Abstract
To assess the association between vitamin D-Binding Protein (VDBP rs7041T>G) and vitamin D receptor (VDR rs1544410G>A) gene polymorphisms with susceptibility to cardiovascular diseases in population from west of Iran. Two hundred forty-nine individuals with cardiovascular disease (92 with aortic and Mitral Valves Calcification (AMVC) and 157 with Coronary Artery Diseases (CAD) that their diseases were confirmed by echocardiography and angiography and unrelated 182 healthy controls (gender and age-matched) were selected for this case–control study. The VDR 1544410G>A, and VDBP 7041T>G genotyping were detected by PCR–RFLP, serum vitamin D and lipid concentrations were measured by ELISA and enzyme assay, respectively. The VDR rs1544410G>A gene is a strong risk factor for CAD (OR = 1.28, p = 0.002) and the dominant genotype (T/G+G/G) of VDBP 7041 T>G SNP plays a protective role (OR = 0.67, p = 0.003) in AMVC development in studied population. In addition, lower level of vitamin D strongly increased the risk of CAD (15 ± 11.02 vs. 21.3 ± 18 μg/L, p = 0.043) and AMVC (12.1 ± 13.1 vs.21.3 ± 18 μg/L, p = 0.014) development in individuals carrying T/T genotype of VDBP 7041 T>G gene polymorphism. There was a strong interaction between A allele VDR rs1544410 and G allele of VDBP rs7041 genes in a protective role (OR = 0.74, p = 0.044) in AMVC patients). CAD and AMVC patients were deficient in vitamin D, i.e. their level of vitamin D was strongly lower than that in the control group. Our findings for the first time indicated that there is a strong association between vitamin D deficiency, lipid profile and the VDR rs1544410G>A and rs7T41>G VDBP genes polymorphisms. These interactions may be one of the important factors for CAD and AMVC incidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases (CVDs) is the first cause of death worldwide [1, 2]. Calcification of the aortic valve or mitral annulus and CAD are major causes of CVD in elderly [3]. Although CAD has a complex etiopathogenesis and a multifactorial origin, it is most commonly caused by atherosclerosis [4]. Although the exact molecular mechanism leading to CVD is unclear, cigarette smoking, hypertension, hyperlipidemia, genetic predisposition, lack of physical activity, obesity and vitamin D (Vit D) deficiency may be a risk factor for incidence of CVD [5, 6].
Vitamin D not only regulates calcium and phosphorous homeostasis, however it also plays important role in physiological functions [6]. Over the past decade, evidences suggested that there was a positive association between low vitamin D level and CVD susceptibility or mortality [6, 7]. Norman et al. reported that although vitamin D is essential for cardiovascular health, its excess could have unintended health consequence, including elastolysis induced by inflammatory processes [8]. In females, vitamin D deficiency is associated with the severity of CAD, but in males, vitamin D status is independently related to CAD susceptibility [9].
The main transport protein of vitamin D in the plasma is vitamin D-binding protein (VDBP) with a molecular weight of (58 kDa). The amount of active vitamin D in the bloodstream depends strongly on the VDBP concentration [10]. The VDBP/25-hydroxy vitamin D [25(OH)D complex formation, its filtration, and reabsorption through vitamin D receptors (VDRs) in renal tubular cells are important factors in providing optimal levels of Vit D in circulation [11].
