Abstract
Hybridizing of different antimicrobial peptides (AMPs) has been a common practice for obtaining novel hybrid AMPs with elevated antibacterial activity but minimized cytotoxicity. The hybrid peptides melittin (1-13)-LL37 (17-30) (M–L) combining the hydrophobic N-teriminal fragment of melittin (M) with the core antibacterial fragment of LL37 (L), was designed for the first time to explore its antibacterial activity and hemolytic activity against bacteria and sheep erythrocyte respectively. Results showed that M–L had an even more potent antibacterial activity against all indicator strains (especially gram-positive bacteria) than M and L, whereas didn’t exhibit hemolytic activity to sheep erythrocytes, implying M–L can be served as a potential therapeutic drug to substitute traditional antibiotics. However the high expense of biosynthesis limited its further research, therefore fusion expression of M–L was carried out in Escherichia coli (E. coli) for overproducing the hybrid peptide so as to solve the problem. The DNA sequence encoding M–L with preferred codons was cloned into the pET-SUMO vector for protein expression in E. coli BL21 (DE3). After IPTG induction, approximately 165 mg soluble fusion protein SUMO-M–L was recovered per liter supernatant of the fermentation ultrasonic lysate using Ni–NTA Sepharose column (92 % purity). And 23 mg recombinant M–L was obtained per liter culture after cleavage of SUMO protease and purification of Ni–NTA Sepharose column. In sum, this research not only supplied an effective approach for overproducing hybrid peptide M–L, but paved the way for its further exploration on pharmaceutical potential and medical importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the increasing microbial resistance to conventional antibiotics has been a major concern worldwide, leading to more effects to develop new safe therapeutic compounds [1, 2]. Antimicrobial peptides (AMPs) are a kind of short amphipathic and cationic polypeptides distributed in nature widely [3–26]. Many literatures have reported that AMPs were important active agents which not only provided a major defense mechanism in bacteria, plants and lower animals before the mobilization of specific immunity, but also inhibited parasitic growth immediately after invasion of the host by microbial agents [3–16]. Moreover, AMPs were reported to be less prone to drug resistance because their mechanism were largely related to the interaction with bacterial cell membrane through several models including “pore-forming”, “barrel-stave”, “carpet” model and so on [8–10], which were completely different from traditional antibiotics targeting five biosynthetic processes of proteins, RNA, DNA, peptidoglycan and folic acid occurring in active growing bacteria [10]. Therefore, AMPs have been recognized as a possible source of pharmaceuticals for the treatment of antibiotic-resistant bacterial infections and immunological disease [12–15].
Melittin (M) isolated from bee venom, is a small linear helical peptide composed of 26 amino acid residues with multiply effects including antibacterial, anti-inflammation, anticancer and so on [16–19]. However, it also has the undesirable property of cytotoxicity to red blood cells at a lower concentration (2 μM) which largely limits its widely application in clinic [16–19]. Thus, a series biochemical approaches have been applied to minimize the cytotoxicity of M including deletion or truncation, substitution at one or more positions and hybridization of different AMPs [16–24]. Among these, hybridization of different AMPs has been a common practice for obtaining novel AMPs with improved properties [20–24]. As reported previously, the hydrophobic N-terminal fragment of M played a profound role for its biological activity, because it not only enabled M to interact with bacteria cell membrane but also governed the extent to which M can partition into the lipid bilayer [25]. So, the fragment was usually used to combine with fragment derived from other AMPs to form novel hybrid peptides with elevated antibacterial activity and minimized cytotocixity, such as HP (2–9) M (1–12) [20], CA (1–8) M (1–12) [21, 22], CA (1–13) M (1–13) [22] and CA (1–7) M (2–9) [23, 24].
LL37 (L), lineal α-helical peptide with 37 amino acid residues isolated from human leukocytes and epithelia, possesses potent antimicrobial activity at low micromolar concentrations (MIC < 10 mM) against a variety of gram-positive and gram-negative bacteria, but also exhibits hemolytic activity against eukaryotic cells by inducing membrane disruption at a slightly higher concentration (25–30 mM, which is three to five times its MIC value) [19, 25–29]. It was believed that the C-terminal helix corresponding to amino acid residues 17–29 is responsible for the antimicrobial, anticancer and antiviral effect [26, 30, 31]. But, there was scarce evidence about it in hybridizing new AMPs.
