Abstract
In both yeast and mammals, the major constituent of the endosomal sorting complex required for transport-II (ESCRT-II) is the VPS22/EAP30 protein, which plays an important role in ubiquitin-mediated degradation of membrane proteins through the multivesicular body pathway. However, the functions of ESCRT-II subunits in plants are largely unknown. In this work, we report the genetic analysis and phenotypic characterization of mutants in OsVPS22 gene, which encodes a functional VPS22 homolog in rice. On the basis of a collection of T-DNA lines, we identified a T-DNA insertion mutant, which showed abnormal segregation ratios; we then found that the T-DNA insertion is located within the sixth intron of the OsVPS22 gene. Compared with the wild type, this vps22 mutant exhibited seedling lethality and severe reduction in shoot and root growth. In addition, the vps22 mutant had a chalky endosperm in the grain. In summary, our data suggest that OsVPS22 may be required for seedling viability and grain filling in rice, thus providing a valuable resource for further exploration of the functions of the ESCRTing machinery in plants.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown that endosomes regulate both the recycling and degradation of plasma membrane (PM) proteins, and they play crucial roles in protein degradation, cytokinesis, endocytosis, vacuolar transport and intracellular signaling [1, 2, 3, 4, 5, 6]. Ubiquitination of PM proteins is a major signal for sorting into multivesicular endosomes and multivesicular bodies by the endosomal sorting complexes required for transport (ESCRTs) [7, 8], which regulate protein trafficking from endosomes to lysosomes. The ESCRT core is composed of three subcomplexes (ESCRT-I to ESCRT-III), which actually perform three distinct but connected functions [9, 10, 11, 12]. The molecular mechanisms of ESCRT-mediated sorting for endosomal and lysosomal degradation are now beginning to emerge; in particular, the isolation of vacuolar protein sorting (vps) mutants in yeast has identified a conserved mechanism for multivesicular body sorting [13]. ESCRT-II, which consists of three subunits (including VPS22, VPS25 and VPS36), is acting as a molecular hub for ESCRT assembly on the endosomal membrane [14, 15, 16]. Plants contain at least one sequence homolog for each ESCRT-II component identified in yeast and mammals, suggesting that these ESCRT-II subunits and their functions are conserved among eukaryotic cells [6]. Although two recent studies provided protein–protein interaction network of Arabidopsis VPS22, an approximate 30 kDa protein containing two predicted coiled-coil domains, which interacts with other components of ESCRT machinery [17, 18], the biological functions of VPS22 have remained largely elusive in plants.
Although rice grain appearance is one of the most important criteria of rice quality, chalky grains have a negative impact on the quality [19, 20]. Chalky grains were categorized into white-cored, milky-white, white-back, white-based, and white-belly types depending on the location of the chalky part in the grain [19, 21, 22]. White-cored grains have chalkiness in the centre of the endosperm, while milky-white grains have a wider part of chalkiness, compared to the white-cored grains [19]. White-based and white-back grains have chalkiness at the basal part and dorsal part of the endosperm, respectively [19]. These differences in the development of the chalky part in grains were considered to be closely related to differences in the patterns of starch accumulation [19, 21]. It was reported that high-temperature stress during grain ripening might result in the formation of chalky grains [19]. In addition, the physiochemical characteristics of chalky rice kernels were confirmed to be associated with genetic factors. Recently, six severe chalky grain mutants were screened from T-DNA insertion mutant pool in rice, and all the mutants were found to have higher contents of fructose, sucrose, and total soluble sugar than the wild-type rice plants [23].
In this study, we identified a rice vps22 mutant generated by T-DNA insertional mutagenesis with white-core grains and a seedling-lethal phenotype. Additionally, we analyzed the genetic and phenotypic characteristics of the vps22 mutant, and we discussed the potential mechanisms for the formation of white-cored and seedling lethality.
Materials and methods
Plant materials and growth conditions
The materials used for transformation were Oryza sativa L. ssp. Japonica cultivar Zhonghua 11. T1 progenies of transformed parental plants were provided by Shanghai Institute of Plant Physiology and Ecology (SIPPE), Chinese Academy of Sciences. The Ti plasmid pDs-Bar1300 as the destination vector for genetic transformation carried maize Ds transposon with an inserted Bar gene, which can confer resistance to commercial herbicide Basta including an effective component of phosphinothricin (PPT). The materials of individual progeny were planted at the Teaching & Experimental Farm of South China Agricultural University. T2 seeds from independent T1 plant was individually harvested and were planted according to the corresponding lines.
