Abstract
Background
Helicobacter pylori infection and heterogeneity in its pathogenesis could describe diversity in the expression of inflammatory genes in the gastric tissue. We aimed to investigate transcriptional alteration of genes linked to gastritis concerning the H. pylori infection status and its virulence factors.
Methods and results
Biopsy samples of 12 infected and 12 non-infected patients with H. pylori that showed moderate chronic gastritis were selected for transcriptional analysis. Genotyping of H. pylori strains was done using PCR and relative expression of inflammatory genes was compared between the infected and non-infected patients using relative quantitative real-time PCR. Positive correlations between transcriptional changes of IL8 with TNF-α and Noxo1 in the infected and TNF-α with Noxo1, MMP7, and Atp4A in the non-infected patients were detected. Six distinct genotypes of H. pylori were detected that showed no correlation with gender, ethnicity, age, endoscopic findings, and transcriptional levels of host genes. Irrespective of the characterized genotypes, our results showed overexpression of TNF-α, MMP7, Noxo1, and ATP4A in the infected and IL-8, Noxo1, and ATP4A in the non-infected patients.
Conclusions
A complexity in transcription of genes respective to the characterized H. pylori genotypes in the infected patients was detected in our study. The observed difference in co-regulation of genes linked to gastritis in the infected and non-infected patients proposed involvement of different regulatory pathways in the inflammation of the gastric tissue in the studied groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastritis is a common disease among humans in different populations. Although this disease can control by therapeutic regimens, its chronic form could lead to cancer. Gastritis is a multifactorial and complex disease associated with both the environment, such as lifestyle, eating habits, and infections and genetic factors [1]. Helicobacter pylori (H. pylori), which is known as the first carcinogenic bacteria, can cause gastritis and chronic inflammation through different mechanisms, including histopathological changes of the gastric tissue that are mediated by its virulence factors, induction of the immune system leading to the infiltration of neutrophils and lymphocytes as well as the production of proinflammatory cytokines, production of substances, such as ammonia, phospholipases, and cytotoxins, which lead to increased risk of malignant alterations of the gastric stem cells [1, 2]. The infection occurs during infancy and may remain silent for decades in the gastric environment throughout life. The infection and pathophysiological abnormalities of chronic gastritis (CG) could lead to the loss of functional glands (atrophy) and replacement of the normal gland and foveolar epithelium with intestinal-type cells, intestinal metaplasia, and progress to dysplasia, and carcinoma [3]. H. pylori also can cause other diseases, such as peptic and duodenal ulcers, mucosa-associated lymphoid tissue lymphoma, or other conditions, such as recurrent aphthous stomatitis, anemia, altered serum levels of lipoproteins, and coronary atherosclerosis in some patients [3,4,5,6,7].
Different virulence factors are attributed to H. pylori that are linked to gastric disorders. Cytotoxic-associated gene A (CagA), vaculotating cytotoxin A (VacA), and several other virulence factors such as outer membrane proteins (OMPs), are involved in H. pylori-induced gastric inflammation via the activation of gene transcription [1, 8]. Interaction of these virulence factors with host cell receptors on gastric epithelial cells has been described in both in vitro and in vivo studies [1]. These interactions trigger intracellular signaling events that result in the release of proinflammatory cytokines and promote bacterial evasion from the acidic environment of the stomach, local mechanical stress, and native immune response [2, 4]. Although infiltration of plasma cells, neutrophils, monocytes, differentiation of the gastric cells to the intestinal type cells, and depletion of the parietal cells in the gastric tissue are among common histological changes occurring following the infection, no congruency exist to link some of these virulence factors with these changes [8].
Many research works have been carried out to find gene expression profiles of gastric cancer in tumor tissues; however, there are very few studies that provide information about gene expression analysis in pre-cancerous tissue, especially in patients with CG, and its link with different genotypes of H. pylori virulence factors. The induction of nuclear factor κB/tumor necrosis factor-alpha (NF-κB/TNF-α) inflammatory pathway is known to be the major route of immune-dependent carcinogenesis in the stomach [9]. Activation of NF-κB by H. pylori induces nuclear translocation, which causes an increase in transcription of NF-κB responsive genes, like interleukin (IL)-8, and up or down-regulation of inflammatory genes [2]. In this pathway, TNF-α, IL-8, nicotinamide dinucleotide phosphate (NADPH) oxidase 1 (NOXO1), Matrix metallopeptidase 7 (MMP-7), and ATPase H+/K+ Transporting Subunit Alpha (ATP4A) cooperate to promote inflammation and histological changes in the gastric tissue [10,11,12].
Heterogeneity in the pathogenicity of the infection among patients seems to depend on the genotype of H. pylori strains carrying different virulence factors, and the extent of induction of genes mediating the activation of this pathway. Understanding this correlation possibly could illustrate observed diversity in the severity of the inflammation and its progress toward cancer. In this study, to describe this relationship, changes in transcription levels of key genes of the NF-kB inflammatory pathway, including TNF-α, IL-8, Noxo1, Atp4aA, and MMP-7 were explored and their link with common virulence genotypes of related isolates was analyzed on gastric biopsy samples of patients with gastric disorders.