In this study, we proposed the hypothesis that the combination of the core antimicrobial fragment of L and the N-terminal fragment of M will amplify antibacterial activity and minimize cytotoxicity compared to template peptides by forming more helical structure in sequence, enhancing the interaction with cell membrane and consequently inserting into cell membrane deeply. Hence, hybrid peptide M–L derived from L (17–30) and M (1–13) was synthesized to investigate its bioactivity. Additionally, the high expense of peptide synthesis limited the further research about its action mechanism and therapeutic application in hospital and feed industry. Therefore, the expression of M–L was also carried out in E. coli using small ubiquitin-like modifier (SUMO) fusion expression system to supply theoretical basis for widely application.
Materials and methods
Bacterial Strains, plasmids and enzymes
Six pathogenic microorganisms were obtained from China Veterinary Culture Collection (CVCC, China). They were Escherichia coli (E. coli) K88, E. coli CVCC245, Staphylococcus aureus (S. aureus) CVCC26003, S. aureus ATCC25923, Micrococcus luteus (M. luteus) CVCC B28001 and Listeria monocytogenes (L. mono.) CVCC1599. The plasmid pET SUMO (Invitrogen, USA) was used as cloning vector. E. coli DH5α and BL21 (Novagen, USA) were cultured and selected in Luria–Bertani (LB) broth containing 50 μg kanamycin (kana) per milliliter at 37 °C as the host for gene manipulation and expression of fusion protein respectively. DNA restriction enzymes, T4 DNA ligase, Taq DNA polymerase and SUMO protease were purchased from Invitrogen (USA).
Design, biological information analysis and synthesis of hybrid peptides
Hybrid peptides derived from selected parental peptides were designed using APD2 (http://aps.unmc.edu/AP/main.php). Protein secondary structure was predicted by Scratch (http://scratch.proteomics.ics.uci.edu/cgi-bin/new_server/sql_predict.cgi) and Jpred 3 (http://www.compbio.dundee.ac.uk/www-jpred/index.html) online. The selected hybrid peptide and parental peptides were synthesized by 9-fluorenylmethoxycarbonyl solid-phase synthesis chemistry and purified by a reverse-phase semi-preparative high performance liquid chromatography (HPLC) (SBS, China) and then stored at −80 °C prior to analysis.
Antibacterial assay
The antibacterial activities were determined as minimal inhibitory concentrations (MICs). Bacterial strains at exponential phase were diluted to the concentration of 5 × 105 cfu/ml with muellerhinton (MH) broth medium, and 180 μl was dispensed per well into 96-well microtiter plate. Susceptibility tests were performed by twofold standard broth microdillution of the test peptides (M–L, M and L) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. Aliquots (20 μl) of each peptide dilution were mixed with 180 μl bacterial suspensions, and the mixture was assayed with Bio-Rad 3700 plate reader by monitoring optical density at 630 nm after 16–18 h incubation at 37 °C. The lowest concentration (highest dilution) required to prevent the growth of bacteria was regarded as MICs [15]. Experiments were performed in triplicate.
Hemolytic assay
The hemolytic activities (HC50) were evaluated by measuring the peptides concentrations that caused 50 % hemolysis of sheep erythrocytes at absorbance of 540 nm (A 540 nm) [32]. Sheep erythrocytes added to 10 mM phosphate-buffered saline (PBS) and 0.1 % (v/v) Triton X-100 (Sigma) were used as negative and positive control respectively. Experiments were performed in triplicate.
PCR amplification and construction of expression vectors
The M–L gene with proper codon for E. coli (http://www.kazusa.or.jp/codon) was synthesized (Invitrogen) and cloned into pMD18-T (TaKaRa, Japan). Plasmid DNA was isolated using a TIAN prep Midi Plasmid Kit (Tiangen, China). The 90 bp gene of M–L was amplified using Taq DNA polymerase (TaKaRa) and primer pairs (Primer F: 5′-GGCATTGGCGCGGTGCT-3′; Primer R: 5′-TCACTATTAGTTGCGCGAAAATCTT-3′). The PCR conditions were 30 cycles of 30 s at 94 °C, 30 s at 54 °C, 45 s at 72 °C after denaturing for 5 min at 94 °C. The PCR products were separated by 2 % gel electrophoresis, purified with a DNA gel extraction kit (Tiangen), and inserted into the linearized pET SUMO plasmid (Invitrogen) by TA cloning using T4 DNA Ligase (TaKaRa). The ligation mixture was transformed into E. coli Mach1™-T1R cells and the recombinant plasmid was verified by PCR amplification and sequencing.
