into CLT. We imagine that these cells further contribute to the remodelling of collagen fibrils within the CLT, resulting in a fully optically-transparent CLT with the shape of human cornea, and with features of tensile strength and visco-elasticity akin to human cornea. Also, the prior presence of non-recipient cells is not important as the replacement of these cells by recipient cells could be expected to proceed apace just as in the case of keratoplasty. Finally, our method has certain additional advantages in that our protocol is simple, inexpensive, completed in about 2 weeks’ time (from acquisition of a corneo-scleral rim) and can be used to generate multiple pieces of CLT through the excision of the CLT and reuse of the same corneo-scleral rim by repeating the same protocol.
Since every corneal transplant surgery provides ready-to-use corneo-scleral rims, such rims can be saved as “off the shelf” products for the creation of CLT. Since each rim can be successively used for creation of at least three CLTs (in our experience), and possibly more, the approach effectively helps to shorten the gap between demand and supply of CLMs/CLTs for transplantation. It is possible to use either the CLM, or the CLT, for patients with corneal blinding diseases, to potentially restore vision in those with corneal fibrosis/scarring and keratoconus, Stevens–Johnson syndrome, unilateral LSCD or for aesthetic purposes. In the case of the CLM, endogenous cells taken from the limbal region can also be used to populate the CLM to generate CLT with minimized risk of immune rejection.
Our results also open up new possibilities and questions that can be addressed in the future: Is there something guiding the morphogenesis of such materials? Is there a morphogenetic field created by the tissue in the corneo-scleral rim which organizes the collagen into a cornea-like shape with a footprint along the inner surface of the rim, in a manner that is different from the polymerization of collagen outside (and above and below) the rim? To what extent is the ECM responsible for determining the shape of the cornea? Does the infiltration and population of a pre-organized ECM by cells, lead to further reorganization of the collagen? To what extent do stem cells provide information about structures, patterns and morphogenesis, and help in formation of CLT?
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Wang EY, Kong X, Wolle M, Gasquet N, Ssekasanvu J, Mariotti SP, Bourne R, Taylor H, Resnikoff S, West S (2023) Global trends in blindness and vision impairment resulting from corneal opacity 1984–2020: a Meta-analysis. Ophthalmology 130:863–871. https://doi.org/10.1016/j.ophtha.2023.03.012
Lagali N, Stenevi U, Claesson M et al (2009) Survival of donor-derived cells in human corneal transplants. Invest Ophthalmol Vis Sci 50:2673–2678. https://doi.org/10.1167/iovs.08-2923
Yu AC, Spena R, Pellegrini M, Bovone C, Busin M (2022) Deep Anterior Lamellar Keratoplasty: current status and future directions. Cornea 41:539–544. https://doi.org/10.1097/ICO.0000000000002840
Crabb RA, Chau EP, Evans MC, Barocas VH, Hubel A (2006) Biomechanical and microstructural characteristics of a collagen film-based corneal stroma equivalent. Tissue Eng 12:1565–1575. https://doi.org/10.1089/ten.2006.12.1565
Duan X, McLaughlin C, Griffith M, Sheardown H (2007) Biofunctionalization of collagen for improved biological response: scaffolds for corneal tissue engineering. Biomaterials 28:78–88. https://doi.org/10.1016/j.biomaterials.2006.08.034
Uchino Y, Shimmura S, Miyashita H et al (2007) Amniotic membrane immobilized poly(vinyl alcohol) hybrid polymer as an artificial cornea scaffold that supports a stratified and differentiated corneal epithelium. J Biomed Mater Res B Appl Biomater 81:201–206. https://doi.org/10.1002/jbm.b.30654
Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF (2017) Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater 49:1–15. https://doi.org/10.1016/j.actbio.2016.11.068