Materials and methods
Patients and samples
The study was performed on adult patients with gastric disorders who referred to endoscopic ward of a general hospital in Tehran, Iran from January to August of 2019. All the patients filled an informed consent form before the sampling. Demographic and clinical information of patients was recorded in a questionnaire. Three biopsy specimens of the patients were obtained during endoscopy from the antrum (the distal region of the stomach), which were used for histological examination, rapid urease test and H. pylori culture, and RNA extraction. Patients who received non-steroidal anti-inflammatory drugs, proton pump inhibitors, or antibiotics within last six weeks, those with a history of the stomach surgery were excluded from this study. To compare transcriptional changes on the H. pylori positive and negative samples, biopsies from patients with acute gastritis, and those that presented mild CG, severe CG, intestinal metaplasia and/or dysplasia were excluded. This study was approved by the ethical committee of the Research Center in Tehran University of Medical Science (Accepted Number, IR.TUMS.SPH.REC.1398.167 1398/7/3).
Histological examination
Biopsy specimens in the pathology department were histologically examined by hematoxylin–eosin staining method and the grade of gastritis was described based on histological parameters and updated Sydney System. Patients samples with CG were selected for further analysis.
Isolation and identification
Each biopsy specimen for culture were kept in a transport medium consisting of thioglycollate with 1.3 g/L Agar (Merck, Germany) with 3% yeast extract (Oxoid, UK) and were transferred to the laboratory in less than 2 h, then were homogenized and cultured on Brucella Agar supplemented with 7% sheep blood, Campylobacter selective supplement (vancomycin 2.0 mg, polymyxin 0.05 mg, trimethoprim 1.0 mg), 10% fetal calf serum, and amphotericin B (2.5 mg/L). Incubation was performed in microaerophilic conditions at 37 °C for 5–7 days. Identification of H. pylori isolates was performed by analyzing colony morphology, Gram staining, positive reactions of oxidase and catalase, and urease activities. Confirmation of the identity was done using specific primers for H. pylori (glmM) by polymerase chain reaction (PCR) as described before [13]. The isolates were preserved in BHI broth containing 20% glycerol and 10% fetal calf serum and stored at − 70 °C.
Genotyping of H. pylori isolates
DNA extraction
Genomic DNA of harvested colonies was extracted as described by Douraghi M [14]. Briefly, harvested colonies of the H. pylori isolates were suspended in 1 ml PBS. After centrifugation (6000 rpm, 5 min), the pellets were resuspended in 50 mM/L NaOH and heated at 100 °C for 20 min. After a quick spin and addition of 1 M Tris–HCl, pH 7.5, centrifuged for 5 min at 3000 rpm. The supernatants containing genomic DNA samples were stored at − 20 °C until used for molecular studies.
Genotyping
In this study, genotyping of the isolates was done by PCR. Primers for detection of cagA, cagL, cagY, vacA (s and m alleles), and iceA1 and iceA2 genes were used in separate reactions as described before [13, 15, 16]. Each genotype was defined according to a distinct pattern of the virulence genes detected as follows, cagA+/−/cagL+/−/cagY+/−/vacA (s1/2/m1/2)/iceA1+/−/A2+/−.
Gene expression analysis
RNA extraction and cDNA synthesis
To analyze the extent of alteration in the transcription of inflammatory genes in patients with CG, RNA extraction of the gastric biopsy samples was done using TRIzol reagent (BlueZol, Iran) following the manufacturer’s guidelines with some modifications. Briefly, the tissue samples were cut into small pieces and mixed thoroughly with 600 μL of the extraction solution (BlueZol, Iran). After the addition of 150 μL chloroform and vigorous vortex for 15 s, the resulting mixture was centrifuged at 12,000 rpm for 15 min at 4 °C. The colorless upper aqueous phase was transferred to a new clean tube and mixed with 400 μL of isopropanol. The mixture was frozen at − 70 °C for 30 min, and the obtained RNA pellet (12,000 rpm, 15 min at 4 °C) was mixed with 1 mL 80% ethanol. A gentle vortex was applied to suspended the white plate at the bottom of the microtube. The extracted RNA in the pellet of the mixture was obtained after centrifuged at 7500 rpm for 5 min at 4 °C. Each pellet was resuspended in 20–30 μL DEPC treated water, and stored at – 70 °C after heat treatment in 60 °C for 5 min. The extracted RNA concentration was measured by quantitative method (Nano Drop™ One Microvolume UV–Vis Spectrophotometers). cDNA synthesis was done after adjustment of RNA concentrations using the easy cDNA synthesis kit (Parstous, Iran), according to the manufacturer’s instruction.