Expression and purification of SUMO-M–L fusion protein
The pET SUMO-M–L plasmid was transformed into competent E. coli BL21 (DE3). The recombinant expression strain was cultivated in LB broth containing 50 μg kana per milliliter (1 % glucose) at 37 °C with shaking (200 rpm) to an optical density (OD600) of 0.6–0.8. Isopropyl β-d-1-thiogalactopyranoside (IPTG) (1.5 mM) was then added to induce the expression of the recombinant fusion protein at 37 °C for 4 h. The cells were harvested by centrifuging at 13,000×g for 2 min, and then resuspended in lysis buffer (50 mM K3PO4, 400 mM NaCl, 100 mM KCl, 10 % glycerol, 0.5 % Triton X-100, 10 mM imidazole, pH 7.8), and disrupted on ice by sonication at 200 W for 45 cycles (2 s working, 8 s free). Afterwards to centrifuge at 13,000×g for 2 min, the supernatant was collected and subjected to purification using a 5 ml Ni–NTA Sepharose column (GE, USA). The eluted fractions were analyzed by Tricine-SDS-PAGE, and dialyzed overnight at 4 °C against water. And the fusion protein content was quantified by bicinchoninic acid (BCA) method with three replicates [33, 34].
Cleavage of SUMO-M–L fusion protein and purification of the recombinant M–L
The SUMO-M–L protein was reacted with SUMO protease (Invitrogen) in 500 μl 10× SUMO protease buffer at 30 °C for 3 h. The recombinant M–L was obtained after the purification of the cleavage mixture in Ni–NTA Sepharose column. The penetrated fractions were analyzed by Tricine-SDS-PAGE.
Results and discussion
Hybridization of different AMPs has been a common practice in improving antibacterial activity and reducing undesirable cytotoxic property [25–29]. In the present research, hybrid peptides M–L were synthesized by chemical method successfully. As shown in Table 1, the synthesized M–L was found to have the correct amino acid sequence and expected molecular weight by MALDI mass spectrometry, and then subjected to antibacterial and hemolytic assay (Table 2).
The antibacterial and hemolytic activities assays were determined as MIC and HC50 using micro-broth dilution method [18]. Results showed that all six indicator were more susceptible to M–L (MIC range 0.95–6.8 μg/ml) than M (MIC range 4.0–28.8 μg/ml) and L (MIC range 12.8–89.6 μg/ml); whereas M–L did not exhibit cytotoxicity (HC50 > 132 μg/ml was determined as no cytotoxicity) in comparison with M (HC50 = 8 μg/ml) and L (HC50 = 32 μg/ml), which were in agreement with the observations in HP (2-9)-M (1-12) (MIC range 0.39–12.5 μg/ml; HC50 > 132 μg/ml) [20], Mdc-hly (MIC range 0.39–6.25 μg/ml) [35], CA (1-8)-M (1-12) (MIC range 0.78–12.5 μg/ml; HC50 = 25 μg/ml) [22, 23] and CA(1-7)-M (2-9) (MIC range 0.25–8 μg/ml) [36], indicating that M–L was as active as above hybrids in antibacterial property.
Generally, a broad range of AMPs derive antimicrobial activity from their structural and physicochemical properties. In present research, the increased antibacterial activity and minimized cytotoxicity of hybrid peptide M–L may be attributed to changes in structure. As the secondary structure type for each residue predicted by Scratch and Jpred (Fig. 1) showed that M–L had higher propensity for helical structure than M and L, particularly in the prediction of Scratch servers, 23 amino acids of M–L took the form of helical structure accounting to 85.19 % which was greater than both M (76.92 %) and L (72.90 %). It was believed that the increased α-helices structure was correlated with stronger antimicrobial activity by allowing AMPs to interact with negative charged membrane potently [10, 14, 18]. Besides, many literatures reported that the proposed antibacterial mechanism of both M and L were pore forming [25, 37]. Hence, we concluded that the elevated helical structure of M–L may broaden the diameter or prolong the duration of pore, resulting in quickly leakage of cell contents, and consequently stronger antibacterial effect.