Previous studies reported that single nucleotide polymorphisms (SNPs) of rs1544410 and rs7041 in VDR and VDBP genes, respectively are potential risk factors for CAD and vascular calcification [8, 12, 13]. The SNPs in the VDR and VDBP genes lead to production of variant proteins with different affinity for vitamin D [13]. The VDBP gene has located on the chromosome 4 and the SNP of rs7041 in exon11 of this gene is significantly associated with low circulating 25(OH)D concentration in different populations [14, 15]. VDR gene is located on chromosome 12 and the rs1544410>A Bsm1 (rs1544410) SNP is located at the 3′ end of the gene [16]. VDR rs1544410G>A SNP does not change the amino acid sequence of the VDR protein, however, it may affect on alteration of mRNA stability and consequently gene expression, also it might change the splicing sites or alter the intronic regulatory elements [16]. The best of our knowledge, the association of VDR rs1544410G>A and VDBP rs7041T>G SNPs with susceptibility to AMVC and CAD has not been studied. Here, the role of VDR rs1544410G>A and VDBP rs7041T>G SNPs in susceptibility to CAD and AMVC and their effects on systolic and diastolic pressure, vitamin D, Ca, phosphate and lipid profile concentrations in circulation in population from west of Iran were studied. In this study, we found for the first time, that there is a strong association between vitamin D deficiency, lipid profile and the VDR rs1544410G>A and rs7041T>G VDBP genes polymorphisms. These interactions may be one of the important factors for CAD and AMVC incidence.
Materials and methods
Ethics Committee of Kermanshah University of Medical Sciences, Iran approved this study according to the principles of the Declaration of Helsinki I. All participants were signed a written informed consent.
Subjects
A total of 249 patients (92 with AMVC and 157 with CAD, mean age, 64.6 ± 9.8 years; range, 40–88 years) were recruited from Imam Ali hospital. The MVC and AVC were diagnosed according to Doppler echocardiography. The criteria for detection of aorta stenosis in mild AVC was the aortic jet velocity 2.5–2.9 (m/s), mean gradient < 25 mmHg and the aortic valve area of 1.5–2 cm2, for moderate AVC the criteria were the aortic jet velocity 3–4 (m/s), mean gradient 25–49 mmHg and the aortic valve area of 1–1.5 cm2. The criteria for detection of severe AVC were the aortic jet velocity > 4 (m/s), mean gradient > 40 mmHg and the area of aortic valve of < 1 cm2.
The criteria for mild MVC was the pulmonary artery pressure < 30 mmHg and mean gradient < 5 mmHg and Mitral valve area > 1.5 cm2, for moderate MVC the criteria were the pulmonary artery pressure 30–40 mmHg and mean gradient 5–10 mmHg and Mitral valve area < 1 cm2.
The CAD was diagnosed by having a greater than 50% diameter stenosis of one or more major coronary vessel as detected by angiography.
The systolic (SBP) and diastolic (DBP) blood pressures and body mass index (BMI) of each individual were measured.
The healthy control volunteers (182, mean age, 63.7 ± 7.2 years; range 47–81 years) were recruited from subjects that were referred to the blood bank of Kermanshah province. The selected individuals were those without CVD and also with the absence of specific diseases (heart and vascular, liver, kidney, diabetes mellitus and major diseases) at their annual medical checkup.
Chemical analyses
Serum lipid profiles included LDL-C, HDL-C, triglyceride (TG), and the serum levels of Ca (mg/dL), Phosphate (mg/dL), urea, creatinine, and fasting blood sugar (FBS) (mg/dL) were determined by the enzymatic method (Pars Azmon kit, Iran), using an automated Erba XL- 600 (Mannheim, Germany).
Serum vitamin D (µg/L) concentration was determined by ELISA according to the manufacturer’s instructions (Monobind Ink Kit, USA).
DNA extraction, VDR 63980G>A, and VDBP 7904T>G genotyping
Phenol chloroform method was used for extracting the genomic DNA from EDTA treated blood samples [17]. The VDR rs1544410G>A and VDBP rs7041T>G genes polymorphisms were determined by PCR–RFLP technique.
The forward 5′-CAACCAAGACTACAAGTACCGCGTCAGTGA-3′ and reverse 5′-AACCAGCGGAAGAGGTCAAGGG-3′ primers were used to identify VDR rs1544410G>A gene polymorphism. The 25 µl PCR reaction mixtures were containing 50–100 ng DNA, primers, and dNTPs. Denaturation of DNA at 95 °C for 5 min was followed by 35 cycles of 95 °C for 1 min, 65.7 °C for 1 min and 72 °C for 1 min. A temperature of 72 °C for 10 min was used as final extension. The size of PCR products was 822 bp [18].