Detection of primer efficiency
The efficiency of primers targeting TNF-α, IL-8, Noxo1, MMP-7, Atp4A, B2M, and ACTB genes was measured before each analysis. The nucleotide sequence of these primers is shown in Table 1.
Relative quantitative real-time PCR
The expression level of IL-8, TNF-α, Noxo1, MMP-7, ATP4A genes in the H. pylori-infected compared with H. pylori non-infected patients with CG was measured using SYBR green quantitative real-time PCR. B2M and ACTB genes were used as endogenous genes as described before [17]. The reaction mixture consisted of 0.5 μL of each primer, 12.5 μL RealQ Plus Master Mix Green (Ampliqon, Denmark), 2 μL of cDNA, and distilled water up to the final volume of 25 μL. The thermal cycling conditions in Rotor Gene 6000 Corbett Sequence Detection System have comprised an initial denaturation step at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 60 s, elongation at 72 °C for 60 s, and a final extension step at 72 °C for 3 min. All the reactions were tested in duplicate. To show the accuracy of the amplification for each gene, primer efficiency, melting curve analysis, and gel electrophoresis were done. Relative gene expressions for all samples were determined from the obtained Ct (Crossing Threshold) values and using the 2−∆∆ct (2^− (Sample ∆ct − Average control group ∆ct) method. Up- and down-regulation were defined based on RQ values ≥ 2 and ≤ 0.5, respectively [18].
Statistical analysis
Statistical analyses and graphical representation of results were performed using SPSS (25 version) and GraphPad Prism7 softwares. The correlation between the relative expression values of IL-8, TNF-α, Noxo1, MMP-7, ATP4A genes in the infected group was evaluated by Spearman's correlation nonparametric test. As well, the correlation between H. pylori genotypes and relative expression values of these genes in the H. pylori-infected patients with CG was evaluated by Kruskal–Wallis nonparametric test. p value ≤ 0.05 was considered statistically significant.
Results
Phenotypic and histological results
A total of 168 volunteer patients with gastric disorders (Male, 41.1% and Female, 58.9%), and a mean age of 46 years ranging from 17–86 years, were included in this study. H. pylori infection was detected in 27.4% (46/168) of the biopsy specimens. Histopathologic analysis showed that 87.5% (147/168) had CG, where 41.49% (61/147) of them presented moderate CG in their histological tests. Atrophy, metaplasia, and dysplasia were not detected in these samples. Among the patients with moderate CG, biopsy samples of the H. pylori-infected and non-infected patients were selected for further analysis. Demographic and pathological information for the patients is presented in supplementary (Table 1). All of the H. pylori isolates in the infected patients showed positive results for common biochemical, enzymatic, and PCR (glmM gene) tests.
Characteristics of H. pylori genotypes
Genotypic characteristics of H. pylori isolates showed six distinct genotypes (Supplementary, Table 1). Statistical analysis showed no correlation between these genotypes and gender, ethnicity, age, and endoscopic findings.
Expression and correlation of IL-8, TNF-α, MMP7, Noxo1 and ATP4A genes in two groups of patients
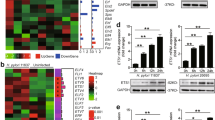
The efficiency of primers targeting TNF-α, IL-8, Noxo1, MMP-7, Atp4A genes was between 0 and 1. Alteration in transcriptional levels of key inflammatory and carcinogenic genes was measured in the gastric tissue samples. The relative expression levels (mean ± SD) of these genes revealed are presented in Table 2. Accordingly, overexpression of TNF-α, MMP7, Noxo1and ATP4A genes in the infected group and IL-8, Noxo1, and ATP4A genes in the non-infected group was detected (Fig. 1). Given that the distribution of data was not normal, Spearman's correlation nonparametric test was used to compare coregulation of different genes linked to the inflammatory pathway in H. pylori-positive and -negative groups. There were positive correlations between the expression of TNF-α and IL-8 genes (r = 0.636, P = 0.026), TNF-α and Noxo1 genes (r = 0.573, P = 0.05), IL-8 and Noxo1 genes (r = 0.601, P = 0.039) in H. pylori-positive group and positive correlations between the expression of TNF-α and MMP-7 genes (r = 0.657, P = 0.020), TNF-α and Noxo1 genes (r = 0.629, P = 0.028), TNF-α and ATP4A genes (r = 0.573, P = 0.05) in H. pylori-negative group that were statistically significant.
