Abstract
Background
Olfaction plays an important role in host-seeking by parasitoids, as they can sense chemical signals using sensitive chemosensory systems. Psyttalia incisi (Silvestri) (Hymenoptera: Braconidae) is the dominant parasitoid of Bactrocera dorsalis (Hendel) in fruit-producing regions of southern China. The olfactory behavior of P. incisi has been extensively studied; however, the chemosensory mechanisms of this species are not fully understood.
Results
Bioinformatics analysis of 64,515 unigenes from the antennal transcriptome of both male and female adults P. incisi identified 87 candidate chemosensory genes. These included 13 odorant-binding proteins (OBPs), seven gustatory receptors (GRs), 55 odorant receptors (ORs), 10 ionotropic receptors (IRs), and two sensory neuron membrane proteins (SNMPs). Phylogenetic trees were constructed to predict evolutionary relationships between these chemosensory genes in hymenopterans. Moreover, the tissue expression profiles of 13 OBPs were analyzed by quantitative real-time PCR, revealing high expression of seven OBPs (1, 3, 6, 7, 8, 12, and 13) in the antennae.
Conclusion
This study represents the first identification of chemosensory genes and the determination of their expression patterns in different tissues of P. incisi. These results contribute to a better understanding of the function of the chemosensory system of this parasitoid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects depend on olfaction to recognize pheromones and volatile compounds, find mates, oviposition sites, and food sources, and avoid natural enemies and toxins [1]. Olfactory receptors are distributed in different parts of an insect’s body, such as the antennae, head, abdomen, wings, and legs [2]. The proteins involved in chemosensory recognition process within the antennal olfactory system include odorant-binding proteins (OBPs), chemosensory proteins (CSPs), odorant receptors (ORs), gustatory receptors (GRs), ionotropic receptors (IRs), and sensory neuron membrane proteins (SNMPs) [3,4,5,6,7]. OBPs, in particular, play a significant role in the detection and recognition of odor molecules [8]. Once bound by OBPs, hydrophobic odor molecules traverse the the insect’s lymphatic system, ultimately reaching the dendritic cell membrane. Subsequently, the olfactory receptors, namely ORs, convert chemical signals into electrical impulses that are transmitted to the nerve center, triggering behavioral responses [4, 9]. Following signal transmission, the ORs dissociate from the odor molecules, which are then degraded by odorant-degrading enzymes, regulating the dynamics of olfactory processing [10, 11].
OBPs are water-soluble acidic proteins that are present in the lymph of chemosensillar [12]. They can be divided into two primary subfamilies: pheromone-binding proteins (PBPs) and general odorant-binding proteins (GOBPs) [13]. Furthermore, OBPs are classified into five groups based on the number of conserved cysteine residues that form an α-helix: typical, plus-C, minus-C, dimer, and atypical [14,15,16]. Typical OBPs, which are the most common, consist of six conserved cysteine residues that form three disulfide bonds, six α-helices, and a hydrophobic pocket responsible for binding small odor molecules [14, 15, 17]. The sequence and structure of OBPs can vary significantly among different insects species. For instance, Trichogramma japonicum (Hymenoptera: Trichogrammatidae) possesses typical OBPs [18], whereas Aenasius bambawalei (Hymenoptera: Encyrtidae) has both typical and minus-C OBPs [19]. OBPs are predominantly expressed in the antennae, head, thorax, abdomen, and legs of insects [20]. Further, the expression of OBPs varies according to the insect’s sex and developmental stage.
OBPs play a crucial role in the olfactory responses of insects by facilitating the transport of hydrophobic odor molecules through an aqueous lymph to odorant receptors [21]. In 1981, Vogt and Riddi-Ford employed an isotopic labeling method to identify an OBP in the antennae of male Antheraea polyphemus (Lepidoptera: Saturniidae) [6]. Subsequently, antennal binding proteins were discovered in Drosophila melanogaster (Diptera: Drosophilidae) and Bombyx mori (Lepidoptera: Bombycidae) [22, 23]. Numerous OBP genes have been identified in D. melanogaster (51) [14], the tobacco cutworm Spodoptera litura (38) (Lepidoptera: Noctuidae) [24], and over 300 in more than 20 parasitoids of Hymenoptera [25]. OBPs are responsible for binding to and transporting odor molecules, thereby regulating olfactory responses [12, 23]. In addition, these proteins have the ability to recognize phytochemical substances and volatile organic compounds emitted by plants. For instance, in A. bambawalei, OBP28 can bind to 15 compounds at pH 7.4, including 1-octen-3-one and diethyl sebacate [26].
Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) poses a significant economic threat to the fruit and vegetable industry worldwide due to its broad range of hosts and high reproductive capacity. It infests over 300 types of produce, including citrus, pomegranate, apple, banana, and cucumber [27]. The primary method for controlling this pest is through chemical insecticide spraying, which contributes to issues such as insecticide resistance, environmental contamination, and food contamination [28]. To mitigate the negative impact of chemical pesticides on human health and the environment, biological control methods utilizing parasitoids have been widely adopted. These approaches are sustainable and do not result in environmental contamination [29]. For instance, following an outbreak of B. dorsalis in 2005 in Fujian Province, China, parasitoids such as Fopius arisanus (Hymenoptera: Braconidae) native to Hawaii, along with other parasitoids including Fopius vandenboschi, Psyttalia incisi, and Diachasmimorpha longicaudata (Hymenoptera: Braconidae), were introduced to reduce the field population of B. dorsalis [30].
Psyttalis incisi is a solitary opiine parasitoid that displays a preference for early larval instars of B. dorsalis [31]. In southern China, P. incisi is the predominant parasitoid species, accounting for 77.6% of the population that targets B. dorsalis in orchards located in Zhangzhou City, Fujian Province, China [31]. Consequently, P. incisi holds significant promise for the biological control of B. dorsalis in this particular region of China [29]. Extensive research has been conducted on various aspects of this species, encompassing studies of fitness [32,33,34], field applicability [29, 35], biology [36,37,38], behavior [39], molecular characterization [40], and mitochondrial genome analysis [41]. The effectiveness of parasitoids as biocontrol agents relies on their ability to seek out hosts using chemical cues [42]. Therefore, it is crucial to comprehend the chemosensory system of P. incisi in order to enhance the efficiency of the biological control measures against B. dorsalis within agricultural ecosystems.
This study analyzed the antennal transcriptome of P. incisi and identified olfactory gene clusters through bioinformatic analysis. The expression patterns of olfactory genes in different tissues were investigated using real-time quantitative PCR (qRT-PCR). Phylogenetic trees were constructed to predict evolutionary relationships among the chemosensory genes of hymenopterans. This study provides the basis for understanding the function of these genes and the host-seeking behavior of P. incisi.
Materials and methods
Insect rearing and sample collection
A strain of B. dorsalis and a strain of P. incisi were obtained from ripe guava fruits collected in Zhangzhou City, Fujian Province, China, in 2004, and were established at the Institute of Beneficial Insects, Fujian Agriculture and Forestry University [31]. Bactrocera dorsalis were allowed to oviposit in perforated plastic bottles, and the eggs were then collected and transferred to a tray containing milled feed for larval development, following the previously described [43]. Adult flies were fed a diet of yeast extract and sugar (1:3, wt/wt) and provided with water. Adult parasitoids were maintained in “Hawaii-type” cages (30 × 30 × 30 cm) and given honey and water as food. To prevent superparasitism, two plates (each containing approximately 1000 larvae) were placed in a cage containing 1000 P. incisi adults (500 females and 500 males) and left for 24 h. Bactrocera dorsalis and P. incisi were reared under a 12 h light, 12 h dark cycle at 25 ± 1 °C and a relative humidity of 65 ± 5% [29].