To observe the phenotype of mutant seedlings, dehusked rice seeds were sterilized and placed in three separate plates. There were at least 12 wild-type and 12 vps22 mutant seedlings in every plate. The seeds were dark-cultivated on MS medium for 3 days at 28 °C, and then treated 12 h of light and 12 h of darkness at 28 °C. The survival and growth of the mutant seedlings was investigated at 4 and 5 days after germination, respectively.
Identification of Basta resistant rice plants
The rice plants at the tillering stage were checked for Basta resistance by smearing one leaf of each plant with herbicide Basta solution. On a sunny day, the healthy leaves with no disease spot at upper portion of plants were smeared with herbicide Basta solution (0.2 % v/v) using Chinese writing brush, and then these treated leaves were marked with a marker pen at the leaf tips. The reaction of plants to Basta was recorded after 5 days. The individual plants with normal leaves were regarded as the resistant plants, while the ones with yellowish or even withered leaves showed sensitive.
Isolation and analysis of flanking sequences of T-DNA
Genomic sequences flanking the T-DNA insertions were isolated by TAIL-PCR [24]. The specific primers were designed using the prime program of primer premier 5. The primers used for TAIL-PCR are shown in Table 1.
The inserting position of T-DNA in rice genome was determined using BLAST (http://www.ncbi.nlm.nih.gov/BLAST), and the gene annotation was gained from rice genome database (http://rapdb.dna.affrc.go.jp/).
Genotypic analysis of T-DNA insertion site
Genotyping of the T2 progeny was performed by PCR. The primer combination of T48F (CGACTTGCTCTACGGTGACA) and T48R (ATGATTGTTTGCGTGCG ATA), was designed in terms of borders and flanking sequences of T-DNA, and employed to amplify the sequence between the two primer binding sites of rice genome, and then a fragment of 625 bp was obtained. In addition, a primer of LB1 (detailed in Table 1) was designed at end of inner T-DNA to be paired with T48R for the PCR amplification, and a specific band of 465 bp was obtained. The primer combinations of T48F, T48R and LB3 (detailed in Table 1) were used to determinate endosperm genotypes, and the specific band of 385 bp was amplified using the primer pair, LB3 and T48-R.
The DNA extraction of leaf and endosperm was conducted using a convenient method. The protocol was carried out as follows: a small piece of endosperm and leaf tissue ground to a fine powder (approximately 10 mg) was incubated with 1000 μl of buffer at 85 or 75 °C for 30 or 60 min, respectively. Following centrifugation at 12, 000 rpm for 10 min, 500 μl of the supernatant was transferred to fresh tubes and the DNA was precipitated with 500 μl of ethanol. DNA was resuspended in 50 or 200 μl of double-distilled water. Extraction buffer: 100 mM Tris–HCl at pH 8.0, 10 mM EDTA at pH 8.0, 1 M KCl.
PCR was performed as follows: 94 °C 5 min, for pre-denaturation; 35 cycles of 94 °C 1 min, 55 °C 1 min, 72 °C 1.5 min; 72 °C 5 min, for final extension. The 20 μl reaction system [1× PCR buffer (Mg2+), 200 μM of each dNTP, 1U Taq enzyme, 0.2 μM of forward and reverse primers and 50–100 ng genomic DNA] was used for amplification.
Results
Mutant lines with abnormal genetic behavior
The T-DNA tagging lines were generated in rice (Oryza sativa L. ssp. japonica cv. Zhonghua 11) by the binary vector pDsBar1300, which provided the resistance to the herbicide Basta. The ratio of resistance to sensitivity should be 3:1 in Basta resistance assay for a single T-DNA insertion. However, the result showed that the Basta resistant plants derived from the T1-48 transgenic lines were fewer, and there were 19 Basta sensitive plants and 40 Basta resistant plants in 59 wild-type plants. The ratio of resistance to sensitivity was 40:19, near the segregation ratio of 2:1 (Table 2). In 551 tested plants of T2 progeny, the 376 plants were Basta resistant, while 175 plants were Basta sensitive. The ratio of Basta resistant to Basta sensitive plants was approximately 2:1 as well (Table 2). Why did the abnormal ratio show? We speculate that the T-DNA insertion resulted in the lethal effects of homozygosity, so the abnormal segregation ratio demonstrated.