Comparison between H. pylori genotypes and relative expression values of genes
As was shown in Fig. 1, relative expression levels of IL-8, TNF α, MMP7, Noxo1, and ATP4A genes in H. pylori-infected patients with defined genotypes were compared. Statistical analysis doesn’t show a correlation between genotypes and the extent of expression for each gene. A link between H. pylori genotype A (cagA+/vacA s1m1) and increased levels of expression for IL-8, TNF-α, and MMP-7 was shown, which were inversely accompanied with the lower level of Noxo1 and ATP4A expression compared with the other genotypes. The lowest level of changes in the transcription of the studied genes compared with the non-infected patients was detected in a patient infected with H. pylori genotype F (cagA-/vacA s2m2). Patients infected with genotype A and C showed the highest level of MMP7 and TNF-α expression compared with other genotypes (1.87 and 3.35-fold vs 0.77 ± 0.34 and 2.52 and 2.55-fold vs 0.47 ± 0.28, respectively). While expression of IL-8, TNF-a, and MMP-7 was higher in biopsy of patients infected with genotype A strains, the expression of ATP4A and Noxo1 genes was higher in the patients infected with a more related genotype (genotype B, 16.91 and 3.76 folds, respectively). The patients infected with genotype C of H. pylori presented the highest transcription level of TNF-α, MMP-7, and Noxo1 genes (2.55, 3.35 and 1.69 folds, respectively) compared to other genotypes (0.88 ± 0.94, 0.96 ± 0.51, and 0.62 ± 0.52).
Discussion
Gastritis is a common disease among humans in different populations. This is estimated that more than half of the world population experience CG in different degree and extent. The role of CG as a serious and insidious illness in the path of gastric carcinogenesis remains largely unknown. H. pylori infection is one of the most important contributors of gastritis that shows great diversity in its genomic content in different geographic locations [1, 15].
Host inflammatory responses are mediated through several mechanisms, including induction of oxidative-reductive stress response pathway, activation of cytotoxic immune cells, B cell activation, and permanent secretion of inflammatory cytokines and chemokines [2]. Although real mechanisms of gastritis and its progression toward gastric cancer after H. pylori infection are poorly known, in vitro studies showed that induction of the nuclear factor-κB (NF-κB) inflammatory pathway is a critical regulator in this relationship. Activation of this pathway could promote immune-dependent inflammation and histological changes in the stomach [9].
A total of 1840 genomics types of H. pylori have been described according to the GenBank database, which shows great diversity in this bacterium that is due to its impaired DNA repair system and evolutionary events. Pathogenicity of this bacterium in strains with intact type 4 secretion system is commonly linked to the expression of CagA, VacA, IceA, Urease, together with adhesions and outer membrane proteins. Finding a link between the genotypes of this bacterium and the extent of inflammatory response in the gastric tissue is hard, since there are several variants of each virulence gene. In this study, our results confirmed this complexity in the studied patients.
Results of our study showed a complexity between the extent of transcription in genes related to inflammation and gastric carcinogenesis and genotypes of the H. pylori isolates. Nearly half of the patients were infected with wild-type genes, while the absence of some virulence genes was detected in other samples. Totally, six different genotypes of H. pylori were detected in biopsy samples of patients with CG, which represented similar diversity as were reported from previous studies in Iran [15]. Genotype A (Cag A+/Cag Y+/Cag L+/Vac A m1s1/ice A1+), which seems to present the highest pathogenicity based on in vitro studies, was detected in 25% of the patients. Histological analysis in the patients infected with this genotype showed similar pathogenicity compared with the patients infected with genotype C, a genotype with low virulence capacity (Cag A−/Cag Y−/Cag L−/Vac A m2s2/ice A1+/A2+). Similar to this finding, in a study by Chiurillo et al., no congruency was detected between genotypes of H. pylori isolates and histological findings [19].
Besides variation in ethnicity of the participants, the observed diversity in genotypes could be explained through impaired DNA repair mechanisms during the replication that delivers several deletions and mutations [19]. In our study, this diversity showed to have no impact on the transcription level of TNF-α in the infected patients compared with non-infected ones. Accordingly, overexpression of TNF-α was detected just for patients infected with the strains belonged to genotype A and C, which show no similarity in their genotypes. In general, a H. pylori strain with genotype A is considered virulent, where expression and secretion of CagA and VacA s1m1 in the gastric tissue is associated with the increased level of TNF-α expression and inflammation [20]. The induction of TNF-α in three infected patients with cagA negative strain carrying an inactive variant of vacA s2m2 allele proposed the involvement of some other virulence factors, host factors, or microbes other than Helicobacter in this interplay. This discrepancy was previously reported by Zabaglia et al. [21]. Although carriage of iceA1/A2 allele in H. pylori isolates with genotype C could explain the induction of TNF-α in the gastric tissue; lack of this induction in a patient infected with H. pylori genotype E has challenged this link. Similar to our finding, in a study by Yamaoka et al. which was done on the gastric tissue, no significant difference in expression of TNF-α was shown between the infected patients with cagA+ and cagA− strains [22]. A positive correlation between the expression of TNF-α gene with IL-8 genes and Noxo1 genes in the H. pylori-positive group and with MMP-7, Noxo1, and ATP4A genes in the H. pylori-negative group confirms its role as a main inflammatory factor that acts as a master switch in establishing between inflammation and cancer [23].