For tissue expression profiling, around 200 male and 200 female P. incisi adults were dissected on ice under a light microscope. The following body parts were collected: antennae (200 pairs), heads without antennae (100), thoraces without legs and wings (100), abdomens (50), and legs (200). In total, three biological replicates were collected, transferred to 1.5 mL centrifuge tubes. The samples were then frozen in liquid nitrogen and stored at − 80 °C until further use.
RNA extraction, cDNA library construction, and Illumina sequencing
Total RNA was extracted from the antennae of female and male adults (20 mg) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The extracted RNA samples were then treated with RNase-free DNase I (TaKaRa, Dalian, Liaoning, China) to remove any contaminating DNA. The concentration and purity of the RNA samples were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). In addition, the integrity of the RNA was assessed by performing 1% agarose gel electrophoresis. The total RNA extracted from the antennae was subsequently frozen in liquid nitrogen and stored at − 80 °C for further use. Alternatively, the RNA samples were transported on dry ice to Sangon Biotech (Shanghai, China) for transcriptome sequencing using the Illumina Hiseq™ platform.
The construction of the cDNA library for each sample was performed using the NEB Next Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s protocol. To summarize the procedure, 10 ug of total RNA from each tissue was used to isolated mRNA using oligo (dT) magnetic beads. The isolated mRNA was then fragmented by incubating with the fragmentation buffer supplied with the kit at 94 °C for 5 min. Next, first-strand cDNA was synthesized using random hexamers and the fragmented mRNA as a template. Subsequently, second-strand cDNA was carried out using a combination of buffer, dNTPs, RNase H, and DNA polymerase I. After performing end-repair, A-tailing, and adaptor ligation, the resulting products were amplified by PCR. The amplified DNA fragments were purified using the QIA Quick PCR Purification Kit (Qiagen, Valencia, CA, USA) to obtain the final sequencing library. The cDNA library was then subjected to sequencing using the Illumina Hiseq™ platform.
De novo assembly and gene annotation
Illumina Hiseq™ raw data files were analyzed with CASAVA and converted to raw reads through base calling. The raw sequencing reads underwent trimming using Trimmomatic, where adaptor sequences, reads with a percentage of unknown nucleotides higher than 10%, and low-quality reads (containing more than 50% bases with Q-value ≤ 20) were removed. Then, de novo transcriptome assembly was performed using three modules within Trinity: Inchworm, Chrysalis, and Butterfly [44]. Redundant sequences were removed, and the longest transcript was designated as unigene.
Unigenes were annotated using BLAST alignment with an E-value cutoff of 1.0 × 10− 5 against seven databases: NCBI non-redundant (NCBI-NR), NCBI nucleotide (NCBI-NT), Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), Protein Family (Pfam), EuKaryotic Ortholog Groups/Clusters of Orthologous Groups (KOG/COG), and SwissProt [45]. The BLAST alignment was performed through the BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Additional parameters were customized according to the system. Based on the best alignment scores, the directions of the unigene sequence were determined, and protein function annotation was carried out. Blast2GO was used for the annotation of Gene Onotology (GO) terms and unigenes [46].
To identify candidate chemosensory genes, transcriptome data from the antennae of male and female adults were screened. Unigenes were annotated by performing BLASTx against NCBI-NR sequences with an E-value cutoff of < 1.0 × 10− 5. Unigenes encoding odorant-binding proteins (OBPs), gustatory receptors (GRs), olfactory receptors (ORs), ionotropic receptors (IRs), chemosensory proteins (CSPs), and sensory neuron membrane proteins (SNMPs) were selected as putative chemosensory genes. All candidate genes were manually verified using BLASTx against the NR database. Relevant parameters such as sequence length, species with the highest homology, gene name, number of entries, E-value, and gene sequence homology were recorded. Open reading frames (ORFs) were predicted using the ORF finder tool (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Signal peptides were predicted using SignalP 5.0 server (https://services.healthtech.dtu.dk/service.php?SignalP-5.0).
Chemosensory gene verification and bioinformatic analysis
The conserved domains and ORFs of candidate chemosensory genes (OBPs, ORs, SNMPs, GRs, and IRs) were manually validated. Amino acid sequences were obtained through BLASTx alignment and coding region prediction. Orthologs were identified, and the degree of homology was calculated using BLASTx (http://blast.ncbi.nlm.nih.gov/blast.cgi). Nucleotide sequences were translated using Expasy (https://web.expasy.org/translate/). The theoretical isoelectric point (pI) and molecular weight (Mw) of the deduced proteins were estimated using ProtParam (https://web.expasy.org/protparam/). Protein domains were predicted using EMBL-EBI services (https://pfam.xfam.org/search#tabview=tab1). Transmembrane domains (TMDs) were identified using TMHMM version 2.0 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0) [47]. All OBP genes reported in this study have complete ORFs. The nucleotide sequences have been deposited in GenBank under accession numbers OP484699 to OP484711.
Phylogenetic analysis
To validate the annotation of candidate genes and identify homologous sequences, we conducted a phylogenetic analysis involving P. incisi, other hymenopteran species, and model insects. GenBank was searched using BLAST to find sequences with the highest homology to the candidate genes. Evolutionary trees were constructed based on nucleic acid sequences from Aphidius gifuensis [48], Cephus cinctus [49], Diachasma alloeum [50], Fopius arisanus [51], Microplitis demolitor [52], Microplitis mediator [53], Chelonus insularis [54], Cotesia glomerata [55] (Hymenoptera: Braconidae), and Nasonia vitripennis (Hymenoptera: Pteromalidae) [56]. Multiple alignments were performed using MAFFT (https://mafft.cbrc.jp/alignment/server/index.html), with the “auto” strategy and customized parameters. Sequences edited was done using BioEdit. The best-fit partition model (Edge-linked) was selected using ModelFinder based on the Akaike information criterion. Maximum likelihood phylogenies were inferred using IQ-TREE under the selected model (Table S1) using the “auto” option for model selection (http://iqtree.cibiv.univie.ac.at/, accessed on 18 October 2021) with 20,000 ultrafast bootstraps and approximate Bayes test [57]. A circular phylogenetic tree was generated and color-coded using iTOL tools (https://itol.embl.de/) and annotated using Adobe Illustrator CC 2018 (Adobe, CA, USA).
Quantitative real-time PCR (qRT-PCR)
Quantitative real-time PCR (qRT-PCR) was used to evaluate the expression levels of candidate OBPs in different tissues of both sexes. Total RNA was extracted from the antennae, head, thorax, abdomen, and legs using aforementioned protocol. Subsequently, cDNA was synthesized from the total RNA using the FastKing gDNA Dispelling RT SuperMix Kit (TIANGEN, Beijing, China) according to the following steps: 10 µg of total RNA from each tissue was utilized for mRNA isolation, and reverse transcriptase (FastKing-RT Enzyme) was used for cDNA synthesis at 42 °C for 15 min. The resulting cDNA was then stored at − 20 °C until further analysis. Each cDNA sample was diluted to a concentration of 200 ng/µL using nuclease-free water. Gene-specific primers were designed with the assistance of Oligo 7.0 software and are listed in Supplementary Table S2. The lengths of the PCR products ranged from 80 to 150 bp. The gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with GenBank Accession OP593307, is a well-known housekeeping gene often used as a normalizer for the expression levels of target genes in different tissues, including antennae, heads, thorax, abdomens and legs of various insects such as Spodoptera frugiperda [58], Trichogramma japonicum [18], Aenasius bambawalei [19], Myzus persicae [59]. To construct a relative standard curve, templates were diluted into a series of five two-fold dilutions (1:1, 1:2, 1:4, 1:8, and 1:16). The amplification efficiency (E) and R2 value of each primer pair were calculated using the slope of the standard curve. The amplification efficiency was calculated as follows: E=[10^(-1/slope)-1] ×100%. Only primers with amplification efficiencies between 90% and 110% were used for subsequent data analysis [60].