The Bsm1 restriction endonuclease was used for digestion of PCR products at 37 °C overnight and the digested PCR products were detected by electrophoresis using a 2 percent agarose gel contained ethidium bromide and photographed under UV transilluminator. The length of digested fragments for homozygote mutant AA was 822 bp, for heterozygote mutant GA were 822 bp, 645 bp, and 177 bp and for homozygote wild GG was 645 bp and 177 bp as shown in Fig. 1.
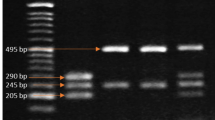
The forward 5′-AAATAATGAGCAAATGAAAGAAGAC-3′ and reverse 5′- CAATAACAGGAAAGAAATGAGTAGA-3′ primers were used to identify VDBP rs7041T>G polymorphism. In brief, the PCR reaction consisted of DNA denaturation at 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 51 °C for 30 secs, and 72 °C for 45 secs, with a final extension at 72 °C for 10 min. After amplification, the PCR products (483 bp) were digested with the HaeIII restriction enzyme. Wild type TT genotype showed a 483 bp product, whereas homozygous mutant GG genotype showed two fragments (298 and 185 bp) and heterozygous TG genotype showed three fragments (483, 298, and 185 bp) (Fig. 2) [19].
A 2% agarose gel electrophoresis patterns for VDBP rs7041T>G gene polymorphism alleles analyzed by PCR–RFLP. Lane 1, 4 and 10, are heterozygous -type (G/T); Lane 2, 3 and 5 homozygous wild type(G/G); Lane 7, 100 bp DNA ladder; Lane 9, undigested PCR product; Lanes 3 and 7, homozygous mutant; Lanes 5, heterozygous mutant (T/T)
Validation of the methods by Sanger sequencing
The presence of VDR rs1544410G>A and VDBP rs7041T>G genes mutations were confirmed by genomic DNA sequencing. To verify the obtained genotypes, Sanger sequencing was also employed as our reference method. For this purpose, some samples from each genotype were subjected to sequencing. The results of Sanger sequencing were determined by Chromas program (Technelysium Pty. Ltd, Helensvale, Queensland, Australia).
Statistical analyses
The χ2 test was used to compared the frequencies of VDR rs1544410G>A and VDBP rs70414T>G in patients with calcification of the aortic and mitral valves and also in CAD patients. The Hardy–Weinberg equilibrium for analyzing the agreement of the frequencies of observed genotype with those expected genotypes was approved using χ2 test. The relative risk of the disease was calculated by Odds ratios (OR) and 95% confidence intervals (CI) was obtained by SPSS logistic regression. The correlation values of serum LDL-C, HDL-C, TG, vitamin D, Ca, Phosphate, FBS, urea, creatinine, MI, age, systolic and diastolic pressures between three groups were calculated using linear regression and an unpaired t test. The quantitative data were compared using t-test, ANOVA and nonparametric independent sample Mann–Whitney analysis. For analyzing the data, the SPSS 16 Software was used. The P value < 0.05 considered as statistical significance.
Results
The results of laboratory tests and demographic characteristics of patients and control group are depicted in Table 1. Comparing CAD and AMCV patients with controls indicated strongly lower serum concentrations of vitamin D (13.5 (7.7–21.2, p = 0.02) and 8.9 (8.6–14.6, p = 0.025) versus 16.2 (5.2–26.2), HDL-C (41 (35–45, p = <0.001) and 44 (39.2–50, p = 0.001 vs. 50 (43–60)), and Ca (9.2 (8.3–9.9, p < 0.001) and 9.2 (8.3–9.9, p < 0.001) vs. 9.4 (8.7–10.1)) and higher levels of LDL-C (95.1 ± 32.9, p = 0.005) and 89.3 (61.3–117.7, p = 0.04) vs. 86 (63.3–110) FBS (100 (90–114.5), p < 0.001 and 98.8 (90–106, p = 0.002) vs. 93 (87.7–99), creatinine (1.14 ± 2.9 vs. 0.8 ± 0.14, p < 0.001), urea 40 (32–50, p < 0.001) and 34 (29.2–39.7, p < 0.001) vs. 29(25–32), respectively (Table 1).