Overexpression of IL-8, as the second important cytokine mediating a role in inflammation, as shown by these authors both in the corpus and antrum of infected patients with cagA+ genotype compared with cagA− ones. This finding is inconsistent with our results since the overexpression was just detected in the infected patients with genotype A. These results are in agreement with Siddique’ (2014) and Audibert’ (2000) findings which showed that the presence of cagA is not associated with increased expression of IL-8 gene [24, 25]. Regardless of defined genotypes, although 2.5-fold higher level of IL-8 transcription was measured in cagA+ compared with cagA− strains in our study, carriage of vacA s1m1 allele was a hallmark for explaining IL-8 overexpression in our samples since this allele was unique in the strains with genotype A, where nearly eightfold higher level of the expression was detected compared with other genotypes.
One unanticipated finding was that the relative expression of the IL-8 gene was lower in the infected patients with H. pylori compared to non-infected patients. This result differs from some published studies [26,27,28,29,30], which suggested that H. pylori infection is not the only factor associated with IL-8 induction in the gastric tissue. Gene polymorphisms in the mediators of NF-κB pathway, induction of TGF-β, IL-10, and Muc-1, and interaction of non-Helicobacter bacteria are among other factors that can influence the expression of IL-8 in patients with gastritis [31]. In the case of studied patients with H. pylori infection, these results suggested that there is a link between genotypes of H. pylori and the level of IL-8 gene expression. According to our knowledge, while no study examined the effect of complete H. pylori genotype on the extent of IL-8 transcription, similar to our results the higher level of IL-8 expression was previously established for H. pylori strains with cagA+/vacA s1m1variant [20, 25, 29].
MMP-7 is a member of the MMP family and a key player in the inflammation process and carcinogenesis, which increase in H. pylori gastritis and early gastric carcinoma [32, 33]. Based on our results, a higher level of the MMP-7 mRNA was detected in the H. pylori-positive group than in the H. pylori-negative group, but this difference was not significant. These results match those observed in earlier studies [32,33,34,35,36]. In the H. pylori-positive group, the patients infected with genotype A and C of H. pylori had higher expression of the MMP-7 gene compared to other genotypes and unlike previous studies, it was found that there was no relationship between the presence of cagA+ H. pylori strains and the increase in MMP-7 gene in patients [36, 37]. The ice A1 and ice A1/A2 seem to be involved in MMP-7 expression that is suggested due to the presence of inactive vacA allele and cagPAI in the stains with genotype C. However, lack of MMP-7 induction in a sample with H. pylori genotype E, which carry this virulence gene, again showed the complexity for the description of this correlation.
H+, K+-adenosine triphosphatase (H+, K+-ATPase) as a proton pump and a marker of parietal cell function is the key pathway mediating the secretion of gastric acid and is affected by H. pylori. H. pylori or its products inhibit the activity of the promoter of the alpha-subunit of H+, K+-ATPase and suppress the expression of H+, K+-ATPase [38, 39]. A few studies have examined the changes in the expression level of the ATP4A gene in human gastric. Our results are almost in line with Kim and Lee’s (2020) findings which showed there were no significant differences in ATP4A mRNA level between H. pylori-negative and H. pylori-positive groups [38]. In our study, downregulation of ATP4A was detected for all genotypes, except genotypes B and E, where overexpression was detected in only one patient. This finding showed that transcription of this gene could be mediated by different pathways, independent of genotypes of H. pylori strains. In previous studies, the effect of existence cagA and type IV secretory system (T4SS), cagL, for repression of ATP4A expression was confirmed in studies of H. pylori cagPAI strains [40,41,42], but in our study, such a relationship was not seen.
NADPH oxidase organizer 1 (NOXO 1) is one of the components forming the NOX1 complex. It is known as a TNF-α-dependent tumor-promoting factor for gastric inflammation [43]. Several reports have shown that Noxo1 expression is significantly upregulated in gastritis as well as the intestinal-type or diffuse-type gastric cancer [43, 44]. In our study, there was no difference in the relative expression of the Noxo1 gene between the two groups of patients. This result may be explained by the fact that all the samples used in our study were from the gastric tissue of patients with CG. In the H. pylori-positive group, the patients infected with genotypes B and C showed higher levels of Noxo1 expression compared with the other genotypes; however, no virulence factor could describe this increased level of the expression. Alteration in the expression level of Noxo1 accompany with TNF-α and IL-8 in the H. pylori-positive group and its link with TNF-α in the H. pylori-negative group represents its role in gastritis irrespective of the type of infections in the gastric tissue. In future studies, determining the role of infection with other microbes could shed light on this interaction.