Recently, several biological systems have been developed in bacterial system for the high-production of AMPs. But due to its antibacterial activity to host cells and sensitive to proteases, fusion expression is commonly applied [38, 39] by conjoining a partner to the N-terminal of AMPs. There are various fusion partners including green fluorescent protein (GFP) [40], inteins [41], neutrally charged polypeptide F4 [42], thioredoxin [43], SUMO [44] and so on. Among these fusion partners, SUMO has been proved to be one of the most effective partners, because it not only has the advantage to facilitate purification of the fusion protein with His6-tag in N-terminal, but also can be removed by SUMO protease specifically, allowing the recombinant protein retain the native N-terminus as a result which is a critical feature to antimicrobial activity [45]. In our study, pET-SUMO expression vector with stronger T7 promoter and SUMO-tag, whose effectiveness had been proved in overproducing AMPs including defensin [46], CM [47] and LQ [48], was applied to express hybrid M–L in E. coli expression system.
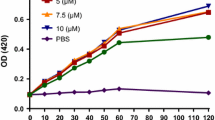
As shown in Fig. 2, the expression plasmid pET-SUMO-M–L which encoding SUMO-M–L transcribed under the control of T7 promoter, was transformed into E. coli BL21 (DE3) for expression. The resultant E. coli BL21 transformants were cultured in LB medium to OD600 0.6-0.8, and then induced with 1.5 mM IPTG for 4 h to express SUMO-M–L. As shown in Fig. 3, an obvious protein band on the gel with a molecular mass of about 17 kDa was detected (calculated molecular mass, 15.2 kDa) during the 4 h of IPTG induction, and the maximum fusion protein was observed at 4 h, constituting up to 37.8 % of all soluble proteins in the ultrasonic lysate supernatants of E. coli BL21/pET-SUMO-M–L transformant (determined by Bandscan 5.0 software (Glyko, Novato, CA, USA).
SDS-PAGE analysis of bacteria lysate in 1, 2, 3, 4 h after 1.5 mM IPTG induction. Lane M, the molecular weight of marker; lane 1, supernatant of non-induced bacteria lysate; lanes 2, 3, 4, 5, 6 are supernatant of 1.5 Mm TPTG-induced bacteria lysate for 0, 1, 2, 3, 4 h respectively. 15 μl samples were loaded onto a 16 % polyacrylamide gel. The arrows indicated fusion protein SUMO-M–L. The molecular weight of fusion protein was approximately agreed with the predicted
The soluble fusion protein SUMO-M–L with His6-tag enabled the affinity purification through Ni–NTA Sepharose affinity column. SDS-PAGE staining with coomassie blue (Fig. 4, lane 1) exhibited that the SUMO-M–L was purified to 92 % (determined by Bandscan 5.0 software (Glyko, Novato, CA, USA). And the content of SUMO-M–L was approximately 165 mg per liter ultrasonic lysate supernatant (tested by BCA method) [33, 34], which was particularly higher than 15.3 mg Trx-Mdc-hly and 32 mg UBI-CA-MA2 using thioredoxin and ubiquitin fusion partner in E. coli respectively [48, 49].
SDS-PAGE analysis of purification of fusion protein SUMO-M–L and the recombinant hybrid peptide M–L. Lane M, the molecular weight marker: Lane 1, the purified fusion protein SUMO-M–L; Lane 2, the purified recombinant hybrid peptide M–L. 20 μl samples were loaded onto a 16 % polyacrylamide gel. Arrow a and b indicated the purified fusion protein SUMO-M–L and recombinant M–L. The molecular weight of the target protein agreed well with the predicted
It was well known that SUMO tag in fusion protein can be efficiently cleaved by SUMO-protease. Therefore, after 3 h digestion of SUMO-M–L with SUMO-protease at 30 °C, approximately 23 mg/L recombinant M–L was affinity purified from the cleavage solution through Ni–NTA Sepharose affinity column, which was also extremely higher than 4.2 mg/L CM4 [42] and 11.2 mg/L cecropin-maganine [49] using intein mediated system and thioredoxin in E. coli respectively, indicating that the pET-SUMO vector was capable to accomplish overproduction of AMPs. SDS-PAGE analysis also confirmed the purification of M–L. As shown in Fig. 4, an obvious protein band (lane 3) on the gel with a molecular mass of about 3.4 kDa was detected in the penetrated liquid (calculated molecular mass, 3.0 kDa). The deviation of molecular mass in SDS-PAGE analysis may contribute to the quality of gel and the parameter of SDS-PAGE. To be notable, the exact molecular weight of M–L was 3,059.91 Da determined by MALDI-TOF (Fig. 5), which was basically consistent with calculated value of 3,056.6 Da.