Fagerholm P, Lagali NS, Merrett K et al (2010) A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med 2:46ra61. https://doi.org/10.1126/scitranslmed.3001022
Fagerholm P, Lagali NS, Ong JA et al (2014) Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 35:2420–2427. https://doi.org/10.1016/j.biomaterials.2013.11.079
Zhang J, Sisley AM, Anderson AJ, Taberner AJ, McGhee CN, Patel DV (2016) Characterization of a Novel Collagen Scaffold for corneal tissue Engineering. Tissue Eng Part C Methods 22:165–172. https://doi.org/10.1089/ten.TEC.2015.0304
Rafat M, Li F, Fagerholm P et al (2008) PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 29:3960–3972. https://doi.org/10.1016/j.biomaterials.2008.06.017
Rafat M, Xeroudaki M, Koulikovska M et al (2016) Composite core-and-skirt collagen hydrogels with differential degradation for corneal therapeutic applications. Biomaterials 83:142–155. https://doi.org/10.1016/j.biomaterials.2016.01.004
Koulikovska M, Rafat M, Petrovski G et al (2015) Enhanced regeneration of corneal tissue via a bioengineered collagen construct implanted by a nondisruptive surgical technique. Tissue Eng Part A 21:1116–1130. https://doi.org/10.1089/ten.TEA.2014.0562
Rafat M, Jabbarvand M, Sharma N et al (2023) Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol 41:70–81. https://doi.org/10.1038/s41587-022-01408-w
Shirzaei Sani E, Kheirkhah A, Rana D et al (2019) Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv 5:eaav1281. https://doi.org/10.1126/sciadv.aav1281
Kilic Bektas C, Burcu A, Gedikoglu G, Telek HH, Ornek F, Hasirci V (2019) Methacrylated gelatin hydrogels as corneal stroma substitutes: in vivo study. J Biomater Sci Polym Ed 30:1803–1821. https://doi.org/10.1080/09205063.2019.1666236
Zhang M, Yang F, Han D et al (2023) 3D bioprinting of corneal decellularized extracellular matrix: GelMA composite hydrogel for corneal stroma engineering. Int J Bioprint 9:774. https://doi.org/10.18063/ijb.774
Jia S, Bu Y, Lau DA et al (2023) Advances in 3D bioprinting technology for functional corneal reconstruction and regeneration. Front Bioeng Biotechnol 10:1065460. https://doi.org/10.3389/fbioe.2022.1065460
Ihanamäki T, Pelliniemi LJ, Vuorio E (2004) Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res 23:403–434. https://doi.org/10.1016/j.preteyeres.2004.04.002
Xiao J, Duan H, Liu Z et al (2011) Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials 32:6962–6971. https://doi.org/10.1016/j.biomaterials.2011.05.084
Mi S, Chen B, Wright B, Connon CJ (2010) Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J Biomed Mater Res A 95:447–453. https://doi.org/10.1002/jbm.a.32861
Mi S, Khutoryanskiy VV, Jones RR, Zhu X, Hamley IW, Connon CJ (2011) Photochemical cross-linking of plastically compressed collagen gel produces an optimal scaffold for corneal tissue engineering. J Biomed Mater Res A 99:1–8. https://doi.org/10.1002/jbm.a.33152
Mi S, Khutoryanskiy VV, Jones RR, Zhu X, Hamley IW, Connon CJ (2008) Hybrid superporous scaffolds: an application for cornea tissue engineering. Crit Rev Biomed Eng 36:441–471. https://doi.org/10.1615/critrevbiomedeng.v36.i5-6.50
Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL (2003) Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci 44:2496–2506. https://doi.org/10.1167/iovs.02-0851
Zaqout S, Becker LL, Kaindl AM (2020) Immunofluorescence staining of paraffin sections step by step. Front Neuroanat 14:582218. https://doi.org/10.3389/fnana.2020.582218
Joseph JP, Gupta N, Miglani C, Nath D, Singh A, Gupta D, Pal A (2022) Unraveling On-demand strain-stiffening in Nanofibrous peptide–polymer conjugates to Mimic Contractility in Actinomyosin Networks. Chem Mater 34:4364–4374. https://doi.org/10.1021/acs.chemmater.1c04301
Beems EM, Van Best JA (1990) Light transmission of the cornea in whole human eyes. Exp Eye Res. 50 (1990) 393–395. https://doi.org/10.1016/0014-4835(90)90140-p
Kinoshita S, Friend J, Thoft RA (1981) Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci 21:434–441
Schuster AK, Fischer JE, Vossmerbaeumer U (2016) Central corneal thickness in Spectral-Domain OCT and associations with ocular and systemic parameters. J Ophthalmol. 2016:2596956. https://doi.org/10.1155/2016/2596956