RT-qPCR was performed using a QuantStudio 3 PCR system (Applied Biosystems, Foster City, CA, USA) in 96-well reaction plates. The reactions were conducted in a 20 µL mixture containing 10 µL of PerfectStart Green qPCR SuperMix (+ Dye II) (TRANS, Beijing, China), 0.4 µL of each primer (10 µM), 2 µL of cDNA (200 ng/µL), and 7.2 µL of nuclease-free water. A negative control using sterile water was included. The reaction conditions were as follows: one cycle at 94 °C for 30 s, followed by 40 cycles at 94 °C for 5 s and 60 °C for 30 s. After 40 cycles, melting curves were analyzed at a temperature range of 60–95 °C to verify the presence of a single gene-specific peak for each gene, without any primer dimer peaks. All reactions were performed independently and in triplicate three times. The relative levels of gene expression among the different samples were measured using the 2−ΔΔCt method [61]. The data are presented as mean ± standard error. Statistical comparison of gene expression was performed using one-way analysis of variance followed by Tukey’s honestly significant difference test. P-values less than 0.05 were considered statistically significant. The statistical analysis was performed using SPSS version 21.0 and GraphPad Prism version 9.0.0 (Inc., La Jolla, CA, USA). The figures were created using Adobe Illustrator CC 2018 (Adobe, CA, USA).
Results
Transcriptome sequence and assembly
The transcriptome analysis of P. incisi adults antennae involved the use of Illumina sequencing, which generated a total of 268,697,486 raw reads. After Trinity assembly, 261,177,254 clean reads were obtained, which were further assembled into 159,379 transcripts. These transcripts comprised 64,515 unigenes, ranging in length from 201 bp to 24,806 bp, with an N50 length of 2347 bp. The mean length of transcripts was 1637 bp, while the average length of unigenes was 876 bp. It is worth mentioning that 21,440 unigenes, accounting for 33.23% of the total, had a length exceeding 1000 bp. The number of transcripts decreased as the length increased, as depicted in Figure S1. A summary of the sequencing and assembly process is provided in Table S3.
Functional annotation and expression level of P. incisi antennae transcriptome
Functional annotation of unigenes involved selecting the proteins with the highest similarity. Out of the 64,515 unigenes, a total of 19,243 (29.83%) had homologous sequences in NCBI-NR database, 12,147 (18.83%) in NCBI-NT, 8,667 (13.43%) in GO, 7,218 (11.19%) in KOG, 6,791 (10.53%) in PFAM, 6,088 (9.44%) in CDD, and 3,738 (5.79%) in KEGG (Table S4). The homologous sequences were determined based on their alignment scores (E-value) in the NR database. The analysis revealed a significant number of homologous genes in Hymenoptera. Among the species with the highest homology, D. alloeum had the highest percentage (56.72%), followed by F. arisanus (14.51%), B. dorsalis (2.82%), Lasius niger (Hymenoptera: Formicidae) (2.23%), Cotesia congregate (Hymenoptera: Braconidae) (1.26%), and M. demolitor (1.07%). The respective number of matched unigenes for these species was 10,914, 2,793, 543, 429, 243, and 205 (Figure S2).
The functional characterization of the transcriptome was performed using GO enrichment analysis. A total of 8,667 unigenes were successfully annotated, and they were found to be enriched in biological processes (816,342), cellular components (192,348), and molecular functions (98,682) (Figure S3).
In the transcriptome of the antennae, a total of 3,738 unigenes were annotated in the KEGG database. These unigenes exhibited enrichment in various categories, including cellular processes (1,040), environmental information processing (1,107), genetic information processing (1,076), metabolism (2,111), organizational systems (1,950), signal transduction (935), translation (474), endocrine system (459), and transport and catabolism (430 unigenes) (Figure S4).
In the KOG database, a total of 7,218 unigenes were annotated and categorized into 25 different categories. Among these categories, the four most significant ones, each with more than 500 unigenes, were T, R, K and O (Figure S5).
Identification of OBPs
Thirteen putative OBPs were identified through a comparative analysis of the antennal transcriptome of P. incisi using BLASTx. The degree of homology to NCBI database sequences from P. incisi and other species ranged from 40.32 to 99.32%. Out of the thirteen identified OBPs, all had complete ORFs and encoded 103 to 167 amino acids. Notably, two OBPs (1 and 13) lacked a signal peptide (Table 1). Among the thirteen OBPs, three (5, 6, and 10) exhibited typical characteristics, while five (1, 4, 8, 11, and 12) posessed a minus-C motif, and the remaining five (2, 3, 7, 9, and 13) displayed plus-C motif.
A BLAST search against the NCBI database revealed that the candidate OBPs shared significant similarities with amino acid sequences from other insect species such as A. gifuensis, C. cinctus, D. alloeum, F. arisanus, M. demolitor, and M. mediator. To further analyze the relationships among these OBPs, a phylogenetic tree was constructed using the maximum likelihood method with 20,000 bootstrap replicates. Clustering was determined based on bootstrap values, with a cutoff of 50% cutoff. The OBP sequences were divided into distinct clusters based on their clustering patterns. Notably, OBP-1 and OBP-6 from P. incisi clustered together with OBP-9 and OBP-4 from C. cinctus, respectively. Furthermore, OBP-3, OBP-4, and OBP-5 from P. incisi clustered with OBP69a-like, OBP69a-like, and OBP83a-like from F. arisanus, respectively (Fig. 1).
Tissue- and sex-specific expression of OBPs
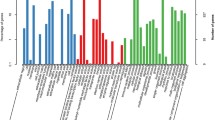
We performed qRT-PCR to examine the expression profiles of the 13 OBPs genes in different tissues. The results showed a diverse distribution pattern of these OBPs among adult wasps. Specifically, seven OBPs exhibited high expression in the antennae. Among them, four OBPs (3, 6, 8, and 12) demonstrated notably elevated expression in the antennae of male adults, displaying a distinct expression profile compared to other tissues. This finding suggests that these OBPs, which are highly expressed in the male antennae, might be associated with mating behavior [62]. On the other hand, three OBPs (1, 7, and 13) showed high expression in the antennae of female adults and may be involved in host-seeking and selecting suitable spawning sites [63]. OBP5 showed prominent expression in the male legs. However, five OBPs (2, 4, 9, 10, and 11) exhibited significantly higher expression in the adult abdomen, indicating that OBPs are also expressed in non-olfactory organs, indicating a potential role beyond olfaction (Fig. 2).