In addition, the values of systolic and diastolic blood pressures were strongly higher in CAD and AMCV patients than control group, p < 0.001 (Table 1).
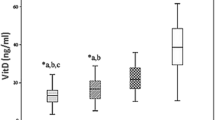
The frequencies of VDR rs1544410G>A and VDBP rs7041T>G genotypes and alleles in CAD and AMVC patients and controls are demonstrated in Table 2. Also, the frequency of VDR rs1544410G>A and VDBP rs7041T>G alleles in CAD and AMVC patients and controls as well as vitamin D level distributions are demonstrated in Figs. 3 and 4, respectively.
Vitamin D distributions among in CAD and AMVC patients and controls is demonstrated in Fig. 4
The overall distribution of VDR 63980G>A genotypes (χ2= 8.9, df = 2, p = 0.011) and alleles (χ2.9, df = 1, p = 0.002) in CAD patients were strongly different compared to controls (Table 2). The presence of dominant (G/A + A/A vs. G/G) genotypes [1.34 (1.05–1.7, p = 0.015)] and the A allele [1.28 (1.09–1.5, p = 0.002, n = 165)] of VDR rs1544410G>A SNP strongly increased the risk of CAD in the population studied. Interestingly, a strong difference was found between VDBP rs7041T>G) genotypes (χ2 = 17.7, df = 2, p < 0.001) and alleles (χ28.4, df = 1, p < 0. 001) frequencies in patients with AMVC and control group (Table 2). Dominant (T/G + G/G) genotype of VDBP rs7041T>G SNP had a trend to decrease the risk of AMVC disease [0.67 (0.51–0.87, p = 0.003)]. The VDBP rs7041T>G genotype and allele frequencies in patients with CAD were similar to the control group (Table 2).
We analyzed the association of GG and GA+AA genotypes of VDR rs1544410 (Table 3), TT and TG+GG genotypes of VDBP rs7041 (Table 4) with serum levels of vitamin D, LDL-C, HDL-C, TC, TG, BMI, Ca, phosphate, FBS, urea, and creatinine, as well as with systolic and diastolic blood pressures in CAD and AMVC patients. As shown in the Table 3, we found that CAD patients with dominant (A/A+A/G) genotype of the VDR rs1544410G>A or with dominant genotype (G/G+G/T vs. TT) of VDBP rs7041T>G SNP had strongly lower levels of HDL-C (40.4 ± 8.1 vs. 51.7 ± 11.8 l p < 0.001 and 39.8 ± 8.04 vs. 52.08 ± 12.7, p < 0.001, respectively) and Ca (9 ± 1.4 vs. 9.4 ± 0.99, p = 0.009 and 8.8 ± 1.5 vs. 9.3 ± 1.1, p = 0.01, respectively) compared to controls.
Both VDR rs1544410 (A/A+A/G) and VDBP rs7041(G/G+G/T) in dominant genetic model strongly increased CAD patients’ systolic and diastolic blood pressures and serum levels of FBS, urea and creatinine.
A strongly lower level of Vit D (15 ± 11.02 vs. 21.3 ± 18, p = 0.043) was detected in CAD patients with TT genotype of VDBP at position 7904 compared to control group (Table 3). Interestingly, in controls there was significantly higher level of HDL-C than the patients with CAD who carrying GG genotype of VDR rs1544410G>A (41.5 ± 7.7 vs. 53.1 ± 11.4, p < 0.001) and TT genotype of VDBP rs1544410T>G (40. 05 ± 7.4 vs. 52.07 ± 12.5, p < 0.001) (Table 3).
The CAD patients with GG genotype of VDR rs1544410G>A and TT genotype of VDBP rs7041T>G had strongly higher concentrations of FBS, Creatinine and urea as well as higher systolic and diastolic blood pressures compared with control subjects (Table 3).