Characterization of the T-DNA insertion site
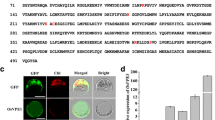
In order to identify the mutated gene, we isolated the genomic DNA sequence flanking T-DNA from the Basta resistant plants using the TAIL-PCR method [24]. This retrieved sequence was then analyzed with the rice genome database and NCBI to identify the tagged gene. BLAST analysis showed that the T-DNA was inserted in the Os09g0529700 gene, referred to as OsVPS22, which is located at the BAC clone AP005682 on chromosome 9. Our comparison of the genomic sequence with the full-length OsVPS22 cDNA clone (AK243545), at KOME (http://www.cdna01.dna.affrc.go.jp), indicated that its primary structure comprises eight exons and seven introns (Fig. 1). The full length genomic sequence of the OsVPS22 gene is 3672 bp and its full length cDNA (AK243545) is 1,652 bp. The T-DNA was inserted into the sixth intron (246 bp upstream of exon seven) of the OsVPS22 gene. The OsVPS22 gene encodes a predicted protein of 252 amino acids that is a homolog of the Vps22 subunit of the yeast ESCRT-II complex.
Structure of the OsVPS22 gene and positions of the T-DNA insertion in the mutant. The OsVPS22 gene contains eight exons and seven introns with the T-DNA inserted into the sixth intron. The untranslated regions are shown in gray rectangle, the exons in black rectangle, and the introns in thick black line. The T-DNA was inserted in the 246 bp upstream region of the seventh exons of OsVPS22 gene. LB T-DNA left border; RB T-DNA right border. Ds 5′: the 5′ end of Ds transposon; Ds 3′: the 3′ end of Ds transposon; Bar Basta resistant gene; LB1 and LB3 binding sites of primers used in PCR (Table 1 for details)
Genotypic analysis of the T-DNA insertion site
Three primers were designed in terms of borders and flanking sequences of T-DNA, in which T48F and T48R were employed to amplify the sequence between the two primer binding sites of rice genome, and then a fragment of 625 bp was produced. In addition, the LB1 primer within T-DNA terminal sequence was designed, and then paired with T48R, and finally a specific band of 465 bp was amplified using the primer pair. According to the numbers and sizes of PCR fragments amplified by two combinations of three primers, the wild type, heterozygous and homozygous T-DNA insertion lines can be identified.
One of the original lines, T1-48, was further self-crossed, and then three Basta resistant progenies were subjected to PCR analysis to determine the genotype of T-DNA insertion site. At seedling stage in rice, 551 T2 plants were examined, and two types of bands were produced (Fig. 2). All Basta sensitive plants had the band of 625 bp, belonging to wild-type plants, and the Basta resistant plants had both bands of 625 and 465 bp, belonging to heterozygous T-DNA insertion plants. The ratio of heterozygous T-DNA insertion plants to wild-type plants was 376:175. Chi square analysis confirmed the 2:1 (heterozygous/wild-type plants) segregation ratio, suggesting that all of the surviving Bast-resistant plants were heterozygous for the OsVPS22 knockout allele. The genotype determination of T-DNA insertion site was consistent with the Basta resistance assay (Table 2). This confirmed that all of the surviving Basta resistant lines were heterozygous for the OsVPS22 knockout. These lines were followed by PCR for three more generations and no homozygous knockout lines were identified. This genetic analysis indicated that the homozygous T-DNA insertion mutants in OsVPS22 were likely lethal.
There exist two putative reasons for lethal phenotype of the vps22 mutants: embryo-defective or seedling lethality. Quantitative and visual examination of the heterozygous OsVPS22 knockout lines revealed no detectable phenotypic difference, including the number of panicles per plant, the number of spikelets per panicle, and the seed setting rate, from those of the wild-type plants (data not shown). We postulated that the OsVPSS22 mutation would fall into the group of seedling lethality. In order to test this postulation, we detected the genotype of rice seeds. Two hundred and seven seeds derived from Basta resistant individuals were analyzed by PCR. The primer combinations of T48F, T48R and LB3 were used to determinate endosperm genotypes of rice, and the specific band of 385 bp was amplified using the primer pair LB3 and T48R. The results showed that the seeds from Basta resistant plants were divided into three types, based on its genotypes (Fig. 3): T-DNA insertion homozygote (Fig. 3, A, B), heterozygote (Fig. 3, D) and wild-type (Fig. 3, C), respectively. Of the 207 seeds derived from Basta resistant individuals, 43 appeared to be homozygous for the T-DNA insertion, suggesting that the seeds of homozygous knockout mutants were obtained from the harvested population. This is consistent with the hypothesis that the OsVPS22 mutation is seedling-lethal.