Relative expression levels of candidate odorant-binding protein (OBP) genes of Psyttalia incisi adults in different tissues by qRT-PCR. Expression was normalized to the housekeeping gene GAPDH using the 2−∆∆Ct method. Data are mean ± SEM. Different letters (a–e) indicate significant differences in means (p < 0.05) between males and females by one-way analysis of variance
Figure 2. Relative expression levels of candidate odorant-binding protein (OBP) genes of Psyttalia incisi adults in different tissues by qRT-PCR. Expression was normalized to the housekeeping gene GAPDH using the 2−∆∆Ct method. Data are mean ± SEM. Different letters (a–e) indicate significant differences in means (p < 0.05) between males and females by one-way analysis of variance.
Identification of GRs
The transcriptome analysis identified seven candidate GR transcripts (GenBank Accessions OP535007 to OP535013) with 2 to 7 transmembrane domains and a degree of homology ranging from 79.10 to 92.68%. Among them, five GRs (2, 4–7) had complete ORFs. The BLASTx alignment revealed that three GRs (3, 5, and 7) clustered with GRs associated with sugar detection (Table S5). The phylogenetic tree constructed using the GR sequences included a putative sugar receptor and a carbon dioxide receptor. Furthermore, the phylogenetic analysis demonstrated that three GRs (3, 5, and 7) clustered with gustatory (sugar) receptor genes, while two GRs (2 and 4) clustered with CO2 receptor genes (Fig. 3).
Phylogenetic tree of P. incisi GR genes. Molecular phylogeny comparing PincGRs with gustatory receptors (GRs) from 6 other insect species: A total of 7 GRs (PincGR1-7) from P. incisi (Pinc) and others from F. arisanus (Fari), D. alloeum (Dall), C. cinctus (Ccin), M. mediator (Mmed), C. insularis (Cins) and A. gifuensis (Agif)
Identification of ORs
We identified 55 putative OR genes (54 typical ORs and one atypical odorant receptor coreceptor [Orco]) from the transcriptome and genome of P. incisi (Table S6) (GenBank Accessions OP956154 to OP956208). The candidate Orco shared a high sequence identity with other conserved insect co-receptors. Compared to typical ORs, Orco was highly conserved in insects, with homology ranging from 50 to 99%. Additionally, amino acid sequence analysis revealed a highly conserved region at the C-terminal [64]. The ORs were named following the convention “ORx,” where the numbers x were assigned in ascending order of coding region length. Alignment of the ORs against the NR database showed homology ranging from 42.72 to 95.74%, and the presence of ORFs (324–1539 bp) encoding 107 to 512 amino acids. Based on sequence characteristics of ORs, the prediction of 0 to 7 transmembrane domains was made, of which four ORs (36, 42, 48, and 49) possessed complete ORFs and exhibited the classical feature of having seven transmembrane domains, characteristic of insect ORs (Table S6). Phylogenetic analysis showed that Orco clustered with C. cinctus Orco. No P. incisi-specific OR family expansion was observed in the phylogenetic tree (Fig. 4).
Phylogenetic tree of P. incisi ORs. Molecular phylogeny comparing PincORs with odorant receptors (ORs) from 5 other insect species: A total of 55 ORs (PincOR1-54 and PincOrco) from P. incisi (Pinc) and others from F. arisanus (Fari), D. alloeum (Dall), M. mediator (Mmed), C. insularis (Cins) and Cotesia glomerata (Cgol). The color of the branches refers to the bootstrap values
Identification of IRs
The transcriptome analysis identified 10 P. incisi IRs (GenBank Accessions OP558961 to OP558970) with zero to four transmembrane domains (Table S7). Among them, seven IRs (2, 4, 6, 7, 8, 9, and 10) had complete ORFs, encoding proteins with 361 to 731 amino acids. When performing a BlastX homology comparison, nine P. incisi IRs (1–7, 9, and 10) exhibited over 83% homology with the IR gene of D. alloeum, while P. incisi IR-8 showed the highest homology (85%) with the IR gene of Meteorus pulchricornis. The IRs from eight different species were classified into subfamilies (Fig. 5). Previous studies have indicated that IR8a and IR25a act as co-receptors for IRs [65]. In our analysis, IR-3, 9, and 10 clustered together with the coreceptor IR25a, with a bootstrap support value of 79, 27, and 65, respectively (Fig. 5). Similarly, two IRs genes were found in C. cinctus, C. insularis, N. vitripennis, and C. glomerata, while one homolog of IR25a was found in the wasps A. gifuensis and D. alloeum. Additionally, P. incisi IR-8 exhibited a match with an N-methyl-D-aspartic acid receptor.
Identification of SNMPs
BLASTx and cluster analysis identified two candidate SNMP transcripts (GenBank Accessions OP558971 and OP558972) (Table S8). The ORFs of SNMP-1a and 1b were found to be 669 bp and 630 bp in length, respectively, indicating that both proteins were not encoded by full-length transcripts. These transcripts exhibited a degree of homology of 86.60% and 96.17% with D. alloeum, respectively, and no transmembrane domains were found. Phylogenetic analysis showed that SNMP1a and SNMP1b clustered together with the SNMP1 group and showed a closer relationship to D. alloeum SNMPs (Fig. 6).
Phylogenetic tree of P. incisi SNMP genes. A total of 2 SNMPs (PincSNMP1a, PincSNMP1b) from P. incisi (Pinc) and others from F. arisanus (Fari), D. alloeum (Dall), C. cinctus (Ccin), C. insularis (Cins), A. gifuensis (Agif), N. vitripennis (Nvit), M. mediatorand (Mmed) and M. demolitor (Mdem). The scale bar represents the 0.1 amino acid substitutions per site
Discussion
The rapid development of genomics and transcriptomics has enabled the identification and characterization of olfactory genes in various parasitoid species, including A. bambawalei [19], F. arisanus [51], M. mediator [53], N. vitripennis [56], E. formosa [60], C. vestalis [66], D. longicaudata [67], and Zele chlorophthalmus [68] (Hymenoptera: Braconidae). The olfactory system plays a crucial role in host-seeking and parasitism for parasitoids. In insects, the antennae serve as the primary olfactory organ and contain a high density of chemosensory receptors [69] Therefore, sequencing the antennal transcriptome is essential for the identification of olfactory genes and serves as the foundation for understanding host-seeking behavior.
The present study involved sequencing and analyzing the antennal transcriptome of the fruit fly parasitoid P. incisi. A total of 64,515 unigenes were identified, with 58.26% of them being longer than 300 bp, demonstrating the high quality and depth of the transcriptome. A homology search using BLASTx against the NR database revealed that the identified P. incisi unigenes shared 56.72% and 14.15% identity with D. alloeum and F. arisanus, respectively (Figure S2), which is potentially due to their common affiliation with the Braconidae family. The number of unigenes annotated in the GO database (8,667) was lower than that in the NCBI-NR database (19,243). GO analysis indicated that P. incisi unigenes were mainly associated with binding and catalytic activities, in line with a previous study [70]. In addition, among the annotated unigenes, 7,218 and 3,738 were identified in the KOG and KEGG databases, respectively. KOG and KEGG analyses showed that a significant enrichment of unigenes involved in signal transduction (Figures S4 and S5), suggesting that potential paralogy or common ancestry with olfactory genes [71].
The transcriptome analysis revealed the presence of 87 chemosensory genes in P. incisi, including 13 OBPs, 55 ORs, 7 GRs, 10 IRs, and 2 SNMPs. The expression patterns of OBPs were further assessed using qRT-PCR. The number of OBP genes in P. incisi was similar to that in F. arisanus (13) [51] and D. alloeum (14) [50], but lower compared to C. vestalis (74) [72], A. bambawalei (54) [19], and M. mediator (20) [53]. OBP-3, OBP-4, and OBP-5 were found to cluster with OBP69a-like, OBP69a-like, and OBP83a-like genes from F. arisanus, respectively (Fig. 1), suggesting a possible functional similarity among these genes.
