Abstract
Background
The most widely used food additive monosodium glutamate (MSG) has been linked to immunopathology. Conversely, quercetin (Q), a naturally occurring flavonoid has been demonstrated to have immunomodulatory functions. Therefore, the purpose of the study is to determine if quercetin can mitigate the deleterious effects of MSG on immune cells, and the possible involvement of TLR, if any.
Methods and Results
This study was conducted on Q, to determine how it affects the inflammatory response triggered by MSG in primary cultured thymocytes and splenocytes from rats (n = 5). Q shielded cells by augmenting cell survival and decreasing lactate dehydrogenase leakage during MSG treatment. It decreased IL-1β, IL-6, IL-8, and TNF-α expression and release by hindering NF-kB activation and by inhibiting the JAK/STAT pathway. Moreover, Q prevented NLRP3 activation, lowered IL-1β production, and promoted an anti-inflammatory response by increasing IL-10 production. Q reduced MSG-induced cellular stress and inflammation by acting as an agonist for PPAR-γ and LXRα, preventing NF-kB activation, and lowering MMP-9 production via increasing TIMP-1. Additionally, Q neutralized free radicals, elevated intracellular antioxidants, and impeded RIPK3, which is involved in inflammation induced by oxidative stress, TNF-α, and TLR agonists in MSG-treated cells. Furthermore, it also modulated TYK2 and the JAK/STAT pathway, which exhibited an anti-inflammatory effect.
Conclusions
MSG exposure is associated with immune cell dysfunction, inflammation, and oxidative stress, and Q modulates TLR to inhibit NF-kB and JAK/STAT pathways, providing therapeutic potential. Further research is warranted to understand Q’s downstream effects and explore its potential clinical applications in inflammation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monosodium glutamate (MSG), a flavoring agent frequently found in food items, has been linked to immunological dysregulation and the escalation of immune-related diseases [1, 2]. Concerns have been raised regarding MSG’s possible impact on immune system function due to the adverse effects of food additives (e.g., MSG) on thymocytes and splenocytes. Thus, it is necessary to investigate methods to reduce the immunological aggravation caused by MSG. MSG has reportedly been linked to cellular assaults by activating pro-inflammatory cytokines and free radicals to cause oxidative stress and an inflammatory response [3, 4]. The nuclear factor (NF)-kB, Janus kinase (JAK), and signal transducer and activator of transcription (STAT) signaling cascades, which are triggered by a variety of events, produce these signaling molecules [5, 6]. On the other hand, damage-associated molecular pattern molecules are generated when there is oxidative stress; as a result, the toll-like receptor (TLR) gets activated which in turn activates the innate immune system during infiltration of toxic chemicals, like MSG. Sudden interruptions in intracellular redox homeostasis can cause a proinflammatory condition. Redox stress can trigger an inflammatory response, but the exact processes by which it does so are still unclear. TLRs may be involved in triggering this reaction [7].
On the contrary, quercetin (Q), a naturally occurring flavonoid that is widely present in a variety of fruits and vegetables, has drawn interest for its possible immunomodulatory properties [2, 8]. It has been demonstrated that a combination therapy including natural substances with anti-inflammatory and antioxidant properties is beneficial in reducing oxidative damage and regulating the immunological response caused by MSG [2]. Quercetin has been shown in several studies to influence immunological responses and control immune cell activities [2, 8, 9]. According to studies, quercetin reduces inflammation brought on by lipopolysaccharide by preventing NF-kB activation via TLR [10, 11]. The effectiveness of quercetin in reducing MSG-induced immunological dysregulation in primary rat thymocytes and splenocytes has not been fully investigated yet. Therefore, the purpose of this investigation is to determine if quercetin has any immunomodulatory effects on MSG-induced aggravation in primary rat thymocytes and splenocytes. To better understand the potential of quercetin in reducing the negative effects of MSG on immune cells, the present study evaluates several immunological markers, such as cell viability, cytokine production, oxidative stress parameters, and gene expression. Therefore, the present study looked at possible mechanisms by which MSG-induced free radicals in primary cultured thymocytes and splenocytes initiate the inflammatory signals to cause immunotoxicity and the possible involvement of TLR, if any, in the activation of the inflammatory response by targeting NF-kB and JAK/STAT signaling pathways.