In this study we also found that patients with AMVC disease who carry the (A/A+A/G) genotype of VDR 63980G>A SNP (dominant genetic model), the (G/G+G/T) genotype of VDBP rs7041T>G SNP in dominant genetic model had strongly lower concentrations of vitamin D and HDL-C than control subjects (Table 4). In addition, AMVC patients with GG genotype of VDR rs1544410G>A or with one or more copy of A allele of VDR at position 63980 had strongly higher concentrations of LDL-C, phosphate, FBS and urea compared to healthy control group (Table 4).
We analyzed the CAD patients in two groups; group 1 patients were those with one vessel stenosis, group 2 were those with two or more vessel stenosis. Patients with AMVC disease were also divided into groups based on involvement of aortic and/or mitral valve calcifications (i.e. 1. aortic or mitral valve calcification 2. aortic and mitral valve calcification). Then, we investigated the distribution and OR for the presence of the VDR rs1544410G>A and VDBP rs7041T>G genotypes in each group according to dominant genetic model and reported the results in Table 5. We found that the overall distribution and OR of VDR rs1544410G>A and VDBP rs7041T>G genotypes in 4 groups of patients were similar (Table 5).
The logistic regression analysis was used to investigate the interaction between A allele of VDR rs1544410G>A and G allele of VDBP rs7041T>G in control group, CAD and AMVC patients and the results are demonstrated in Table 6. A strongly association between VDR rs1544410 A and VDBP rs7041, G alleles was observed in AMVC patients (χ2 = 9.2, df = 3, p = 0. 027). As depicted in Table 6, the concomitant presence of the G allele of VDBP rs7041 and the A allele of VDR rs1544410 strongly decreased the risk of AMVC disease by 0.74 (0.58–1, p = 0.044) times.
We also divided the CAD, AMVC patient and control groups based on vitamin D deficiency into 4 groups as shown in Table 7. The distribution of all 4 groups, severe deficiency (< 10 ng/mL), deficient 10 < and < 20 ng/mL, insufficient 20 < and < 30 and sufficient individuals with > 30 ng/mL of Vit D [20].
Deficiency of vitamin D in CAD (χ2 = 10.5, df = 3, p = 0.01) and AMVC patients (χ23.9, df = 3, p = 0.003) was strongly higher than controls. In addition, the frequency of severe deficiency (< 10 ng/mL) of Vit D in AMVC patients (55.4%) was higher compared to CAD patients (35%, p = 0.01). Vitamin D distribution among CAD and AMVC patients and controls is demonstrated in Fig. 4.
Discussion
Coronary artery disease in conjunction with AMVC are leading cause of death especially in the elderly [21, 22]. These disorders happen as a result of interaction between genetic predisposition and environmental factors [22]. One meta analysis about a comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease reported that 2213 variants (7.6% in dels) have a strong correlation (p < 5 × 10 − 8) with CAD with a low false discovery rate (FDR q-value < 2.1 × 10 − 4) [23]. Although our results demonstrated that vitamin D deficiency had the strong genetic association with increased the risk of CAD and AMVC development in our studied population, Manousaki et al. failed to show such an association [24]. These findings suggested that although the role of genetic in CVD susceptibility is confirmed, there is no approved evidence correlated with vitamin D deficiency, VDR and VDBP genetic variations with increased the risk of CVD. Association of vitamin D deficiency with autoimmune diseases, certain cancers, osteoporosis, hypertension, preeclampsia, diabetes, and hyperlipidemia has already been reported [25, 26].
Recently, evidence has demonstrated that the deficiency of vitamin D is correlated with the prevalence of CVD [25]. The genetic variation of VDBP, a major transporter of vitamin D metabolites, has been demonstrated to alter serum 25(OH) vitamin D levels [9]. According to our knowledge, there are only a few reports regarding the association of VDR rs1544410G>A and VDBP rs7041T>G genes polymorphisms with susceptibility to CAD and AMVC. In the present study, for the first time, we have focused on the role of SNPs of VDR rs1544410G>A and VDBP rs7041T>G genes in susceptibility to CAD, AMVC and their effects on systolic and diastolic pressures, vitamin D, Ca, phosphate and lipid profile in a population from west of Iran. We detected that distribution of genotypes and alleles of VDR rs1544410G>A polymorphism was strongly higher in CAD patients and they enhanced the risk of CAD. The present study also suggests that the A allele of VDR gene at position rs1544410 could be a suitable marker for prediction of CAD development.