The RT-qPCR analysis showed that seven OBPs (1, 3, 6, 7, 8, 12, and 13) exhibited high expression levels in the antennae, suggesting their potential involvement in recognizing host odors or pheromones. This finding is consistent with the gene expression profiles observed in other insects like A. bambawalei [19] and E. formosa [60]. In addition, OBP-1, OBP-7, and OBP-13 showed high expression specifically in the female antennae (Fig. 2), suggesting their potential roles in mate and host finding. Moreover, the highly expressed OBP genes in the antennae may also play a role in recognizing herbivore-induced plant volatiles [63]. Interestingly, five OBPs (2, 4, 9, 10, and 11) exhibited significantly higher expression levels in the adult abdomen, suggesting their potential involvement in detecting sex pheromones, as demonstrated in Sclerodermus sp [73]. . Moreover, the expression of OBP-5 was found to be higher in male legs compared to other body parts, which is consistent with the tissue-specific expression of OBP2 in Plutella xylostella, suggesting a potential involvement in chemotaxis [74]. Several P. incisi OBPs were found to be exclusively or predominantly expressed in the antennae, highlighting their specific roles in olfactory perception. Furthermore, the expression of certain OBP genes exhibited sexual dimorphism, indicating functional differences between males and females.
The number of GRs in the antennae varies considerably among species—33 in F. arisanus [51], 23 in Z. chlorophthalmus [68], and seven in P. incisi—presumably because the antennae are not the major olfactory organ. This variability may reflect differences in the ability to detect sugars, carbon dioxide, and pheromones. The phylogenetic analysis indicates that GR2 and GR6 may be involved in carbon dioxide sensing, while GR3, GR5, and GR7 may be implicated in sugar detection. Other GRs may be involved in different chemosensory processes. Since gustatory sensilla are mainly distributed in mouthparts (proboscises and labial palps), antennae, wings, legs, and ovipositor [75], it is necessary to identify other GR genes from the transcriptome of these additional tissues to better understand chemotaxis.
ORs are important chemoreceptors that primarily detect sex pheromones and other odorants [4]. The number of ORs in P. incisi was much lower (55) compared to F. arisanus (157) [51], Meteorus, pulchricornis (99) [76], and N. vitripennis (269) [56], but higher than C. vestalis (9) [63]. Variations in the number of OR genes could be attributed to differences in sequencing methods and depth. Through nucleic acid sequence analysis, several OR and Orco genes were identified. Phylogenetic analyses showed that most OR sequences clustered with those of D. alloeum and F. arisanus. The Orco receptors are conserved among insects [64]. One Orco sequence clustered with the highly conserved Orco of C. insularis, suggesting that they may have a similar role in odorant detection.
IR genes were first identified in the genome of D. melanogaster, and these genes are abundantly expressed in sensory neurons without ORs and GRs, playing a role in detecting various odor combinations [3]. The number of IRs in the antennae of P. incisi was similar to C. cinctus (16) [49] and Macrocentrus cingulum (13) [77]. IR sequences clustered into five groups, including IR3 and IR9, while IR10 clustered with the coreceptor IR25a, indicating that IRs within each group may have a similar function in the antennae.
SNMPs are classified as SNMP1 or SNMP2. SNMP1 is expressed in the dendritic membranes of olfactory receptor neurons, while SNMP2 is expressed in Sertoli cells or lymph [78]. In this study, two SNMPs were identified, and phylogenetic analysis showed that SNMP1a and SNMP1b clustered together with the SNMP1 group.
CSPs are involved in various such as chemosensory function, lipid transport, immunity, insecticide resistance, and xenobiotic degradation. While no CSP genes were found in P. incisi, they have been identified in other body parts such as antennae, head, thorax and wings in other species [79]. The number of chemosensory genes can vary depending on the species, body part, and testing method used.
In this study, we sequenced and assembled the transcriptome of P. incisi antennae and functionally annotated a total of 64,515 unigenes. Among these, 87 chemosensory genes were identified, including 13 OBPs, 55 ORs, 7 GRs, 10 IRs, and 2 SNMPs. We compared these genes with sequences from other insects, which enhanced our understanding of the olfactory systems in parasitoids and provided important insights into exploring the binding characteristics of OBPs in P. incisi. However, further investigations into olfactory mechanisms in P. incisi should be conducted using alternative methods such as protein expression profiling, fluorescence polarization competition, and molecular docking.
Conclusion
Eighty-seven chemosensory genes, including 13 OBPs, 55 ORs, 7 GRs, 10 IRs, and 2 SNMPs, were identified in the antennal transcriptome of P. incisi. These genes were further studied by phylogenetic and bioinformatics methods. The expression patterns of OBPs were also examined through qRT-PCR. This study significantly contributed to our understanding of P. incisi’s the chemosensory system. Moreover, it provided a foundation for developing attractants to monitor the population dynamics of parasitoid in the field and improve the efficacy of biological control for B. dorsalis.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- OBPs:
-
Odorant-binding proteins
- GRs:
-
Gustatory receptors
- ORs:
-
Odorant receptors
- IRs:
-
Ionotropic receptors
- SNMPs:
-
Sensory neuron membrane proteins
- CSPs:
-
Chemosensory proteins
- PBPs:
-
Pheromone-binding proteins
- GOBPs:
-
General odorant-binding proteins
- NCBI:
-
NR-NCBI non-redundant
- NCBI:
-
NT-NCBI nucleotide
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- Pfam:
-
Protein Family
- KOG/COG:
-
EuKaryotic Ortholog Groups/Clusters of Orthologous Groups
- ORFs:
-
Open reading frames
- TMDs:
-
Transmembrane domains
- pI:
-
Theoretical isoelectric point
- Mw:
-
Molecular weight
- TMDs:
-
Transmembrane domains
References
Schmidt HR, Benton R (2020) Molecular mechanisms of olfactory detection in insects: beyond receptors. Open Biol 10:200252. https://doi.org/10.1098/rsob.200252
Kwon HW, Lu T, Rutzler M, Zwiebel LJ (2006) Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA 103:13526–13531. https://doi.org/10.1073/pnas.0601107103
Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136:149–162. https://doi.org/10.1016/j.cell.2008.12.001
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58:373–391. https://doi.org/10.1146/annurev-ento-120811-153635