Conclusion
In general, in this study, we investigated the impact of H. pylori infection and its characterized combined virulence genotypes on transcriptional changes of genes linked to the inflammatory pathway in the gastric tissue of the infected compared with non-infected patients with CG. Our results showed complexity in the transcription of genes link to the inflammatory pathways in the gastric tissue respective to the characterized H. pylori genotypes. A direct relationship with overexpression of IL-8, TNF-α, and MMP-7 genes and downregulation of Noxo-1 and ATP4A was detected in the samples of patients infected with hypervirulent strains with genotype A compared with other genotypes. Irrespective of genotypes of H. pylori, our results showed a significant positive correlation between transcriptional changes of IL8 in conjunction with Noxo1 and/or TNF-α in H. pylori-infected patients that was different from the characterized correlation in the transcription of TNF-α and ATP4A/Noxo1/MMP-7 genes in H. pylori-negative patients with gastritis. This finding proposed the involvement of some other host factors, such as gene polymorphisms in the mediators of the NF-κB pathway, the interplay of the anti-inflammatory pathway, and the interaction of non-Helicobacter bacteria in this regard. More studies on a larger number of samples, especially those with characterized microbiota and related host immunogenetics and transcriptional data could provide valuable documents about this interaction.
References
Varbanova M, Frauenschläger K, Malfertheiner P (2014) Chronic gastritis—an update. Best Pract Res Clin Gastroenterol 28(6):1031–1042. https://doi.org/10.1016/j.bpg.2014.10.005
Qadri Q, Rasool R, Gulzar G, Naqash S, Shah ZAH (2014) pylori infection, inflammation and gastric cancer. J Gastrointest Cancer 45(2):126–132. https://doi.org/10.1007/s12029-014-9583-1
Carrasco G, Corvalan AH (2013) Helicobacter pylori-induced chronic gastritis and assessing risks for gastric cancer. Gastroenterol Res Pract. https://doi.org/10.1155/2013/393015
Ricci V, Romano M, Boquet P (2011) Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol 17(11):1383. https://doi.org/10.3748/wjg.v17.i11.1383
Riggio MP, Lennon A, Wray D (2000) Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med 29(10):507–513. https://doi.org/10.1034/j.1600-0714.2000.291005.x
Jia E-Z, Zhao F-J, Hao B, Zhu T-B, Wang L-S, Chen B et al (2009) Helicobacter pylori infection is associated with decreased serum levels of high density lipoprotein, but not with the severity of coronary atherosclerosis. Lipids Health Dis 8(1):1–7. https://doi.org/10.1186/1476-511X-8-59
Farsak B, Yildirir A, Akyön Y, Pinar A, Öç M, Böke E et al (2000) Detection of Chlamydia pneumoniae and Helicobacter pylori DNA in human atherosclerotic plaques by PCR. J Clin Microbiol 38(12):4408–4411. https://doi.org/10.1128/JCM.38.12.4408-4411.2000
Correa P, Piazuelo MB (2012) The gastric precancerous cascade. J Dig Dis 13(1):2–9. https://doi.org/10.1111/j.1751-2980.2011.00550.x
Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K et al (2000) Implication of NF-κB in Helicobacter pylori-associated gastritis. Am J Gastroenterol 95(10):2768–2776. https://doi.org/10.1016/S0002-9270(00)01096-0
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2(1):1–9. https://doi.org/10.1038/sigtrans.2017.23
Williams RA, Timmis J, Qwarnstrom EE (2014) Computational models of the NF-KB signalling pathway. Computation 2(4):131–158. https://doi.org/10.3390/computation2040131
Hammond CE, Beeson C, Suarez G, Peek RM Jr, Backert S, Smolka AJ (2015) Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion. Am J Physiol Gastrointest Liver Physiol 309(3):G193–G201. https://doi.org/10.1152/ajpgi.00099.2015
Yadegar A, Mobarez AM, Alebouyeh M, Mirzaei T, Kwok T, Zali MR (2014) Clinical relevance of cagL gene and virulence genotypes with disease outcomes in a Helicobacter pylori infected population from Iran. World J Microbiol Biotechnol 30(9):2481–2490. https://doi.org/10.1007/s11274-014-1673-5
Saberi S, Douraghi M, Azadmanesh K, Shokrgozar MA, Zeraati H, Hosseini ME et al (2012) A potential association between Helicobacter pylori CagA EPIYA and multimerization motifs with cytokeratin 18 cleavage rate during early apoptosis. Helicobacter 17(5):350–357. https://doi.org/10.1111/j.1523-5378.2012.00954.x
Vaziri F, Peerayeh SN, Alebouyeh M, Mirzaei T, Yamaoka Y, Molaei M et al (2013) Diversity of Helicobacter pylori genotypes in Iranian patients with different gastroduodenal disorders. World J Gastroenterol 19(34):5685. https://doi.org/10.3748/wjg.v19.i34.5685