DeMali KA, Williams TR (1994) Determination of the content of water in bovine corneas by differential scanning calorimetry and thermogravimetric analysis. Ophthalmic Res. 26 (1994) 105–109. https://doi.org/10.1159/000267399
Pircher M, Götzinger E, Leitgeb R, Fercher A, Hitzenberger C (2003) Measurement and imaging of water concentration in human cornea with differential absorption optical coherence tomography. Opt Express 11(18):2190–2197. https://doi.org/10.1364/oe.11.002190
Hashimoto Y, Funamoto S, Sasaki S, Negishi J, Honda T, Hattori S, Nam K, Kimura T, Mochizuki M, Kobayashi H, Kishida A (2015) Corneal regeneration by Deep Anterior Lamellar Keratoplasty (DALK) using decellularized corneal matrix. PLoS ONE 10(7):e0131989. https://doi.org/10.1371/journal.pone.0131989
Kivanany PB, Grose KC, Tippani M, Su S, Petroll WM (2018) Assessment of corneal stromal remodeling and regeneration after Photorefractive Keratectomy. Sci Rep 8(1):12580. https://doi.org/10.1038/s41598-018-30372-2
Mirazul Islam M, Cėpla V, He C, Edin J, Rakickas T, Kobuch K, Ruželė Ž, Bruce Jackson W, Rafat M, Lohmann CP, Valiokas R, Griffith M (2015) Functional fabrication of recombinant human collagen-phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater Acta Biomater 12:70–80. https://doi.org/10.1016/j.actbio.2014.10.035
Last JA, Thomasy SM, Croasdale CR, Russell P, Murphy CJ (2012) Compliance profile of the human cornea as measured by atomic force microscopy. Micron 43:1293–1298. https://doi.org/10.1016/j.micron.2012.02.014
Søndergaard AP, Ivarsen A, Hjortdal J (2013) Corneal resistance to shear force after UVA-riboflavin cross-linking. Invest Ophthalmol Vis Sci 54:5059–5069. https://doi.org/10.1167/iovs.12-10710
Ramier A, Eltony AM, Chen Y, Clouser F, Birkenfeld JS, Watts A, Yun SH (2020) In vivo measurement of shear modulus of the human cornea using optical coherence elastography. Sci Rep 10:17366. https://doi.org/10.1038/s41598-020-74383-4
Zeng Y, Yang J, Huang K, Lee Z, Lee X (2001) A comparison of biomechanical properties between human and porcine cornea. J Biomech 34:533–537. https://doi.org/10.1016/s0021-9290(00)00219-0
Hatami-Marbini H (2014) Viscoelastic shear properties of the corneal stroma. J Biomech 47(3):723–728. https://doi.org/10.1016/j.jbiomech.2013.11.019
Chen F, Le P, Fernandes-Cunha GM, Heilshorn SC, Myung D (2020) Bio-orthogonally crosslinked hyaluronate-collagen hydrogel for suture-free corneal defect repair. Biomaterials 255:120176. https://doi.org/10.1016/j.biomaterials.2020.120176
Acknowledgements
We would like to thank Ms. Nandita and Dr Anirban Roy Choudhury (IMTECH, Chandigarh), and Mr. Joydip De and Dr Santanu Pal (IISER, Mohali) for initial help with rheometry.
Funding
None.
Author information
Authors and Affiliations
Contributions
MS and MLG discovered the phenomenon of generation of CLM within corneo-scleral rims, and characterized the CLM through SEM and OCT experiments. SK and MLG transformed the CLM into CLT through crosslinking experiments and cellularization, and characterized the CLT through rheology, transmission, thermogravimetric analysis, SEM, OCT and histology, with PG overseeing transmission, TGA, and rheology. UNS evaluated the cellularization of the CLT. PCG and JR organized for the corneo-scleral rims and OCT measurements, with JR also involved in the overall evaluation of the CLM for potential use in surgery. NM and AP contributed to the performance and analyses of rheological experiments. AC and SM contributed to the performance and analyses of TGA experiments.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki. The study protocols were approved by the Institutional Ethics Committee of PGIMER.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M., Kaur, S., Mavlankar, N.A. et al. Use of discarded corneo-scleral rims to create cornea-like tissue. Mol Biol Rep 51, 391 (2024). https://doi.org/10.1007/s11033-024-09321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09321-y