Liu GX, Ma HM, Xie HY, Xuan N, Guo X, Fan ZX, Rajashekar B, Arnaud P, Offmann B, Picimbon JF (2016) Biotype characterization, developmental profiling, insecticide response and binding property of Bemisia tabaci chemosensory proteins: role of CSP in insect defense. PLoS ONE 11:e0154706. https://doi.org/10.1371/journal.pone.0154706
Vogt RG, Riddiford LM (1981) Pheromone binding and inactivation by moth antennae. Nature 293:161–163. https://doi.org/10.1038/293161a0
Younus F, Chertemps T, Pearce SL, Pandey G, Bozzolan F, Coppin CW, Russell RJ, Maibeche-Coisne M, Oakeshott JG (2014) Identification of candidate odorant degrading gene enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem Mol Biol 53:30–43. https://doi.org/10.1016/j.ibmb.2014.07.003
Swarup S, Williams TI, Anholt RRH (2011) Functional dissection of odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav 10:648–657. https://doi.org/10.1111/j.1601-183X.2011.00704.x
Hall SE, Floriano WB, Vaidehi N, Goddard WA (2004) Predicted 3-D structures for mouse I7 and rat I7 olfactory receptors and comparison of predicted odor recognition profiles with experiment. Chem Senses 29:595–616. https://doi.org/10.1093/chemse/bjh063
Zhang YX, Wang WL, Li MY, Li SG, Liu S (2017) Identification of putative carboxylesterase and aldehyde oxidase genes from the antennae of the rice leaffolder, Cnaphalocrocis Medinalis (Lepidoptera: Pyralidae). J Asia-Pac Entomol 20:907–913. https://doi.org/10.1016/j.aspen.2017.06.001
Durand N, Carot-Sans G, Bozzolan F, Rosell G, Siaussat D, Debernard S, Chertemps T, Maïbèche-Coisne M (2011) Degradation of pheromone and plant volatile components by a same odorant-degrading enzyme in the cotton leafworm, Spodoptera littoralis. PLoS ONE 6:e29147. https://doi.org/10.1371/journal.pone.0029147
Pelosi P, Zhou JJ, Ban LP, Calvello M (2006) Soluble proteins in insect chemical communication. Cell Mol Life Sci 63:1658–1676. https://doi.org/10.1007/s00018-005-5607-0
Peng G, Leal WS (2001) Identification and cloning of a pheromone-binding protein from the oriental beetle, Exomala Orientalis. J Chem Ecol 27:2183–2192. https://doi.org/10.1023/a:1012270602288
Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA (2002) Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res 12:1357–1369. https://doi.org/10.1101/gr.239402
Zhou JJ, Robertson G, He XL, Dufour S, Hooper AM, Pickett JA, Keep NH, Field LM (2009) Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J Mol Biol 389:529–545. https://doi.org/10.1016/j.jmb.2009.04.015
Venthur H, Mutis A, Zhou JJ, Quiroz A (2014) Ligand binding and homology modelling of insect odorant-binding proteins. Physiol Entomol 39:183–198. https://doi.org/10.1111/phen.12066
Honson NS, Gong Y, Plettner E (2005) Chapter nine-structure and function of insect odorant and pheromone-binding proteins (OBPs and PBPs) and chemosensory-specific proteins (CSPs). Recent Adv Phytochem 39:227–268. https://doi.org/10.1016/S0079-9920(05)80010-3
Wu JD, Shen ZC, Hua HQ, Zhang F, Li YX (2017) Identification and sex expression profiling of odorant-binding protein genes in Trichogramma japonicum (Hymenoptera: Trichogrammatidae) using RNA-Seq. Appl Entomol Zool 52:623–633. https://doi.org/10.1007/s13355-017-0516-x
Nie XP, Li QL, Xu C, Li DZ, Zhang Z, Wang MQ, Zhou AM, Li SQ (2018) Antennal transcriptome and odorant binding protein expression profiles of an invasive mealybug and its parasitoid. J Appl Entomol 142:149–161. https://doi.org/10.1111/jen.12417
Zhang ZK, Zhang Y, Wu SY, Lei ZR (2017) Recent advances in odorant binding proteins of insects. J Environ Entomol 39:713–720. https://doi.org/10.3969/j.issn.1674-0858.2017.02.29
Larter NK, Sun JS, Carlson JR (2016) Organization and function of Drosophila odorant binding proteins. Elife 5:e20242. https://doi.org/10.7554/eLife.20242
Pikielny CW, Hasan G, Rouyer F, Rosbash M (1994) Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron 12:35–49. https://doi.org/10.1016/0896-6273(94)90150-3
Krieger J, von Nickisch-Rosenegk E, Mameli M, Pelosi P, Breer H (1996) Binding proteins from the antennae of Bombyx mori. Insect Biochem Mol Biol 26:297–307. https://doi.org/10.1016/0965-1748(95)00096-8
Gu SH, Zhou JJ, Gao S, Wang DH, Li XC, Guo YY, Zhang YJ (2015) Identification and comparative expression analysis of odorant binding protein genes in the tobacco cutworm Spodoptera litura. Sci Rep 5:13800. https://doi.org/10.1038/srep13800
He YY, Wang K, Zhang YJ, Wu QJ, Wang SI (2019) Research progress of olfactory protein of parasitoid wasps. J Environ Entomol 41:1232–1243. http://doi.org/CNKI:SUN:KCTD 0.2019-06-012
Xu C, Li QL, Qu XB, Chen J, Zhou AM (2020) Ant-hemipteran association decreases parasitism of Phenacoccus solenopsis by endoparasitoid Aenasius bambawalei. Ecol Entomol 45:290–299. https://doi.org/10.1111/een.12797
Liu H, Zhang DJ, Xu YJ, Wang L, Cheng DF, Qi YX, Zeng L, Lu YY (2019) Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J Integr Agric 18:771–787. https://doi.org/10.1016/s2095-3119(18)62015-5
Díaz-Fleischer F, Pérez-Staples D, Cabrera-Mireles H, Montoya P, Liedo P (2017) Novel insecticides and bait stations for the control of Anastrepha fruit flies in mango orchards. J Pest Sci 90:865–872. https://doi.org/10.1007/s10340-017-0834-3
Lin J, Yang DQ, Hao XX, Cai PM, Guo YQ, Shi S, Liu CM, Ji QE (2021) Effect of cold storage on the quality of Psyttalia incisi (Hymenoptera: Braconidae), a larval parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects 12:558. https://doi.org/10.3390/insects12060558
Yang JQ, Cai PM, Chen J, Zhang HH, Wang C, Xiang HJ, Wu J, Yang YC, Chen JH, Ji QE, Song DB (2018) Interspecific competition between Fopius arisanus and Psyttalia incisi (Hymenoptera: Braconidae), parasitoids of Bactrocera dorsalis (Diptera: Tephritidae). Biol Control 121:183–189. https://doi.org/10.1016/j.biocontrol.2018.02.003
Liang GH, Wu Y, Chen JH (2006) Seasonal incidence of Bactrocera dorsalis and its parasitoids in the field. J Southwest Forestry College 26:72–74. https://doi.org/10.3969/j.issn.2095-1914.2006.06.019
Carmichael AE, Wharton RA, Clarke AR (2005) Opiine parasitoids (Hymenoptera: Braconidae) of tropical fruit flies (Diptera: Tephritidae) of the Australian and South Pacific region. Bull Entomol Res 95:545–569. https://doi.org/10.1079/ber2005383
Bokonon-Ganta AH, McQuate GT, Messing RH (2007) Natural establishment of a parasitoid complex on Bactrocera latifrons (Diptera: Tephritidae) in Hawaii. Biol Control 42:365–373. https://doi.org/10.1016/j.biocontrol.2007.05.019