Phenotype analysis of the rice vps22 mutant
To determine the effect of the OsVPSS22 mutation on endosperm development, we investigated the seed appearances and the transverse sections of grains to reveal the endosperm traits. The central portion of the dehusked seeds of mutants showed the endosperm with a dark central portion (Fig. 4B), whereas the wild-type control seeds showed a transparent normal endosperm on an illuminator (Fig. 4A). Notably, in the mutant endosperms, a dark central portion is commonly evident and is characteristic of a floury white-core endosperm. Transverse section of the mature seeds of rice mutant further revealed that the central portion of the endosperm showed floury or floury-like phenotype (Fig. 4D), whereas the seeds of wild-type rice plants showed normal development (Fig. 4C).
Morphology of the endosperm in the vps22 mutants and wild-type. A Dehusked seeds of wild-type rice plants, B dehusked seeds of rice vps22 mutants, with dark speck, C transverse section in the endosperm of wild-type rice plants, D transverse section in the endosperm of rice vps22 mutants, with central-chalkiness. White scale bar represents 1.1 mm
To investigate the effect of the OsVPS22 mutation on seedling development, we conducted the germination assays of wild-type and mutants. The sterilized seeds were cultivated on MS medium for three days in darkness at 28 °C, and then treated 12 h in light and 12 h in the dark at 28 °C. The survival and growth of the mutant seedlings was investigated at 5 and 10 days after germination, respectively. In spite of the fact that there were no significant differences between the coleoptile growth of osvps22 and that of wild-type plants, mutant seedlings had severely retarded shoots compared with non-mutant siblings five days after germination (Fig. 5A, C).When compared to wild-type siblings ten days after germination, vps22 mutant seedlings had fewer and severely stunted seminal roots (Fig. 5B, D). Furthermore, Compared with control shoots, shoot elongation of vps22 mutants was inhibited, eventually resulting in the seedling lethality. Therefore, the OsVPS22 also is one of genes relative to the seedling lethality.
Comparison of seed germination between the vps22 mutant and wild-type. A Wild-type seedlings 5 days after germination, B wild-type seedlings 10 days after germination, C vps22 seedlings 5 days after germination, D vps22 seedlings 10 days after germination. White scale bars represent 10 mm (A, B) and 2 mm (C, D), respectively
Discussion
The rice vps22 is a novel mutant closely related to endosperm development
Chalkiness (i.e. an opaque white condition of parts of the endosperm) is one of the three most important character qualities in rice, which is affected by the genetic and environmental factors [25]. High-temperature stress during rice grain-filling facilitates the formation of chalky grains [19]. Over a half of chalky grains induced by the high-temperature were classified into the white-cored/milky-white grain with a chalky part around the centre of endosperm, which were formed from the early to middle stages of grain filling in the high-temperature stress, when the ability for starch synthesis was still active [19]. At maturity, single rounded as well as compound amyloplasts were present at the chalky part [19, 26], which was supposed to be the result of abnormal plastid initiation or starch granule initiation. According to the results, starch degradation by α-amylase was not the cause of the formation of the white-cored/milky-white type of chalkiness under high-temperature stress [19].
Previous studies have led to the cloning of several genes from mutants showing chalky endosperm, such as flo2 [27], flo4 [28] and flo5 [29, 30]. The FLOURY ENDOSPERM2 (FLO2) gene was predicted to encode a protein composed of 1720 amino acid residues with a tetratricopeptide repeat motif and involved in regulation of production of the storage proteins as well as storage starch in rice endosperm [27]. The flo2 grains had white and floury endosperm. The flo4 rice mutant showed that the white-core endosperm phenotype was generated by T-DNA insertion into the fifth intron of the OsPPDKB gene encoding pyruvate orthophosphate dikinase (PPDK) [28]. The flo5 rice mutant showed the similar white-core endosperm phenotype, which was generated by T-DNA/Tos17 insertion into the starch synthase IIIa (SSIIIa) gene which is the second major SS isozyme in activity levels next to SSI in developing rice endosperm [29, 30]. Furthermore, it was reported that loss of GW2 and GIF1 function also resulted in chalky rice grain. The rice GIF1 (GRAIN INCOMPLETE FILLING 1) gene that encodes a cell-wall invertase was required for carbon partitioning during early grain-filling [31]. GW2, a QTL that controls rice grain width and weight, encodes a RING protein with E3 ubiquitin ligase activity and significantly increases percentage of chalky rice kernels as well as grain width and weight [32]. These recent studies suggested that there is a complex regulatory network working for the formation of chalky grains.