Materials and methods
Chemicals and reagents
Quercetin was acquired from Sigma-Aldrich, MO, USA, while monosodium glutamate was bought from SRL, India. The study utilized high-grade chemicals and reagents from Merck, Germany, and Sigma Aldrich, USA. Gibco, USA provided all the cell culture medium, buffer, and other reagents.
Experimental animals
Five male albino Wistar rats that were ≥90 days old and weighed 110 ± 10 g were utilized in the investigation for each group for the isolation and primary culture of thymocytes and splenocytes (n = 5). Each of the experiments was carried out in line with ethical standards advised by the Serampore College (affiliated to University of Calcutta) Institutional Animal Ethics Committee (IAEC), West Bengal, India (Project Approval No. 25/P/S/SC/IAEC/2019), and registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India (Register No. 1946/PO/Re/S/17/CPCSEA).
Isolation and culture of thymocytes and splenocytes
The rats’ thymus and spleens were removed and cleaned in ice-cold D-PBS. Collagenase was used to mince the thymus tissues in a cell culture dish, and Alsever’s solution was used to macerate the spleen pieces. Both cell suspensions underwent centrifugation, filtering, and RBC lysis buffer incubation. The thymocytes and splenocytes were collected in RPMI-1640 medium with 10% fetal bovine serum, 100 µg/mL penicillin, and 100 U/mL streptomycin after being washed with D-PBS. Primary thymocytes and splenocytes were cultured in a 5% CO2 incubator at 37 °C. MSG and quercetin were separately dissolved and diluted using RPMI-1640 [2]. This work was performed initially in 8 groups: control, MSG (100 mM) [12, 13], quercetin (5 μM (Q1), 10 μM (Q2), and 20 μM (Q3), respectively) [10], MSG + Q1, MSG + Q2, and MSG + Q3. The experimental plan is depicted in Fig. 1A (Experimental period: 24 h).
Experimental plan (BioRender was used to make the Figure: https://biorender.com) A; the restorative role of quercetin on MSG-induced cellular toxicity via LDH leakage from the thymocytes and splenocytes B–E: B and C-cytotoxicity and cell viability of thymocytes, D and E-cytotoxicity and cell viability of splenocytes. Significance level based on Mann–Whitney U multiple comparison tests: Control vs. MSG: a, Control vs. Q1/Q2/Q3: b, Q1 vs. Q2 vs. Q3: c, MSG vs. MSG + Q1: d, MSG vs. MSG + Q2: e, MSG vs. MSG + Q3: f, MSG + Q1 vs. MSG + Q2 vs. MSG + Q3: g (*p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant)
Biochemical assay
According to the manufacturer’s instructions, lactate dehydrogenase (LDH) was determined using a standard kit as a cytotoxicity assay from Span Diagnostics Ltd. in Mumbai, India.
Cell viability assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) test was used to assess the viability of the cells by the instructions provided by the MTT assay kit from Thermo Fisher Scientific, Waltham, Massachusetts, USA. In short, thymocytes and splenocytes were seeded individually in 96-well plates at a density of 1 × 104 cells/well and subjected to three different quercetin doses (final concentration at 5 μM, 10 μM, and 20 μM, respectively) with or without MSG (final concentration at 100 mM) for 24 h. Next, 20 μL of MTT (5 mg/mL) was added to each well, and each well was incubated for an additional 4 hours at 37 °C with 5% CO2. Following the removal of the medium, 150 μL of dimethyl sulfoxide (DMSO) was given to each well to dissolve the insoluble formazan crystals in the living cells. The absorbance at 450 nm was then measured with a microplate reader to determine the formazan concentrations. Relative to the untreated control group, which was taken to be 100%, cell viability was expressed.
Estimation of pro-inflammatory and anti-inflammatory cytokines
Commercially available rat enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource, San Diego, CA, USA) were used to determine the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-1β, IL-10, matrix metalloproteinases (MMP)-9, and tissue inhibitors of metalloproteinases (TIMP)-1, respectively.
Determination of intracellular reactive oxygen species (iROS)
Utilizing DCFH-DA with excitation at 488 nm (blue filter) by the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA), iROS were quantified using flow cytometry. A 527/32 nm bandpass filter was used to record the emission, and the results were then examined [2, 3].