Vargas RI, Stark JD, Uchida GK, Purcell M (1993) Opiine parasitoids (Hymenoptera: Braconidae) of oriental fruit fly (Diptera: Tephritidae) on Kauai Island, Hawaii: is land wide relative abundance and parasitism rates in wild and orchard guava habitats. Environ Entomol 22:246–253. https://doi.org/10.1093/ee/22.1.246
Gu XH, Cai PM, Yang YC, Yang QY, Yao MY, Idrees A, Ji QG, Yang JQ, Chen JH (2018) The response of four braconid parasitoid species to methyl eugenol: optimization of a biocontrol tactic to suppress Bactrocera dorsalis. Biol Control 122:101–108. https://doi.org/10.1016/j.biocontrol.2018.04.002
John DS, Tim TYW, Roge RIV, Ronald KT (1992) Survival, longevity, and reproduction of Tephritid fruit fly parasitoids (Hymenoptera: Braconidae) reared from fruit flies exposed to Azadirachtin. J Econ Entomol 85:1125–1129. https://doi.org/10.1093/jee/85.4.1125
Bautista RC, Harris EJ (1997) Effects of multiparasitism on the parasitization behavior and progeny development of oriental fruit fly parasitoids (Hymenoptera: Braconidae). J Econ Entomol 90:757–764. https://doi.org/10.1093/jee/90.3.757
Ramadan MM, Wong TTY, Wong MA (1991) Influence of parasitoid size and age on male mating success of opiinae (Hymenoptera: Braconidae), larval parasitoids of fruit flies (Diptera: Tephritidae). Biol Control 1:248–255. https://doi.org/10.1016/1049-9644(91)90074-A
Vargas RI, Stark JD, Prokopy RJ, Green TA (1991) Response of oriental fruit fly (Diptera: Tephritidae) and associated parasitoids (Hymenoptera: Braconidae) to different-color spheres. J Econ Entomol 84:1503–1507. https://doi.org/10.1093/jee/84.5.1503
Shariff S, Ibrahim NJ, Md-Zain BM, Idris AB, Suhana Y, Roff MN, Yaakop S (2014) Multiplex PCR in determination of Opiinae parasitoids of fruit flies, Bactrocera sp., infesting star fruit and guava. J Insect Sci 14:7. https://doi.org/10.1093/jis/14.1.7
Yang DQ, Hao XX, Jiang LL, Chou TL, Lin X, Yue GQ, Xiao K, Lin J, Ji QE, Cai PM (2022) The complete mitochondrial genome of Psyttalia incisi (Silvestri, 1916) (Hymenoptera: Braconidae). Mitochondrial DNA B 7:1038–1040. https://doi.org/10.1080/23802359.2022.2081942
Cai PM, Song YZ, Huo D, Lin J, Zhang HM, Zhang ZH, Xiao CM, Huang FM, Ji QG (2020) Chemical cues induced from fly-oviposition mediate the host-seeking behaviour of Fopius Arisanus (Hymenoptera: Braconidae), an effective egg parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a tritrophic context. Insects 11:231. https://doi.org/10.3390/insects11040231
Chang CL, Vargas RI, Caceres C, Jang E, Cho IK (2006) Development and assessment of a liquid larval diet for Bactrocera dorsalis (Diptera: Tephritidae). Ann Entomol Soc Am 99:1191–1198. https://doi.org/10.1603/0013-8746(2006)99[1191:Daaoal]2.0.Co;2
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng QD, Chen ZH, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–U130. https://doi.org/10.1038/nbt.1883
Anderson I, Brass A (1998) Searching DNA databases for similarities to DNA sequences: when is a match significant? Bioinformatics 14:349–356. https://doi.org/10.1093/bioinformatics/14.4.349
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatic 21:3674–3676. https://doi.org/10.1093/bioinformatics/bti610
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol 305:567–580. https://doi.org/10.1006/jmbi.2000.4315
Fan J, Zhang Q, Xu QX, Xue WX, Han ZL, Sun JR, Chen JL (2018) Differential expression analysis of olfactory genes based on a combination of sequencing platforms and behavioral investigations in Aphidius gifuensis. Front Physiol 9:1679. https://doi.org/10.3389/fphys.2018.01679
Robertson HM, Waterhouse RM, Walden KKO, Ruzzante L, Reijnders MJMF, Coates BS, Legeai F, Gress JC, Biyiklioglu S, Weaver DK, Wanner KW, Budak H (2018) Genome sequence of the wheat stem sawfly, Cephus cinctus, representing an early-branching lineage of the Hymenoptera, illuminates evolution of Hymenopteran chemoreceptors. Genome Biol Evol 10:2997–3011. https://doi.org/10.1093/gbe/evy232
Tvedte ES, Walden KKO, McElroy KE, Werren JH, Forbes AA, Hood GR, Logsdon JM, Feder JL, Robertson HM (2019) Genome of the parasitoid wasp Diachasma Alloeum, an emerging model for ecological speciation and transitions to asexual reproduction. Genome Biol Evol 11:2767–2773. https://doi.org/10.1093/gbe/evz205
Calla B, Sim SB, Hall B, DeRego T, Liang GH, Geib SM (2015) Transcriptome of the egg parasitoid Fopius arisanus: an important biocontrol tool for Tephritid fruit fly suppression. Gigascience 4:36. https://doi.org/10.1186/s13742-015-0075-4
Wang SN, Shan S, Zheng Y, Peng Y, Lu ZY, Yang YQ, Li RJ, Zhang YJ, Guo YY (2017) Gene structure and expression characteristic of a novel odorant receptor gene cluster in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). Insect Mol Biol 26:420–431. https://doi.org/10.1111/imb.12306
Zhang SA, Zhang YJ, Su HH, Gao XW, Guo YY (2009) Identification and expression pattern of putative odorant-binding proteins and chemosensory proteins in antennae of the Microplitis mediator (Hymenoptera: Braconidae). Chem Senses 34:503–512. https://doi.org/10.1093/chemse/bjp027
Liu JF, Zhao HY, Song YF, Yu YC, Yang MF (2022) A chromosome-level genome assembly of the parasitic wasp Chelonus Formosanus Sonan 1932 (Hymenoptera: Braconidae). Genome Biol Evol 14. https://doi.org/10.1093/gbe/evac006
Wang Q, Gu H, Dorn S (2004) Genetic relationship between olfactory response and fitness in Cotesia glomerata (L). Heredity 92:579–584. https://doi.org/10.1038/sj.hdy.6800464
Robertson HM, Gadau J, Wanner KW, O’Brochta D, Field LM (2010) The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia Vitripennis. Insect Mol Biol 19:121–136. https://doi.org/10.1111/j.1365-2583.2009.00979.x
Zhang D, Gao FL, Jakovlic I, Zou H, Zhang J, Li WX, Wang GT (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour 20:348–355. https://doi.org/10.1111/1755-0998.13096
Liu XL, Wu ZR, Liao W, Zhang XQ, Pei YW, Lu M (2023) The binding affinity of two general odorant binding proteins in Spodoptera frugiperda to general volatiles and insecticides. Int J Biol Macromol 252:126338. https://doi.org/10.1016/j.ijbiomac.2023.126338
Liu JT, Xie JX, Khashaveh A, Zhou JJ, Zhang YJ, Dong H, Cong B, Gu SH (2022) Identification and tissue expression profiles of odorant receptor genes in the green peach aphid Myzus persicae. Insects 5:398. https://doi.org/10.3390/insects13050398
He YY, Wang K, Zhang YJ, Wu QJ, Wang SI (2020) Analysis of the antennal transcriptome and odorant-binding protein expression profiles of the parasitoid wasp Encarsia formosa. Genomics 112:2291–2301. https://doi.org/10.1016/j.ygeno.2019.12.025
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Zhang L, Feng YQ, Ren LL, Luo YQ, Wang F, Zong SX (2015) Sensilla on antenna, ovipositor and leg of Eriborus Applicitus (Hymenoptera: Ichneumonidae), a parasitoid wasp of Holcocerus Insularis Staudinger (Lepidoptera: Cossidae). Acta Zool 96:253–263. https://doi.org/10.1111/azo.12073