At the same time, many researchers conducted QTL analyses of the chalkiness characteristics, discovered 36 gene loci to be related with chalkiness, which were mapped to eleven chromosomes but the fourth chromosome [25, 33, 34, 35, 36, 37, 38, 39]. Especially, on chromosome 9, qPGWC-9, one QTL for percentage of grains with chalkiness (PGWC), was consistently identified in several environments [36, 38]. The qPGWC-9 was mapped to an interval between markers RM219-RM296 on chromosome 9 [36]. Thus, the qPGWC-9 was mapped to about 3-Mb interval (7.89-10.78 Mb) according to the Nipponbare genome (http://www.gramene.org). The analysis of genes based on their physical position on the chromosome suggests that the OsVPS22 gene (20.74 Mb) is different from the qPGWC-9.
The OsVPS22 gene on chromosome 9 may be one of many genes closely related to chalky endosperm, whose loss leads to the white-core chalkiness (Fig. 4). The rice vps22 mutant is distinct from previous chalky endosperm mutants and may provide a clue for the identification of genes involved in the grain chalkiness.
Seedling lethality and OsVPS22 gene
The ESCRT core consists of three subcomplexes, ESCRT-I, -II, and -III, which performed three distinct but connected functions [12]. Recent studies showed that the deubiquitinating enzyme AMSH3 interacts with AtVPS2.1 and AtVPS24.1 and regulates their localization [9], but little is known about the differential function of these two ESCRT-III subunits. VPS22, one of three subunits of ESCRT-II, is essential for the stability of the ESCRT-II complex, which is required for multivesicular body (MVB) formation and sorting of endosomal cargo proteins into MVBs [15, 16, 40]. The MVB pathway mediates delivery of transmembrane proteins into the lumen of the lysosome/vacuole for degradation. The ESCRT-II complex is involved in the recruitment of the ESCRT-III complex [14]. Thus, it is quite reasonable that disrupting these two sequential processes should result in some similar phenotypes. Putative homologs of all the main ESCRT and ESCRT-related proteins have been identified in plants [6]. Notably, the roles of ESCRT-III-related protein CHMP1 have been studied to date in maize [41] and in Arabidopsis [3, 12, 43]. The ESCRT-III -related CHMP1 proteins with conserved endosomal functions mediate multivesicular body sorting of auxin carriers, which have been shown to to be important for auxin transport and distribution in plants [42], and required for plant development in Arabidopsis [43]. Because of abnormal auxin gradients resulting from mislocalization of auxin carriers, the double mutant of chmp1a chmp1b died before or shortly after germination [43]. The presence of early seedling lethality in the vps22 mutant, which compromised in polar auxin transport, resembled that of the chmp1 mutant. In fact, the phenotypic alterations seen in the vps22 mutant, such as retarding growth of shoot and root, are commonly related to defects in polar auxin transport or in auxin response [44]. Therefore, the knockout of the OsVPS22 gene probably leads to the defects in auxin transport and results in seedling lethality in the vps22 mutants.
The seedling lethality mutants generated by T-DNA/Ds insertion with a wide range of phenotypes were classified as affecting pigmentation and/or morphology [45]. There were 407 lines segregating in seedling lethality, more than 30 mutants had tiny or abnormal root growth [45]. In this study, the vps22 mutant showing a seedling lethality in rice (Fig. 5), which attributes to the mutant that lacks roots or has reduced root systems, indicating that the OsVPS22 gene is responsible for the mutant phenotypes and also is essential for seedling viability. Although phenotypic defects of the mutants described in this article, such as the impaired growth of shoot and root, which can be linked to deficient auxin transport, it is reasonable to think that the mutant defects might be due to alterations in the localization and degradation rate of multiple membrane proteins.