Ta LH, Hansen LM, Sause WE, Shiva O, Millstein A, Ottemann KM et al (2012) Conserved transcriptional unit organization of the cag pathogenicity island among Helicobacter pylori strains. Front Cell Infect Microbiol 2:46. https://doi.org/10.3389/fcimb.2012.00046
Wisnieski F, Calcagno DQ, Leal MF, dos Santos LC, de Oliveira GC, Chen ES et al (2013) Reference genes for quantitative RT-PCR data in gastric tissues and cell lines. World J Gastroenterol 19(41):7121. https://doi.org/10.3748/wjg.v19.i41.7121
Festuccia C, Gravina GL, Giorgio C, Mancini A, Pellegrini C, Colapietro A et al (2018) UniPR1331, a small molecule targeting Eph/ephrin interaction, prolongs survival in glioblastoma and potentiates the effect of antiangiogenic therapy in mice. Oncotarget 9(36):24347. https://doi.org/10.18632/oncotarget.25272
Chiurillo MA, Moran Y, Cañas M, Valderrama E, Granda N, Sayegh M et al (2013) Genotyping of Helicobacter pylori virulence-associated genes shows high diversity of strains infecting patients in western Venezuela. Int J Infect Dis 17(9):e750–e756. https://doi.org/10.1016/j.ijid.2013.03.004
Augusto AC, Miguel F, Mendonça S, Pedrazzoli J Jr, Gurgueira SA (2007) Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin Biochem 40(9–10):615–622. https://doi.org/10.1016/j.clinbiochem.2007.03.014
Zabaglia LM, Ferraz MA, Pereira WN, Orcini WA, de Labio RW, Neto AC et al (2015) Lack of association among TNF-α gene expression,-308 polymorphism (G > A) and virulence markers of Helicobacter pylori. J Venom Anim Toxins Incl Trop Dis 21(1):1–7. https://doi.org/10.1186/s40409-015-0054-3
Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J (1996) Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110(6):1744–1752
Wu Y, Zhou B (2010) TNF-α/NF-κ B/Snail pathway in cancer cell migration and invasion. Br J Cancer 102(4):639–644. https://doi.org/10.1038/sj.bjc.6605530
Siddique I, Al-Qabandi A, Al-Ali J, Alazmi W, Memon A, Mustafa AS et al (2014) Association between Helicobacter pylori genotypes and severity of chronic gastritis, peptic ulcer disease and gastric mucosal interleukin-8 levels: Evidence from a study in the Middle East. Gut Pathog 6(1):1–10. https://doi.org/10.1186/s13099-014-0041-1
Audibert C, Janvier B, Grignon B, Salaüna L, Burucoa C, Lecron J-C et al (2000) Correlation between IL-8 induction, cagA status and vacA genotypes in 153 French Helicobacter pylori isolates. Res Microbiol 151(3):191–200. https://doi.org/10.1016/S0923-2508(00)00139-X
Crabtree J, Wyatt J, Trejdosiewicz L, Peichl P, Nichols P, Ramsay N et al (1994) Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol 47(1):61–66. https://doi.org/10.1136/jcp.47.1.61
Moss S, Legon S, Davies J, Calam J (1994) Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut 35(11):1567–1570. https://doi.org/10.1136/gut.35.11.1567
Sharma SA, Tummuru MK, Blaser MJ, Kerr LD (1998) Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J Immunol 160(5):2401–2407
Bartchewsky W Jr, Martini MR, Masiero M, Squassoni AC, Alvarez MC, Ladeira MS et al (2009) Effect of Helicobacter pylori infection on IL-8, IL-1β and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol 44(2):153–161. https://doi.org/10.1080/00365520802530853
Outlioua A, Badre W, Desterke C, Echarki Z, El Hammani N, Rabhi M et al (2020) Gastric IL-1β, IL-8, and IL-17A expression in Moroccan patients infected with Helicobacter pylori may be a predictive signature of severe pathological stages. Cytokine 126:154893. https://doi.org/10.1016/j.cyto.2019.154893
Bornschein J, Kandulski A, Selgrad M, Malfertheiner P (2010) From gastric inflammation to gastric cancer. Dig Dis 28(4–5):609–614. https://doi.org/10.1159/000320061
Lu L, Ma G, Liu X, Sun R, Wang Q, Liu M et al (2017) Correlation between GDF15, MMP7 and gastric cancer and its prognosis. Eur Rev Med Pharmacol Sci 21(3):535–541
Gontar Siregar SH, Sitepu R (2016) Serum IL-10, MMP-7, MMP-9 levels in Helicobacter pylori infection and correlation with degree of gastritis. Open Access Maced J Med Sci 4(3):359. https://doi.org/10.3889/oamjms.2016.099
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3(12):a005058. https://doi.org/10.1101/cshperspect.a005058
Wroblewski LE, Noble P-J, Pagliocca A, Pritchard DM, Hart CA, Campbell F et al (2003) Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 116(14):3017–3026. https://doi.org/10.1242/jcs.00518
Sadeghiani M, Bagheri N, Shahi H, Reiisi S, Rahimian G, Rashidi R et al (2017) cag Pathogenicity island-dependent upregulation of matrix metalloproteinase-7 in infected patients with Helicobacter pylori. J Immunoassay Immunochem 38(6):595–607. https://doi.org/10.1080/15321819.2017.1351372
Crawford HC, Krishna US, Israel DA, Matrisian LM, Washington MK, Peek RM Jr (2003) Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 125(4):1125–1136. https://doi.org/10.1016/S0016-5085(03)01206-X