Estimation of free radicals and antioxidants
The thiobarbituric acid (TBA) assay [14] and Griess reaction [15], respectively, were used to quantify free radicals such as TBA reactive substances (TBARS) and nitric oxide (NO). Utilizing the nitroblue tetrazolium (NBT) [16], Beer’s [17], and Elman methods [18], antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) were assessed.
Real-time quantitative PCR
Thymocytes and splenocytes were seeded in a 6-well plate at a density of 1 × 106 cells/well and treated for 24 h with quercetin at final concentrations of 5 μM, 10 μM, and 20 μM, respectively, with or without MSG (100 mM). After that, PBS buffer was used to wash the cells three times. The Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract RNA from thymocytes and splenocytes by the manufacturer’s instructions. Thermo Fisher Scientific, Waltham, Massachusetts, USA, ND-1000 spectrophotometer was used to determine the amounts of the extracted RNA at 260 nm wavelength. Reverse transcribes RNA into cDNA using the high-capacity cDNA reverse transcription Kit from Applied Biosystems in Foster City, California. Real-time PCR was carried out using the SYBR Green Real-time PCR Master Mixes (Invitrogen). mRNA levels of IL-1β, IL-6, IL-8, TNF-α, IkB, NF-kB (p50 and p65), peroxisome proliferator-activated receptor (PPAR)-γ, liver X receptor (LXR) α, TLR4, JAK1, JAK2, JAK3, tyrosine kinase 2 (TYK2), STAT1, STAT2, STAT3, STAT4, STAT5, STAT6, Toll/IL-1 receptor (TIR) domain-containing adapter inducing interferon β (TRIF), IL-1 receptor-associated kinase (IRAK) 4, receptor-interacting protein kinase 3 (RIPK3), and nod-like receptor protein 3 (NLRP3) in thymocytes and splenocytes were evaluated by real-time quantitative PCR. The 2−△△Ct technique was utilized to determine the mRNA levels of these targets, and they were then normalized against the internal reference gene β-actin [2]. The primers employed in this investigation were made available in Supplementary Material 1.
Statistical analysis by StatsDirect 3.0 software
The data were presented as Mean ± SE. Statsdirect 3.0 (United Kingdom) was utilized to perform the Kruskal–Wallis nonparametric analysis of variance and the Mann-Whitney U multiple comparison tests. When p ≤ 0.05, differences were considered significant.
Results
Restorative impact of quercetin on thymocytes and splenocytes against the cytotoxic effects of MSG
This study revealed that MSG affects thymocytes and splenocytes negatively, as evidenced by LDH leakage and reduced cell viability (Fig. 1B-E). Cell viability indicates the general health and survival of the cells, whereas LDH leakage acts as a marker of cellular damage as shown by cytotoxicity [19]. However, the inclusion of quercetin as a preventive supplement in vitro significantly reversed the cytotoxic effects of MSG. The amount of intracellular LDH leaked when exposed to MSG increased noticeably in the absence of quercetin. This leaking is a sign of damaged cells and weakened membrane integrity as an indication of cytotoxicity. In addition, cell viability was dramatically decreased, indicating a detrimental effect on the cells’ general health and capacity to survive. On the other hand, cytotoxicity was dramatically decreased by reducing the LDH leakage when quercetin was added to the media. Here, quercetin protects thymocytes and splenocytes by avoiding cellular deterioration and preserving membrane integrity to increase cell survival. Additionally, neither the quercetin groups alone compared to the control group nor the three different dosages of quercetin alone showed any statistically significant changes. This shows that quercetin conserves the general well-being and survival of thymocytes and splenocytes in addition to protecting against cellular damage.