In conclusion, a novel hybrid peptide M–L was designed and testified as active as other hybrids in antibacterial activity, but did not exhibit cytotoxicity. Moreover, a effective approach was created for overproducing recombinant M–L using SUMO fusion expression system in E. coli, which paved the way for widely application in practice. But up to now, we mainly went in for preliminary study on antibacterial activity, whether the hybrid peptide M–L will deserve an opportunity as clinical alternatives against the rising threat of widespread dissemination of panresistant, (including colistin), it is necessary to carry out further exploration on antibacterial activity against acinetobacter baumannii strains and multidrug resistant strains, action mechanism and efficacy in vivo prior to application.
References
Hwang I-S, Hwang J-S, Hwang JH, Choi H, Lee E, Kim Y, Lee DG (2013) Synergistic effect and antibiofilm activity between the antimicrobial peptide coprisin and conventional antibiotics against opportunistic bacteria. Curr Microbiol 66(1):56–60
Lu XM, Jin XB, Zhu JY, Mei HF, Ma Y, Chu FJ, Wang Y, Li XB (2010) Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl Microbiol Biotechnol 87(6):2169–2176
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Bowles DJ (1990) Defense-related proteins in higher plants. Annu Rev Biochem 59:873–907
Brown KL, Hancock REW (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18(1):24–30
Zaiou M (2007) Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med 85(4):317–329
Boman HG (1995) Peptide antibiotics and their roles in innate immune. Annu Rev Immunol 13:61–92
Bonucci A, Balducci E, Pistolesi S, Pogni R (2013) The defensin–lipid interaction: insights on the binding states of the human antimicrobial peptide HNP-1 to model bacterial membranes. Biochim Biophys Acta 1828(2):758–764
Reddy KVR, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66(4):236–248
Finlay BB, Hancock RE (2004) Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol 2:497–504
Hancock RE, Patrzykat A (2002) Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets-Infectious Disord 2:79–83
Hancock REW, Sahl H-G (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Hwang IS, Hwang JS, Hwang JH, Choi H, Lee E, Kim Y, Lee DG (2013) Synergistic effect and antibiofilm activity between the antimicrobial peptide coprisin and conventional antibiotics against opportunistic bacteria. Curr Microbiol 66(1):56–60
Kreil G, Bachmayer H (1971) Biosynthesis of melittin, a toxic peptide from bee venom detection of a possible precursor. Eur J Biochem 20:344–350
Habermann E, Jentsch J (1967) Sequence analysis of melittin from tryptic and peptic degradation products. Hoppe Seylers Z Physiol Chem 348:37–50
Shin SY, Lee MK, Kim KL, Hahm KS (1997) Structure–antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J Pept Res 50:279–285
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta 1778(2):357–375
Hyung KK, Dong GL, Yoonk YP, Hee NK, Bo HC, Cheol HC, Hahm KS (2002) Antibacterial activities of peptides designed as hybrids of antimicrobial peptides. Biotechnol Lett 24(5):347–353
Lee DG, Shin SY, Maeng CY, Hahm KS (1998) Cecropin A-melittin hybrid peptide exerts its antifungal effects by damaging on the plasma membranes of Trichosporon beigelii. Biotechnol. Lett. 20(3):211–214
Shin SY, Rang JH, Hahm KS (1999) Structure-antibacterial, antitumor and hemolytic activity relationships of cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J Pept Res 53:82–90
Saugar JM, Rodriguez-Hernandez MJ, de la Torre BG, Pachon-Ibanez ME, Fernandez-Reyes M, Andreu D, Pachon J, Rivas L (2006) Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50(4):1251–1256
Bastos M, Bai G, Gomes P, Andreu D, Goormaghtigh E, Prieto M (2008) Energetics and partition of two cecropin–melittin hybrid peptides to model membranes of different composition. Biophys J 94(6):2128–2141
Cowland JB, Johnsen AH, Borregaard N (1995) HCAP-18, a cathelin/probactenecin-like protein of human neutrophil specific granules. FEBS Lett 368:173–176
Vandamme D, Landuyt B, Luyten W, Schoofs L (2012) A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell Immunol 280:22–35
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI (1998) Activities of ll-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 42(9):2206–2214