References
Carlton JG, Martin-Serrano J (2007) Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316:1908–1912
Katzmann DJ, Babst M, Emr SD (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145–155
Otegui MS, Spitzer C (2008) Endosomal functions in plants. Traffic 9:1589–1598
Schellmann S, Pimpl P (2009) Coats of endosomal protein sorting: retromer and ESCRT. Curr Opin Plant Biol 12:670–676
Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, Schumacher K (2011) Multivesicular Bodies Mature from the Trans-Golgi Network/Early Endosome in Arabidopsis. Plant Cell 23:3463–3481
Spitzer C, Schellmann S, Sabovljevic A, Shahriari M, Keshavaiah C, Bechtold N, Herzog M, Müller S, Hanisch F, Hülskamp M (2006) The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 133:4679–4689
Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458:445–452
Reyes FC, Buono R, Otegui MS (2011) Plant endosomal trafficking pathways. Curr Opin Plant Biol 14:666–673
Katsiarimpa A, Anzenberger F, Schlager N, Neubert S, Hauser MT, Schwechheimer C, Isono E (2011) The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell 23:3026–3040
Shahriari M, Hülskamp M, Schellmann S (2010) Seeds of Arabidopsis plants expressing dominant-negative AtSKD1 under control of the GL2 promoter show a transparent testa phenotype and a mucilage defect. Plant Signal Behav 5:1308–1310
Shahriari M, Keshavaiah C, Scheuring D, Sabovljevic A, Pimpl P, Häusler RE, Hülskamp M, Schellmann S (2010) The AAA-type ATPase AtSKD1 contributes to vacuolar maintenance of Arabidopsis thaliana. Plant J 64:71–85
Winter V, Hauser M (2006) Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci 11:115–123
Katzmann DJ, Odorizzi G, Emr SD (2002) Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Bio 3:893–905
Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD (2002) Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3:283–289
Hierro A, Sun J, Rusnak AS, Kim J, Prag G, Emr SD, Hurley JH (2004) Structure of the ESCRT-II endosomal trafficking complex. Nature 431:221–225
Teo H, Perisic O, González B, Williams RL (2004) ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev Cell 7:559–569
Richardson LGL, Howard ASM, Khuu N, Gidda SK, McCartney A, Morphy BJ, Mullen RT (2011) Protein–protein interaction network and subcellular localization of the Arabidopsis thaliana ESCRT machinery. Front Plant Sci 2:20
Shahriari M, Richter K, Keshavaiah C, Sabovljevic A, Huelskamp M, Schellmann S (2011) The Arabidopsis ESCRT protein–protein interaction network. Plant Mol Biol 76:85–96
Ishimaru T, Horigane AK, Ida M, Iwasawa N, San-oh YA, Nakazono M, Nishizawa NK, Masumura T, Kondo M, Yoshida M (2009) Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. J Cereal Sci 50:166–174
Kim SS, Lee SE, Kim OW, Kim DC (2000) Physicochemical characteristics of chalky kernels and their effects on sensory quality of cooked rice. Cereal Chem 77:376–379
Nagato K, Ebata M (1965) Effects of high temperature during ripening period on the development and the quality of rice kernels. Proc Crop Sci Soc Jpn 34:59–65
Tashiro T, Wardlaw IF (1991) The effect of high temperature on kernel dimensions and the type and occurrence of kernel damage in rice. Aust J Agric Res 42:485–496
Jin TY, Li H, Guo T, Liu XL, Su N, Wu FQ, Wan JM (2010) Analysis of physiological and biochemical characteristics of six mutants with stable high percentage of chalkiness in rice grains. Acta Agron Sin 36:121–132
Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
He P, Li SG, Qian Q, Ma YQ, Li JZ, Wang WM, Chen Y, Zhu LH (1999) Genetic analysis of rice grain quality. Theor Appl Genet 98:502–508
Lisle AJ, Martin M, Fitzgerald MA (2000) Chalky and translucent rice grains differ in starch composition and structure and cooking properties. Cereal Chem 77:627–632
She KC, Kusano H, Koizumi K, Yamakawa H, Hakata M, Imamura T, Fukuda M, Naitoa N, Tsurumaki Y, Yaeshima M, Tsuge T, Matsumoto K, Kudoh M, Itoh E, Kikuchi S, Kishimoto N, Yazaki J, Ando T, Yano M, Aoyama T, Sasaki T, Satoh H, Shimada H (2010) A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell 22:3280–3294