Quercetin mitigates the inflammatory response by regulating the cytokine level
It has been demonstrated that quercetin protects against the inflammatory response that MSG causes in thymocytes and splenocytes. TNF-α, IL-6, IL-8, IL-1β, IL-10, MMP-9, and TIMP-1 cytokines and inflammatory enzymes are modulated to provide their protective effects (Fig. 2). In MSG-induced inflammation, quercetin has been shown to inhibit the production of TNF-α, a pro-inflammatory cytokine. Quercetin aids in reducing inflammation by preventing the release of TNF-α [10]. During MSG-induced inflammation, quercetin has been demonstrated to reduce the production of IL-6, another pro-inflammatory cytokine, in thymocytes and splenocytes. The anti-inflammatory effects of quercetin are influenced by this decline in IL-6 levels [8, 9]. The generation of IL-8, a chemokine involved in attracting immune cells to the site of inflammation, has been reported to be inhibited by quercetin [10]. Quercetin aids in modulating the inflammatory response by lowering IL-8 levels. IL-1β, a pro-inflammatory cytokine involved in the onset and severity of inflammation, is produced by cells less frequently when quercetin is present [20]. Quercetin helps mitigate the inflammatory response by suppressing the production of IL-1β. There is evidence that quercetin stimulates IL-10 production, a cytokine that inhibits inflammation [21]. The pro-inflammatory cytokines are balanced by IL-10, which functions as a negative regulator of inflammation. Quercetin provides anti-inflammatory effects by increasing the synthesis of IL-10. According to studies, quercetin lowers the levels of MMP-9, an enzyme implicated in inflammation and tissue remodeling [20]. Quercetin assists in preventing excessive tissue deterioration and inflammation by blocking MMP-9. It has been established that quercetin raises the levels of TIMP-1, an MMP-9 inhibitor [22]. Quercetin serves to control MMP-9 activity and uphold cellular homeostasis by increasing the synthesis of TIMP-1. It has been shown that quercetin protects thymocytes and splenocytes against MSG-induced inflammation by modulating cytokine and chemokine levels.
Role of quercetin in MSG-induced altered cytokine levels A–D: A, B-thymocytes culture, C-D-splenocytes culture. Significance level based on Mann–Whitney U multiple comparison tests: Control vs. MSG: a, MSG vs. MSG + Q1: d, MSG vs. MSG + Q2: e, MSG vs. MSG + Q3: f, MSG + Q1 vs. MSG + Q2 vs. MSG + Q3: g (*p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant)
Quercetin attenuates the generation of iROS in MSG-treated cells to counterbalance free radicals and intracellular antioxidants
This study offers molecular insights into the antioxidant properties of quercetin, which protects thymocytes and splenocytes from the oxidative stress caused by MSG. The effects of MSG administration on thymocyte and splenocyte cultures included higher levels of NO, TBARS (Fig. 3B and C), and iROS (Fig. 3A). Additionally, a decrease in antioxidant enzymes, such as GSH, CAT, and superoxide dismutase (SOD), was also found after MSG exposure (Fig. 3B and C). These results show that oxidative stress is induced in the cells by MSG, which is strongly supported by a recent in vivo investigation [1, 2]. Nevertheless, these effects were completely reversed when quercetin was added as a preventive supplement. Quercetin has antioxidant capabilities since it drastically decreases intracellular ROS levels. Quercetin supplementation also induced a considerable drop in TBARS and NO levels, indicating that it can lessen the oxidative injury from MSG. The levels of antioxidant enzymes, including CAT, GSH, and SOD, were also recovered by quercetin supplementation. This shows that the natural antioxidant defense systems were strengthened by quercetin, shielding thymocytes and splenocytes from oxidative damage brought on by MSG.