Consistent with our results, VDR polymorphism has been shown to strongly increase the risk of calcific aortic valve stenosis in German [27]. Elevated risk of CAD in Caucasian population associated with VDR Bsm1 allele and genotype has also been reported by Shuai et al. [12]. However, in contrast to our results, Alizadeh et al. failed to indicate such an association in East-Asians population [28]. In addition, no strong association has been found between VDR SNP/haplotypes with either Vit D or CAD in Indian population [20].
In addition, higher systolic and diastolic blood pressure and FBS, urea and creatinine levels in CAD patients was strongly correlated with wild and heterozygote genotypes of VDR rs1544410G>A gene. Our results indicated that GG and GA+AA genotypes of VDR at position rs1544410 were considerably associated with higher levels of phosphate, FBS, urea and also higher systolic and diastolic blood pressures in patients with AMVC compared to control group.
We also found that the VDBP rs7041T>G gene polymorphism had strongly protective role against the incidence of AMVC in Iranian population. The dominant genotype T/G+G/G genotype and G allele of VDBP at position rs7041 had protective role against the incidence of AMVC. Changes in VDBP protein due to rs7041T>G mutation might be responsible for its protective role in AMVC. Decreased risk for AMVC susceptibility was observed in presence of both G allele or VDBP and the A allele of VDR rs1544410. There is no study demonstrating the influence of VDBP rs7041T>G gene polymorphism in CAD or in AMVC susceptibility. In the report of Chan et al. the effect of 4 SNPs, rs4588, rs7041, rs2282679, rs1155563 of VDBP gene were investigated and their findings indicated that these SNPs were correlated with serum concentration of 25(OH)D and genetically deprived vitamin D predisposes the individuals with CAD to increased atrial fibrillation (AF) [29].
The high serum levels of FBS, urea and creatinine serum level and systolic and diastolic blood pressures in CAD patients compared to control were also associated with the VDBP rs7041 TT genotype in dominant genetic model. Further, the increased BMI and LDL-C concentration in CAD and in AMVC patients were associated with T/G+G/G genotype of VDBP 7rs7041T>G.
Our results also indicated that serum concentration of vitamin D in CAD and in AMVC patients was lower than in control subjects. The presence of TT and T/G+G/G genotypes of VDBP rs7041T>G strongly affected the vitamin D concentration in CAD and AMVC patients compared to their corresponding control groups. AMVC patients with VDR rs1544410 GG and G/A+A/A genotypes had also lower level of vitamin D concentration than their corresponding control subjects. Our findings suggest that correlation of deficiency of vitamin D with VDR rs1544410>A and VDBP gene rs7041T>G polymorphisms may be one of the important factors that increase the CAD and AMVC incidence in population from west of Iran.
Daffara et al. reported the impact of VDBP polymorphisms rs7041 and rs4588 on the coronary artery disease extent in Italian population and they found that the status of 25(OH) D but no genetic variants of VDBP were correlated with CAD [30]. Mostafavi et al. indicated that although the deficiency of vitamin D was detected in 60.2% of the 224 Iranian patients with coronary artery stenosis, low level of vitamin D did not correlate with persistence, extent, and severity of the disease [31]. According to the report of Akin, Shanker, Young and Sun et al. Vitamin D deficiency was strongly increased the risk of coronary artery stenosis and CAD among populations from Turkey, Colorado (United States), India and china [20, 32,33,34], respectively. These inconsistent findings may be due to factors associated with the study designs, ethnicity, sample size, and other CAD and AMVC risk factors. Further study is needed to confirm this correlation.