Nishimura O, Brillada C, Yazawa S, Maffei ME, Arimura G (2012) Transcriptome pyrosequencing of the parasitoid wasp Cotesia vestalis: genes involved in the antennal odorant-sensory system. PLoS ONE 7:e50664. https://doi.org/10.1371/journal.pone.0050664
Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, von Heijne G, Nilsson I (2007) Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett 581:5601–5604. https://doi.org/10.1016/j.febslet.2007.11.007
Wang YL, Chen Q, Guo JQ, Li J, Wang JT, Wen M, Zhao HB, Ren BZ (2017) Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus. Insect Biochem Mol Biol 89:58–70. https://doi.org/10.1016/j.ibmb.2017.09.001
Wu GX, Su RR, Ouyang HL, Zheng XL, Lu W, Wang XY (2022) Antennal transcriptome analysis and identification of olfactory genes in Glenea Cantor Fabricius (Cerambycidae: Lamiinae). Insects 13:553. https://doi.org/10.3390/insects13060553
Tang LD, Liu JM, Liu LH, Yu YH, Zhao HY, Lu W (2020) De novo transcriptome identifies olfactory genes in Diachasmimorpha longicaudata (Ashmead). Genes 11:144. https://doi.org/10.3390/genes11020144
Wang YT, Xu LB, Duan LQ, Yu LB, Cui J, Cao YY, Zhao YA (2021) Transcriptome sequencing and identification of the Zele chlorophthalmus olfactory related protein gene. Chin J Appl Entomol 58:846–855. https://doi.org/10.7679/j.issn.2095-1353.2021.082
Li ZQ, Zhang S, Luo JY, Wang SB, Wang CY, Lv LM, Dong SL, Cui JJ (2015) Identification and expression pattern of candidate olfactory genes in Chrysoperla sinica by antennal transcriptome analysis. Comp Biochem Physiol Part D: Genomics Proteomics 15:28–38. https://doi.org/10.1016/j.cbd.2015.05.002
Qi YX, Teng ZW, Gao LF, Wu SF, Huang J, Ye GY, Fang Q (2015) Transcriptome analysis of an endoparasitoid wasp Cotesia chilonis (Hymenoptera: Braconidae) reveals genes involved in successful parasitism. Arch Insect Biochem Physiol 88:203–221. https://doi.org/10.1002/arch.21214
Wu ZC, Ye J, Qian JL, Purba ER, Zhang QH, Zhang LW, Mang D (2022) Identification and expression profile of chemosensory receptor genes in Aromia Bungii (Faldermann) antennal transcriptome. Insects 13:96. https://doi.org/10.3390/insects13010096
Liu YP, Du LX, Zhu Y, Yang SY, Zhou Q, Wang G, Liu Y (2020) Identification and sex-biased profiles of candidate olfactory genes in the antennal transcriptome of the parasitoid wasp Cotesia vestalis. Comp Biochem Physiol Part D: Genomics Proteomics 34:100657. https://doi.org/10.1016/j.cbd.2020.100657
Zhou CX, Tang YL, Min SF, Wang MQ (2015) Analysis of antennal transcriptome and odorant binding protein expression profiles of the recently identified parasitoid wasp, Sclerodermus Sp. Comp Biochem Physiol Part D: Genomics Proteomics 16:10–19. https://doi.org/10.1016/j.cbd.2015.06.003
Cheng XJ, Cai LJ, Zheng LS, Qin JM, Huang YP, You MS (2016) Cloning, expression profiling and binding characterization of the OBP2 gene in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol Sin 59:365–376. https://doi.org/10.16380/j.kcxb.2016.04.001
Zhang YF, van Loon JJA, Wang CZ (2010) Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (Hubner). J Exp Biol 213:2889–2895. https://doi.org/10.1242/jeb.042705
Sheng S, Liao CW, Zheng Y, Zhou Y, Zhou Y, Xu Y, Song WM, He P, Zhang J, Wu FA (2017) Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. Comp Biochem Physiol Part D: Genomics Proteomics 22:20–31. https://doi.org/10.1016/j.cbd.2017.01.002
Ahmed T, Zhang TT, Wang ZY, He KL, Bai SX (2016) Identification and expression pattern analysis of chemosensory receptor genes in the Macrocentrus cingulum (Hymenoptera: Braconidae) antennae. Eur J Entomol 113:76–83. https://doi.org/10.14411/eje.2016.009
Du LX, Liu Y, Wang GR (2016) Molecular mechanisms of signal transduction in the peripheral olfactory system of insects. Sci Sin Vitae 46:573–583. https://doi.org/10.1360/n052016-00163
Lu DG, Li XR, Liu XX, Zhang QW (2007) Identification and molecular cloning of putative odorant-binding proteins and chemosensory protein from the bethylid wasp, Scleroderma Guani Xiao Et Wu. J Chem Ecol 33:1359–1375. https://doi.org/10.1007/s10886-007-9310-5
Acknowledgements
We thank Ms. Shumei Wang, Institute of Biological Control, Fujian Agriculture and Forestry University, Fuzhou, China, for kindly rearing and providing parasitoids and fruit flies.
Funding
This research was supported by the advanced Talents Introduction Project of Wuyi University (YJ201910); the Key Laboratory of Biopesticide and Chemical Biology, Ministry of Education, Fujian Agriculture and Forestry University (Keylab2020-02, Keylab2021-05); Key Project of Nanping Natural Science Foundation (N2023J004); Key Technological Innovation and Industrialization Project (2023XQ019); Special Funds for Technological Representative (NP2021KTS04).
Author information
Authors and Affiliations
Contributions
Methodology, D.Y., Q.J., J.Y. and P.C.; performed the experiments, D.Y., L.J., G.Y., X.H. and K.X.; analyzed the data, D.Y., L.J., Y.H. and D.L.; drafted the manuscript, D.Y. and P.C.; revised the manuscript, D.Y., J.Y. and P.C. All the authors read and approved the publication. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2024_9281_MOESM1_ESM.docx
Supplementary Material 1: Table S1: Model of phylogentic trees; Table S2: Primers for qRT-PCR of PincOBP genes in P. incisi; Table S3: Summary of P. incisi antennae transcriptome; Table S4: Gene annotation ratio; Table S5: BLASTx alignment of gustatory receptor genes of Psyttalia incisi and other insect species; Table S6: BLASTx alignment of odorant receptor genes of Psyttalia incisi and other insect species; Table S7: BLASTx alignment of ionotropic receptor (IR) genes of Psyttalia incisi and other insect species; Table S8: BLASTx alignment of sensory neuron membrane protein (SNMP) genes of Psyttalia incisi and other insect species. Figure S1: Distribution of unigene size in the P. incisi transcriptome assembly; Figure S2: Pie chart of homologous species distributionin in NCBI-NR database; Figure S3: Gene ontology (GO) classification of unigenes; Figure S4: KEGG classification of P. incisi unigenes; Figure S5: KOG classification of P. incisi unigenes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, D., Li, D., Jiang, L. et al. Antennal transcriptome analysis of Psyttalia incisi (silvestri) (Hymenoptera: Braconidae): identification and tissue expression profiling of candidate odorant-binding protein genes. Mol Biol Rep 51, 333 (2024). https://doi.org/10.1007/s11033-024-09281-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09281-3