In vitro antioxidant activity of quercetin on MSG-induced generation of iROS to promote oxidative damage in the thymocytes and splenocytes: generation of iROS in thymocytes and splenocytes A, free radicals, and antioxidant levels of thymocytes B and splenocytes C. Significance level based on Mann–Whitney U multiple comparison tests: Control vs. MSG: a, MSG vs. MSG + Q1: d, MSG vs. MSG + Q2: e, MSG vs. MSG + Q3: f, MSG + Q1 vs. MSG + Q2 vs. MSG + Q3: g (*p < 0.05, **p < 0.01, ***p < 0.001)
Immunomodulatory role of quercetin on MSG-induced inflammation by suppressing NF‑kB, JAK/STAT through TLR
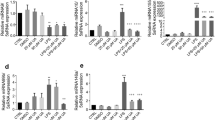
The mRNA levels changed as a result of MSG administration in both thymocytes and splenocytes causing an inflammatory response. In response to MSG treatment, IL-1β, IL-6, IL-8, and TNF-α mRNA levels increased (Figs. 4 and 5). This shows that MSG causes thymocytes and splenocytes to produce an inflammatory response. IkBα and NF-kB (p50 and p65) (Figs. 4 and 5), two molecules involved in the NF-kB signaling pathway, were shown to have elevated mRNA levels. This implies that NF-kB signaling, a crucial pathway involved in the synthesis of pro-inflammatory cytokines, is activated. Furthermore, there was a reduction in the mRNA levels of the anti-inflammatory transcription factors PPAR-γ and LXRα (Figs. 4 and 5). The inflammatory response can become more pronounced as a result of this decline. Additionally, the mRNA levels of JAK/STAT pathway subunits JAK1, JAK2, JAK3, TYK2, STAT1, STAT2, STAT3, STAT4, STAT5, and STAT6 (Figs. 4 and 5) were investigated. In cytokine signaling and immunological responses, these elements are essential. The findings revealed changes in their mRNA levels, suggesting possible dysregulation of JAK/STAT signaling in response to inflammation brought on by MSG. Additionally, the mRNA levels of the TLR4, TRIF, IRAK4, RIPK3, and NLRP3 (Figs. 4 and 5) were measured. The results imply that TLR is activated in response to the late activation of NF-kB and JAK/STAT induced by MSG.
Quercetin attenuated MSG-induced inflammation by regulating the mRNA expression level in the thymocytes A–B. Significance level based on Mann–Whitney U multiple comparison tests: Control vs. MSG: a, MSG vs. MSG + Q1: d, MSG vs. MSG + Q2: e, MSG vs. MSG + Q3: f, MSG + Q1 vs. MSG + Q2 vs. MSG + Q3: g (*p < 0.05, **p < 0.01)
Quercetin modulated the mRNA expression level in the splenocytes to reduce the inflammation caused by MSG A–B. Significance level based on Mann–Whitney U multiple comparison tests: Control vs. MSG: a, MSG vs. MSG + Q1: d, MSG vs. MSG + Q2: e, MSG vs. MSG + Q3: f, MSG + Q1 vs. MSG + Q2 vs. MSG + Q3: g (*p < 0.05, **p < 0.01)
Notably, quercetin supplementation suggested possible anti-inflammatory benefits against MSG-induced inflammation in primary cultured thymocytes and splenocytes. The pro-inflammatory IL-1β, IL-6, IL-8, and TNF-α were significantly downregulated by quercetin treatment (Figs. 4 and 5). Quercetin thereby reduced the inflammatory reaction produced by MSG. The mRNA levels of multiple signaling molecules implicated in inflammatory pathways were also modulated by quercetin treatment. Additionally, the quercetin supplementation resulted in a reduction in IkBα and NF-kB (p50 and p65) mRNA levels, indicating its capacity to suppress NF-kB activation (Figs. 4 and 5). The downregulation of inflammatory mediators may be aided by this inhibition. Additionally, the application of quercetin increased PPAR-γ and LXRα (Figs. 4 and 5) mRNA levels, demonstrating its capacity to promote the production of anti-inflammatory transcription factors. Additionally, quercetin administration resulted in a reduction in TLR4 mRNA levels (Figs. 4 and 5), indicating its capacity to modify TLR signaling and perhaps lessen the response to inflammation. In response to quercetin administration, IkBα, NF-kB (p50 and p65), TLR4, TRIF, IRAK4, RIPK3, and NLRP3 (Figs. 4 and 5) mRNA levels decreased. This suggests that quercetin may interfere with the NF-kB and TLR signaling pathways to produce its protective effects. According to these results, MSG causes inflammation in thymocytes and splenocytes, as seen by changes in mRNA levels. The findings demonstrate that MSG-induced inflammation in thymocytes and splenocytes involves NF-kB, JAK/STAT, and TLR signaling. By modulating mRNA expression, quercetin is demonstrated to exert its protective effects by modulating these pathways. In the context of inflammation caused by MSG, these findings offer physiological insights into the possible anti-inflammatory actions of quercetin.