Conclusion
This study showed that SNPs at position rs1544410 of VDR and at position rs7041 of VDBP genes may be risk factors for CAD development and SNP at position 7904 of VDBP is protective for susceptibility to AMVC, respectively. In addition, these SNPs were associated with vitamin D deficiency in CAD and AMVC patients. Both VDR rs1544410G>A and VDBP 7041T>G SNPs had strong effect on FBS, urea and creatinine concentrations in CAD and AMVC patients.
References
Yeh HL, Kuo LT, Sung FC, Yeh CC (2018) Association between Polymorphisms of Antioxidant Gene (MnSOD, CAT, and GPx1) and Risk of Coronary Artery Disease. Biomed Res Int 2018:5086869
Vaisi-Raygani A, Rahimi Z, Entezami H, Kharrazi H, Bahrhemand F, Tavilani H et al (2008) Butyrylcholinesterase K variants increase the risk of coronary artery disease in the population of western Iran. Scand J Clin Lab Invest 68(2):123–129
Wang AY, Ho SS, Wang M, Liu EK, Ho S, Li PK et al (2005) Cardiac valvular calcification as a marker of atherosclerosis and arterial calcification in end-stage renal disease. Arch Intern Med 165(3):327–332
Martinez PF, Okoshi MP (2018) Genetic risk in coronary artery disease. Arq Bras Cardiol 111(1):62–63
Hu N, Wang J, Hu P, Li Z (2018) Investigation of Renalase gene rs2576178 polymorphism in patients with coronary artery disease. Biosci Rep 38(5):BSR20180839
Mirhosseini N, Rainsbury J, Kimball SM (2018) Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med 5:87
Hao Y, Chen Y (2019) Vitamin D levels and vitamin D receptor variants are associated with chronic heart failure in Chinese patients. J Clin Lab Anal 33:e22847
Norman PE, Powell JT (2005) Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler Thromb Vasc Biol 25(1):39–46
Stakisaitis D, Lesauskaite V, Girdauskaite M, Janulionis E, Ulys A, Benetis R (2016) Investigation of vitamin D-binding protein polymorphism impact on coronary artery disease and relationship with longevity: own data and a review. Int J Endocrinol 2016:8347379
Abdella NA, Mojiminiyi OA (2018) Vitamin D-binding protein clearance ratio is significantly associated with glycemic status and diabetes complications in a predominantly vitamin D-deficient population. J Diabetes Res 2018:6239158
Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL (2011) Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 96(1):142–149
Lu S, Guo S, Hu F, Guo Y, Yan L, Ma W et al (2016) The associations between the polymorphisms of vitamin D receptor and coronary artery disease: a systematic review and meta-analysis. Medicine (Baltimore) 95(21):e3467
Amadori D, Serra P, Masalu N, Pangan A, Scarpi E, Bugingo AM et al (2017) Vitamin D receptor polymorphisms or serum levels as key drivers of breast cancer development? The question of the vitamin D pathway. Oncotarget 8(8):13142–13156
Santos BR, Lecke SB, Spritzer PM (2017) Genetic variant in vitamin D-binding protein is associated with metabolic syndrome and lower 25-hydroxyvitamin D levels in polycystic ovary syndrome: a cross-sectional study. PLoS ONE 12(3):e0173695
Chen H, Zhang L, He Z, Zhong X, Zhang J, Li M et al (2015) Vitamin D binding protein gene polymorphisms and chronic obstructive pulmonary disease: a meta-analysis. J Thorac Dis 7(8):1423–1440
Triantos C, Aggeletopoulou I, Kalafateli M, Spantidea PI, Vourli G, Diamantopoulou G et al (2018) Prognostic significance of vitamin D receptor (VDR) gene polymorphisms in liver cirrhosis. Sci Rep 8(1):14065
Bahrehmand F, Vaisi-Raygani A, Kiani A, Rahimi Z, Tavilani H, Navabi SJ, Shakiba E, Hassanzadeh N, Pourmotabbed T (2012) Matrix metalloproteinase-2 functional promoter polymorphism G1575A is associated with elevated circulatory MMP-2 levels and increased risk of cardiovascular disease in systemic lupus erythematosus patients. Lupus 21(6):616–624