Kim HJ, Kim N, Park JH, Choi S, Shin CM, Lee OJ (2020) Helicobacter pylori eradication induced constant decrease in interleukin-1B expression over more than 5 years in patients with gastric cancer and dysplasia. Gut Liver 14(6):735. https://doi.org/10.5009/gnl19312
Yao X, Smolka AJ (2019) Gastric parietal cell physiology and Helicobacter pylori–induced disease. Gastroenterology 156(8):2158–2173. https://doi.org/10.1053/j.gastro.2019.02.036
Saha A, Backert S, Hammond CE, Gooz M, Smolka AJ (2010) Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase α subunit. Gastroenterology 139(1):239–248. https://doi.org/10.1053/j.gastro.2010.03.036
Chang W-L, Yeh Y-C, Sheu B-S (2018) The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci 25(1):1–9. https://doi.org/10.1186/s12929-018-0466-9
Saha A, Hammond CE, Trojanowska M (2008) Helicobacter pylori-induced H, K-ATPase α-subunit gene repression is mediated by NF-κB p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol 294(3):G795–G807. https://doi.org/10.1152/ajpgi.00431.2007
Oshima H, Ishikawa T, Yoshida G, Naoi K, Maeda Y, Naka K et al (2014) TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 33(29):3820–3829. https://doi.org/10.1038/onc.2013.356
Echizen K, Horiuchi K, Aoki Y, Yamada Y, Minamoto T, Oshima H et al (2019) NF-κB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene 38(22):4250–4263. https://doi.org/10.1038/s41388-019-0702-0
Lou X, Zhu H, Ning L, Li C, Li S, Du H et al (2019) EZH2 regulates intestinal inflammation and necroptosis through the JNK signaling pathway in intestinal epithelial cells. Dig Dis Sci 64(12):3518–3527. https://doi.org/10.1007/s10620-019-05705-4
Ohki R, Yamamoto K, Mano H, Lee RT, Ikeda U, Shimada K (2002) Identification of mechanically induced genes in human monocytic cells by DNA microarrays. J Hypertens 20(4):685–691
Binato R, Santos EC, Boroni M, Demachki S, Assumpção P, Abdelhay E (2018) A common molecular signature of intestinal-type gastric carcinoma indicates processes related to gastric carcinogenesis. Oncotarget 9(7):7359. https://doi.org/10.18632/oncotarget.23670
Rubach M, Lang R, Hofmann T, Somoza V (2008) Time-dependent component-specific regulation of gastric acid secretion-related proteins by roasted coffee constituents. Ann N Y Acad Sci 1126(1):310–314. https://doi.org/10.1196/annals.1433.061
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):1–12. https://doi.org/10.1186/gb-2002-3-7-research0034
Steinau M, Rajeevan MS, Unger ER (2006) DNA and RNA references for qRT-PCR assays in exfoliated cervical cells. J Mol Diagn 8(1):113–118. https://doi.org/10.2353/jmoldx.2006.050088
Acknowledgements
The authors would like to thank Prof. Abdollah Karimi, Prof. Fatemeh Fallah and all colleagues of Pediatric Infections Research Center (PIRC), Research Institute for Children’s Health, Mofid Children's Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their cooperation in this study. The authors of this study also thank the kindly support of gastroenterology and pathology units of Firoozgar hospital, Iran University of Medical Sciences, Tehran, Iran.
Funding
This study was funded by a Ph.D. grant from the Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
We declare that all the authors fulfilled the authorship criteria and all authors read and approved the final version of the manuscript. Seyedeh Zohre Mirbagheri, do main part of the experiments and wrote initial draft of the manuscript; Ronak Bakhtiari co-supervised the study and provide the fund; Masoud Alebouyeh designed the study, supervised the research, reviewed and revised the manuscript; Hashem Fakhre Yaseri, do endoscopy and provide the gastric biopsy samples and pathology reports; Abbas Rahimi Foroushani and Seyyed Saeed Eshraghi were counselors of this study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study was approved by the ethical committee of the Research Center in Tehran University of Medical Science (accepted Number, IR.TUMS.SPH.REC.1398.167 1398/7/3) and an informed consent form was obtained from all the patients.
Informed consent
The authors included in the study consent to this manuscript to participate and for publication.
Consent for publication
The authors declare that they consent for publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mirbagheri, S.Z., Bakhtiari, R., Fakhre Yaseri, H. et al. Transcriptional alteration of genes linked to gastritis concerning Helicobacter pylori infection status and its virulence factors. Mol Biol Rep 48, 6481–6489 (2021). https://doi.org/10.1007/s11033-021-06654-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06654-w