Discussion
Quercetin modulates several signaling pathways, transcription factors, and enzyme activities to reduce the inflammatory response that MSG causes in thymocytes and splenocytes. Quercetin reduces the leakage of LDH to promote cell survival, protecting cells from MSG-induced cytotoxicity [23], which was similarly seen in the current investigation. By regulating several cytokines and inflammatory enzymes, quercetin also showed a protective impact in reducing the inflammatory response in the MSG-treated group. The release of TNF-α is inhibited by quercetin blocking the activation of NF-kB [24]. In MSG-treated thymocytes and splenocytes, quercetin decreases the production and release of IL-6, IL-8, TNF-α, and IL-1β, decreasing the inflammatory response, which is well supported by previous studies [2, 10]. Quercetin also reduced IL-6 production by impeding the JAK/STAT signaling pathway, which helped alleviate the inflammation induced by MSG. Additionally, the formation and release of IL-1β are controlled by the NLRP3 inflammasome [25]. Furthermore, TRIF regulates the activation of NLRP3 and IL-1β in response to TLR ligands, which in turn activates NF-kB through TLR4 and releases inflammatory mediators [26, 27]. TLR signaling in this situation entails activating IRAK4 to further affect NF-kB activation [28, 29].
On the other hand, quercetin reduces inflammation by inhibiting NLRP3 activation in MSG-exposed cells, which in turn reduces IL-1β production. Quercetin also increases the synthesis of IL-10 by suppressing NLRP3 activation, which helps develop an anti-inflammatory milieu [2, 30]. Furthermore, by serving as anti-inflammatory transcription factors, PPAR-γ and LXRα can both be activated to limit the immune response and inflammation [10]. Similar results were obtained in the current investigation, which demonstrates quercetin’s ability to decrease inflammation by activating PPAR-γ and LXRα as agonist molecules, which subsequently hinder the activation of NF-kB. Additionally, PPAR-γ activation reduces NF-kB, which in turn decreases MMP9 release [31]. Quercetin induces TIMP-1 to inhibit MMP-9 through the activation of PPAR-γ, which in turn inhibits MMP-9 in MSG-treated cells, thus reducing cellular assaults and inflammation.
A pro-inflammatory condition can result from disturbances in the redox balance [1,2,3,4, 7]. The link between oxidation and inflammation is multifaceted, ranging from cellular harm brought on by oxidative stress that triggers TLR4-mediated inflammation and cellular damage to precisely regulated signaling by iROS during TLR4 activation that activates the NF-kB and JAK/STAT signaling pathways [7]. According to this study, quercetin strengthens the cellular antioxidant defense system by neutralizing free radicals and increasing the activity of antioxidant enzymes like SOD and CAT. Additionally, quercetin raises intracellular levels of GSH, a cellular redox buffer that is essential for neutralizing iROS and preserving cellular redox equilibrium. The activation of RIPK3 by TNF-α, TLR agonists, and oxidative stress increases inflammation by activating NF-kB and NLRP3 inflammasomes [32]; it was significantly restored by quercetin. This work was well corroborated with the recent study where quercetin regulates the TLR4-mediated NF-kB activation by MSG to cause inflammatory response in rat models [2]. Furthermore, the anti-inflammatory effects of quercetin on inflammatory cells have been linked to its regulation of TYK2 and JAK/STAT [33]. Here, quercetin markedly diminished TYK2 and JAK/STAT signaling components that were activated by MSG in thymocytes and splenocytes. This study explored the processes behind the benefits of quercetin that were identified, but it did not completely define the downstream consequences and possible therapeutic applications. It may be possible to design treatments to prevent the negative effects of MSG on immunological function by understanding the immunomodulatory characteristics of quercetin in the setting of MSG-induced immune aggravation. Additionally, examining quercetin’s potential as an immunomodulatory drug may have wider ramifications for treating immune-related conditions linked to MSG usage. Furthermore, to find out if quercetin is safe and effective as a treatment for human illnesses related to inflammation, more investigation is required, including clinical studies.
Conclusions
As a result, it can be argued that quercetin modulates TLR to regulate the NF-kB and JAK/STAT signaling pathways involved in inflammation brought on by MSG in primary cultured thymocytes and splenocytes (Fig. 6). The therapeutic immunomodulatory potential of quercetin in reducing immune cell dysfunction, inflammation, and oxidative stress linked to MSG exposure is explored mechanistically in this work. To completely comprehend the downstream effects of quercetin on these signaling pathways and to investigate its therapeutic uses in inflammation-related disorders, more study is required.