Hong YJ, Kang ES, Ji MJ, Choi HJ, Oh T, Koong SS et al (2015) Association between Bsm1 Polymorphism in vitamin D receptor gene and diabetic retinopathy of type 2 diabetes in korean population. Endocrinol Metab (Seoul) 30(4):469–474
Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP et al (2010) Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J 35(5):1106–1112
Shanker J, Maitra A, Arvind P, Nair J, Dash D, Manchiganti R et al (2011) Role of vitamin D levels and vitamin D receptor polymorphisms in relation to coronary artery disease: the Indian atherosclerosis research study. Coron Artery Dis 22(5):324–332
Paradis JM, Fried J, Nazif T, Kirtane A, Harjai K, Khalique O et al (2014) Aortic stenosis and coronary artery disease: what do we know? What don’t we know? A comprehensive review of the literature with proposed treatment algorithms. Eur Heart J 35(31):2069–2082
Alizadeh S, Djafarian K, Alizadeh H, Mohseni R, Shab-Bidar S (2017) Common variants of vitamin D receptor gene polymorphisms and susceptibility to coronary artery disease: a systematic review and meta-analysis. J Nutrigenet Nutrigenomics 10(1–2):9–18
Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S et al (2015) A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 7(10):1121–1130
Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB (2016) Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet 9(4):349–356
Hossein-Nezhad A, Eshaghi SM, Maghbooli Z, Mirzaei K, Shirzad M, Curletto B et al (2014) The role of vitamin D deficiency and vitamin d receptor genotypes on the degree of collateralization in patients with suspected coronary artery disease. Biomed Res Int 2014:304250
Rezavand N, Tabarok S, Rahimi Z, Vaisi-Raygani A, Mohammadi E, Rahimi Z (2018) The effect of VDR gene polymorphisms and vitamin D level on blood pressure, risk of preeclampsia, gestational age, and body mass index. J Cell Biochem Nov 120(4):6441–6448
Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P (2001) The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart 85(6):635–638
Hassani H, Djafarian K (2017) Vitamin D receptor gene polymorphisms and risk of coronary artery disease: findings from a meta-analysis with 5259 cases. Razi J Med Sci 24(160):79–89
Chan YH, Yiu KH, Hai JJ, Chan PH, Lam TH, Cowling BJ et al (2017) Genetically deprived vitamin D exposure predisposes to atrial fibrillation. Europace 19(suppl_4):iv25–iv31
Daffara V, Verdoia M, Rolla R, Nardin M, Marino P, Bellomo G et al (2017) Impact of polymorphism rs7041 and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr Metab Cardiovasc Dis 27(9):775–783
Mostafavi A, Khavandi S, Jafarnejad S, Naderian MM, Akbarzadeh M, Farbod Z (2018) Evaluation of correlation between serum levels of vitamin D and coronary artery disease: an existing debate. J Clini Diag Res 12(8):1–4
Akin F, Ayca B, Kose N, Duran M, Sari M, Uysal OK et al (2012) Serum vitamin D levels are independently associated with severity of coronary artery disease. J Investig Med 60(6):869–873
Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA et al (2011) Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care 34(2):454–458
Sun H, Long SR, Li X, Ge H, Liu X, Wang T et al (2019) Serum vitamin D deficiency and vitamin D receptor gene polymorphism are associated with increased risk of cardiovascular disease in a Chinese rural population. Nutr Res 61:13–21
Acknowledgements
This study was funded by NIMAD, Iran; Grant #958709.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiani, A., Mohamadi-Nori, E., Vaisi-Raygani, A. et al. Vitamin D-binding protein and vitamin D receptor genotypes and 25-hydroxyvitamin D levels are associated with development of aortic and mitral valve calcification and coronary artery diseases. Mol Biol Rep 46, 5225–5236 (2019). https://doi.org/10.1007/s11033-019-04979-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04979-1