Lai YP, Gallo RL (2008) AMPed up immunity: how antimicrobial peptides have muliple roles in immune defense. Trends Immunol 30(3):131–141
Splith K, Neundorf I (2011) Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J 40(4):387–397
Li X, Li Y, Han H, Miller DW, Wang G (2006) Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc 128:5776–5785
Wang G (2008) Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283:32637–32643
Tian ZG, Dong TT, Teng D, Yang YI, Wang JH (2009) Design and characterization of novel hybrid peptides from LFB15(W4,10), HP(2-20), and cecropin A based on structure parameters by computer-aided method. Appl Microbiol Biotechnol 82(6):1097–1103
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 50(1):76–85
Osnes T, Sandstad O, Skar V, Osnes M, Kierulf P (1993) Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand J Clin Lab Invest 53(7):757–763
Lu XM, Jin XB, Zhu JY, Mei HF, Ma Y, Chu FJ, Wang Y, Li XB (2010) Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl Microbiol Biotechnol 87(6):2169–2176
Andrea G, Oscar C, Wojciech K, Giuseppina D’A, Carmela S, Maria SDP, Jerzy Ł, Giorgio Scalise (2003) Comparative activities of cecropin A, melittin, and cecropin A–melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24:1315–1318
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide ll-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(3):501–513
Beaulieu L, Groleau D, Miguez CB, Jetté JF, Aomari H, Subirade M (2005) Production of pediocin PA-1 in the methyl-otrophic yeast Pichia pastoris reveals unexpected inhibition of its biological activity due to the presence of collagen-like material. Protein Expr Purif 43:111–125
Yu F, Wang J, Zhang P, Hong Y, Liu W (2010) Fusion expression of cecropin B-like antibacterial peptide in Escherichia coli and preparation of its antiserum. Biotechnol Lett 32:669–673
Kishimoto K, Fujimoto S, Matsumoto K, Yamamo Y, Morishima I (2002) Protein purification, cDNA cloning and gene expression of attacin, an antibacterial protein, from eri-silkworm, Samia cynthia ricini. Insect Biochem Mol Biol 32(8):881–887
Chen YQ, Zhang SQ, Li BC, Qiu W, Jiao B, Zhang J, Diao ZY (2008) Expression of a cytotoxic cationic antibacterial peptide in Escherichia coli using two fusion partners. Protein Expr Purif 57(2):303–311
Lee JH, Kim JH, Hwang SW, Lee WJ, Yoon HK, Lee HS, Hong SS (2000) High-level expression of antimicrobial peptide mediated by a fusion partner reinforcing formation of inclusion bodies. Biochem Biophys Res Commun 277(3):575–580
Tenno T, Goda N, Tateishi Y, Tochio H, Mishima M, Hayashi H, Shirakawa M, Hiroaki H (2004) Highthroughput construction, method for expression vector of peptides for NMR study suited for isotopic labeling. Protein Eng Des Sel 174:305–314
Raymond J, Peroutka III, Orcutt Steven J, Strickler James E, Butt Tauseef R (2005) SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes heterologous gene expression in E. coli. Methods Mol Biol 705:15–30
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif 43:1–9
Bommarius B, Jenssen H, Elliott M, Kindrachuk J, Pasupuleti M, Gieren H, Jaeger KE, Hancock REW, Kalman D (2010) Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides 31:1957–1965
Li JF, Zhang J, Song R, Zhang JX, Shen Y, Zhang SQ (2009) Production of a cytotoxic cationic antibacterial peptide in Escherichia coli using SUMO fusion partner. Appl Microbiol Biotechnol 84:383–388
Ma Q, Yu Z, Han B, Wang Q, Zhang R (2012) Expression and purification of lacticin Q by small ubiquitin-related modifier fusion in Escherichia coli. J Microbiol 50(2):326–331
Xu X, Jin F, Yu X, Ren S, Hu J, Zhang W (2007) High-level expression of the recombinant hybrid peptide cecropinA(1–8)–magainin2(1–12) with an ubiquitin fusion partner in Escherichia coli. Protein Expr Purif 55(1):175–182
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 31272476) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110008110002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, R., Wang, Q., Zheng, Z. et al. Design, characterization and expression of a novel hybrid peptides melittin (1–13)-LL37 (17–30). Mol Biol Rep 41, 4163–4169 (2014). https://doi.org/10.1007/s11033-013-2900-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2900-0