Hypothetical target pathway by which quercetin mitigates inflammation by inhibiting the activation of NF-kB and JAK/STAT via TLR in MSG-treated primary cultured thymocytes and splenocytes; apricot color with round shape: monosodium glutamate (MSG), red cross: inhibited/blocked/suppressed by quercetin (Q), (→): activation, (⊥): inhibition (BioRender was used to make the Figure: https://biorender.com)
Data availability
Data available within the article or its supplementary materials.
Abbreviations
- CAT:
-
Catalase
- CPCSEA:
-
Committee for the purpose of control and supervision of experiments on animals
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- GSH:
-
Glutathione
- IAEC:
-
Institutional animal ethics committee
- IL:
-
Interleukin
- IRAK4:
-
IL-1 receptor-associated kinase 4
- iROS:
-
Intracellular reactive oxygen species
- JAK:
-
Janus kinase
- LDH:
-
Lactate dehydrogenase
- LXR:
-
Liver X receptor
- MMP:
-
Matrix metalloproteinases
- MSG:
-
Monosodium glutamate
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)
- NBT:
-
Nitroblue tetrazolium
- NF-kB:
-
Nuclear factor kappa B
- NLRP3:
-
Nod-like receptor protein 3
- NO:
-
Nitric oxide
- PPAR:
-
Peroxisome proliferator-activated receptor
- Q:
-
Quercetin
- RIPK3:
-
Receptor-interacting protein kinase 3
- SOD:
-
Superoxide dismutase
- STAT:
-
Signal transducer and activator of transcription
- TBA:
-
Thiobarbituric acid
- TBARS:
-
TBA reactive substances
- TIMP:
-
Tissue inhibitors of metalloproteinases
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TRIF:
-
Toll/IL-1 receptor domain containing adapter inducing interferonβ
- TYK2:
-
Tyrosine kinase 2
References
Das D, Banerjee A, Bhattacharjee A, Mukherjee S, Maji BK (2022) Dietary food additive monosodium glutamate with or without high-lipid diet induces spleen anomaly: a mechanistic approach on rat model. Open Life Sci 17(1):22–31
Das D, Banerjee A, Manna K, Sarkar D, Shil A, Sikdar M, Mukherje S, Maji BK (2023) Quercetin counteracts monosodium glutamate to mitigate immunosuppression in the thymus and spleen via redox-guided cellular signaling. Phytomedicine. https://doi.org/10.1016/j.phymed.2023.155226
Banerjee A, Das D, Paul R, Roy S, Das U, Saha S, Dey S, Adhikary A, Mukherjee S, Maji BK (2020) Mechanistic study of attenuation of monosodium glutamate mixed high lipid diet induced systemic damage in rats by Coccinia grandis. Sci Rep 10(1):15443
Banerjee A, Mukherjee S, Maji BK (2021) Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: an overview. Toxicol Rep 8:938–961
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. https://doi.org/10.1016/j.bbadis.2016.11.005
Liu X, Yin S, Chen Y, Wu Y, Zheng W, Dong H, Bai Y, Qin Y, Li J, Feng S, Zhao P (2018) LPS–induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF–κB, STAT3 or AP–1 activation. Mol Med Rep 17(4):5484–5491
Gill R, Tsung A, Billiar T (2010) Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med 48(9):1121–1132
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y (2016) Quercetin, inflammation and immunity. Nutrients 8(3):167
Mlcek J, Jurikova T, Skrovankova S, Sochor J (2016) Quercetin and its anti-allergic Immune response. Molecules 21(5):623
Xiong G, Ji W, Wang F, Zhang F, Xue P, Cheng M, Sun Y, Wang X, Zhang T (2019) Quercetin inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. Biomed Res Int. https://doi.org/10.1155/2019/6282635
Bhaskar S, Helen A (2016) Quercetin modulates toll-like receptor-mediated protein kinase signaling pathways in oxLDL-challenged human PBMCs and regulates TLR-activated atherosclerotic inflammation in hypercholesterolemic rats. Mol Cell Biochem 423(1–2):53–65
Pavlović V, Cekić S, Kocić G, Sokolović D, Zivković V (2007) Effect of monosodium glutamate on apoptosis and Bcl-2/Bax protein level in rat thymocyte culture. Physiol Res 56(5):619–626
Baciu D, Salageanu A, Visan T (2020) In vitro evaluation of the antiproliferative effect, cytotoxicity and proinflammatory activity of the food additive monosodium glutamate on RAW 264.7 cell line. Rev Chim 71:443–448
Wills ED (1987) Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B (eds) Biochemical toxicology: a practical approach. IRL Press, Oxford, England, pp 138–140