Kang H, Park S, Matsuoka M, An G (2005) White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J 42:901–911
Fujita N, Yoshida M, Kondo T, Saito K, Utsumi Y, Tokunaga T, Nishi A, Satoh H, Park J, Jane J, Miyao A, Hirochika H, Nakamura Y (2007) Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol 144:2009–2023
Ryoo N, Yu C, Park C, Baik M, Park IM, Cho M, Bhoo SH, An G, Hahn T, Jeon J (2007) Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep 26:1083–1095
Wang ET, Wang JJ, Zhu XD, Hao W, Wang LY, Li Q, Zhang LX, He W, Lu BR, Lin HX, Ma H, Zhang GQ, He ZH (2008) Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat Genet 40:1370–1374
Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39:623–630
Guo T, Liu XL, Wan XY, Weng JF, Liu SJ, Liu X, Chen MJ, Li JJ, Su N, Wu FQ, Cheng ZJ, Guo XP, Lei CL, Wang JL, Jiang L, Wan JM (2011) Identification of a stable quantitative trait locus for percentage grains with white chalkiness in rice (Oryza sativa). J Integr Plant Biol 53:598–607
Li ZF, Wan JM, Xia JF, Zhai HQ (2003) Mapping quantitative trait loci underlying appearance quality of rice grains (Oryza sativa L.). Acta Genet Sin 30:251–259
Li JM, Xiao JH, Grandillo S, Jiang LY, Wan YZ, Deng QY, Yuan LP, McCouch SR (2004) QTL detection for rice grain quality traits using an interspecific backcross population derived from cultivated Asian (O. sativa L.) and African (O. glaberrima S.) rice. Genome 47:697–704
Liu JF, Kui LM, Zhu ZF, Tan LB, Wang GJ, Li QW, Shu JH, Sun CQ (2007) Identification of QTLs associated with processing quality and appearance quality of common wild rice (Oryza rufipogon Griff.). J Agric Biotechnol 15:90–96
Tan YF, Xing YZ, Li JX, Yu SB, Xu CG, Zhang QF (2000) Genetic bases of appearance quality of rice grains in Shanyou 63, an elite rice hybrid. Theor Appl Genet 101:823–829
Wan XY, Wan JM, Weng JF, Jiang L, Bi JC, Wang CM, Zhai HQ (2005) Stability of QTLs for rice grain dimension and endosperm chalkiness characteristics across eight environments. Theor Appl Genet 110:1334–1346
Zhou LJ, Chen LM, Jiang L, Zhang WW, Liu LL, Liu X, Zhao ZG, Liu SJ, Zhang LJ, Wang JK, Wan JM (2009) Fine mapping of the grain chalkiness QTL qPGWC-7 in rice (Oryza sativa L.). Theor Appl Genet 118:581–590
Malerød L, Stuffers S, Brech A, Stenmark H (2007) Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic 8:1617–1629
Shen B, Li C, Min Z, Meeley RB, Tarczynski MC, Olsen OA (2003) sal1 determines the number of aleurone cell layers in maize endosperm and encodes a class E vacuolar sorting protein. Proc Natl Acad Sci USA 100:6552–6557
Wiśniewska J, Xu J, Seifertová D, Brewer PB, Růžička K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883
Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, Otegui MS (2009) The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21:749–766
Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12:160–168
Budziszewski GJ, Lewis SP, Glover LW, Reineke J, Jones G, Ziemnik LS, Lonowski J, Nyfeler B, Aux G, Zhou Q, McElver J, Patton DA, Martienssen R, Grossniklaus U, Ma H, Law M, Levin JZ (2001) Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics 159:1765–1778
Acknowledgments
We are grateful to S. Hohmann and other anonymous reviewers for helpful suggestions, critical reading of the manuscript, and stimulating discussions. This work was supported by the National Natural Science Foundation of China (Nos. 30900884 and 31272491), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20094404120011), and the Natural Science Foundation of Guangdong province, China (No. 9451064201003804).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiang-Qian Zhang and Pei Hou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, XQ., Hou, P., Zhu, HT. et al. Knockout of the VPS22 component of the ESCRT-II complex in rice (Oryza sativa L.) causes chalky endosperm and early seedling lethality. Mol Biol Rep 40, 3475–3481 (2013). https://doi.org/10.1007/s11033-012-2422-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2422-1