Raso GM, Meli R, Gualillo O, Pacilio M, Di Carlo R (1999) Prolactin induction of nitric oxide synthase in rat C6 glioma cells. J Neurochem 73(6):2272–2277
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Beers RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Banerjee A, Maji BK, Chattopadhyay A (2021) Terminalia arjuna induced testicular assault through Leydig cell derangement: an in vitro approach. J Complement Integr Med 18(3):627–631
Cheng SC, Huang WC, Pang S, Wu JH, Cheng YH (2019) Quercetin inhibits the production of IL-1β-Induced Inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int J Mol Sci 20(12):2957
Mkhize NVP, Qulu L, Mabandla MV (2017) The effect of quercetin on pro- and anti-inflammatory cytokines in a prenatally stressed rat model of febrile seizures. J Exp Neurosci 11:1179069517704668
Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X (2019) Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed Pharmacother 114:108839
Bao D, Wang J, Pang X, Liu H (2017) Protective effect of quercetin against oxidative stress-Induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules 22(7):1122
Chen T, Zhang X, Zhu G, Liu H, Chen J, Wang Y, He X (2020) Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 99(38):e22241
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20(13):3328
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183(2):787–791
Kawai T, Akira S (2007) Signaling to NF-kappaB by toll-like receptors. Trends Mol Med 13(11):460–469
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023
Hao Y, Ma J, Wang J, Yu X, Li Z, Wu S, Tian S, Ma H, He S, Zhang X (2023) Synthesis and evaluation of dihydrofuro[2,3-b]pyridine derivatives as potent IRAK4 inhibitors. Eur J Med Chem 258:115616
Aghababaei F, Hadidi M (2023) Recent advances in potential health benefits of Quercetin. Pharmaceuticals (Basel) 16(7):1020
Luo X, Wu J, Wu G (2021) PPARγ activation suppresses the expression of MMP9 by downregulating NF-κB post intracerebral hemorrhage. Neurosci Lett 752:135770
Xue S, Cao ZX, Wang JN, Zhao QX, Han J, Yang WJ, Sun T (2022) Receptor-interacting protein kinase 3 inhibition relieves mechanical Allodynia and suppresses NLRP3 inflammasome and NF-κB in a rat model of spinal cord injury. Front Mol Neurosci 15:861312
Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A (2016) Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients 8(9):529
Acknowledgements
To conduct this study, the authors would like to express their appreciation to the Principal of Serampore College who provided administrative support and encouragement. Additionally, the CSIR-Indian Institute of Chemical Biology (CSIR-IICB, Kolkata) and the Centre for Research in Nanoscience and Nanotechnology (University of Calcutta) were acknowledged for their technical support during this study. The graphical abstract, Figs. 1A and 6 were created with BioRender.com.
Funding
The authors are grateful to the Post Graduate Project Fund of the Department of Physiology, Serampore College, India for funding this study.
Author information
Authors and Affiliations
Contributions
All of the authors have affirmed their submission and accepted responsibility for the whole content of this submitted work. DD and AB were equally responsible for the concept and experimental design, performing experiments, analyzing the data, writing the original draft, and reviewing it for confirmation of submission. SM was responsible for providing specific chemicals and reagents and also reviewed the final draft of the manuscript. BKM was responsible for the concept and experimental design, providing instrumental and infrastructural support, reviewing the original draft, data analysis, and final approval for submission of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that may have influenced the work reported in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, D., Banerjee, A., Mukherjee, S. et al. Quercetin inhibits NF-kB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: an in vitro approach. Mol Biol Rep 51, 277 (2024). https://doi.org/10.1007/s11033-024-09245-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09245-7