Abstract
SEPT4 belongs to the Septin family with multiple functions in cell division, cytoskeletal organization and other processes. This study aims to investigate the relationship between SEPT4_i1 isoform and human hepatocellular carcinoma (HCC). We showed that over-expression of SEPT4_i1 in HCC cells was able to sensitize cells to serum starvation-induced apoptosis. By contrast, knockdown of SEPT4_i1 expression in HCC cells was able to rescue cells from apoptosis induced by serum deprivation and to promote cell growth. Expressional analysis of SEPT4_i1 in tumor tissues further revealed that SEPT4_i1 was significantly down-regulated in human HCC tissues. Taken together, these data suggests a tumor suppressor role of SEPT4_i1 in HCC through regulating HCC cell apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septins comprise a family of cytoskeletal filament-forming GTPases and whose overall structure are evolutionarily conserved from yeast to human [1–3]. They were originally discovered in budding yeast in a screen for mutations blocking cell cycle progression [4]. Human septins are known to function in cytokinesis [5, 6], vesicle trafficking [7], interaction with actin filaments and microtubule networks [8] and DNA damage-related checkpoint response [9]. For instance, dominant negative SEPT2 mutant could prevent the completion of cell division [10]. Over-expression of SEPT7 was able to suppress U251 glioma cell growth [11].
SEPT4 resides at chromosome 17q23 and is widely expressed in normal human tissues including brain, heart, liver, lymphocyte and testes [12]. SEPT4 plays diverse physiological functions in many processes. Expression of murine SEPT4 is required for morphology and motility of the sperm flagellum [13]. In SEPT4-knockout male mice, both cortical organization and intraflagellar transport are disrupted in spermatozoa [13, 14]. SEPT4 also interacts with SEPT8 in platelet and exhibits a regulatory role in platelet granular secretion [15]. The roles of SEPT4 in various neurological diseases have also been widely studied. For instance, SEPT4 together with SEPT1 and SEPT2 are accumulated in taubased filamentous deposits known as neurofibrillary tangles and glial fibrils in Alzheimer’s disease [16]. SEPT4 protein is also involved in the formation of cytoplasmic inclusions and induction of cell death in α-synuclein associated with neurodegenerative disorders [17]. Enhanced expression of SEPT4 has been reported in substantia nigra and amygdale in Parkinson’s disease [18]. However, there remain controversies in the involvement of SEPT4 in tumors. While SEPT4 shows up-regulated expression level in colorectal cancer [19], down-regulated expression of SEPT4 is also observed in other cancers such as adenocarcinoma of lung [20].
In this study, we investigated the biological roles of SEPT4_i1 (GeneBank accession no. NM_004574.3), an isoform of SEPT4 with an open reading frame of 1434 nucleotides encoding 478 amino acids, in human HCC. Over-expression of SEPT4_i1 in HCC cells suppressed colony formation and sensitized HCC cells to serum starvation-induced apoptosis. Knocking down of SEPT4_i1 in HCC cells not only conferred cells higher resistance to serum deprivation-induced apoptosis, but also promoted cell growth. Moreover, the pro-apoptotic role of SEPT4_i1 correlated with its expression pattern in HCC tissues. Both western blot and immunohistochemical analysis revealed a significantly down-regulated expression pattern of SEPT4_i1 in HCC tissues.
Materials and Methods
Plasmid and siRNA transfection
Four human HCC cell lines SMMC-7721, HepG2, SK-Hep1 and QGY-7703 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO-BRL) containing 10% fetal calf serum (GIBCO-BRL). The SEPT4_i1 cDNA was kindly gifted by Professor Makoto Kinoshita (Kyoto University, Japan). SEPT4_i1 cDNA was then subcloned into mammalian expression vector pDsRed1-C1 and pcDNA3.1a(−) (Invitrogen) respectively. The siRNA oligos targeting SEPT4 were: SEPT4 siRNA-1, GGAACGGAAUCGCAACAAAtt; SEPT4 siRNA-2, CCAGAAACUGAGAAGCUUAtt. Both siRNAs and control siRNA were synthesized by GenePharma, Inc. (Shanghai, P. R. China). Both plasmids and siRNA oligos were transiently transfected into HCC cells by using Lipofectamine 2000 reagents (Invitrogen) according to manufacturer’s instructions. For flow cytometric analysis, plasmids were transfected together with plasmid pBB14, which expresses integral membrane green fluorescence protein (GFP) as a transfection marker. Cells with green fluorescence were considered to be positive transfectants and were selected in the following analysis.
Nuclear staining with DAPI
Cells were seeded onto sterile glass coverslips placed in 6-well plate at 2.5 × 105 cells per well. After 36 h transfection, cells were washed, fixed with 4% paraformaldehyde (Sigma) and permeated with 0.2% Tritonx-100 in PBS for 10 min at room temperature. Cells were then washed with PBS, and then subjected to DAPI staining (4,6-diamidino-2-phenylindole, 1 μg/ml) for 20 min at room temperature. Images were acquired by LEICA DC 500 camera.
Western blot analysis
Protein extracts were separated by 8–12% SDS-PAGE, and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies against different proteins at 4°C overnight, followed by incubation of horseradish peroxidase-conjugated secondary antibody. Immunoreactivity was visualized by enhanced chemiluminescence (Santa Cruz). The antibodies used were Anti-SEPT4 antibody (sc-20179, Santa Cruz), anti-β-actin antibody (Sigma), goat anti rabbit IgG antibody and rabbit anti-mouse IgG antibody (Calbiochem), biotinylated anti-rabbit and anti-mouse secondary antibody (Boster).
Sub-G1 analysis by flow cytometry
Sub-G1 distribution were determined by staining cells with propidium iodide (PI, Ex/Em = 488 nm/617 nm, Sigma). In brief, cells were harvested and fixed in ice-cold 70% ethanol for more than 2 h. After treatment with RNaseA (70 μg/ml, Gibco) for 30 min, cells were stained with PI for 15 min in dark at room temperature. 10,000 events were collected for each sample with a FACSCalibur flow cytometer (Becton–Dickinson) and analyzed using CellQuest software or ModFit software. The data is presented as the percentage of sub-G1 cells in respect to total cells. In the serum starvation assay, 18 h post-transfection, cells were washed and treated with 0.25% serum-containing media (starvation medium) for an additional 36 h before further analysis.
Annexin-V staining
Apoptosis was analyzed with the Annexin-V-PE apoptosis detection kit I (BD Pharmingen). According to manufacturer’s protocol, cells were harvested, washed and stained with Annexin-V-PE for 15 min in dark at room temperature. 10,000 events for each sample were collected and analyzed with a FACSCalibur flow cytometer (Becton–Dickinson). The data is presented as the percentage of apoptotic cells (Annexin-V positive and 7-AAD negative cells) in respect to total cells. In the serum starvation assay, 18 h post-transfection, cells were washed and treated with 0.25% serum-containing media (starvation medium) for an additional 36 h before further analysis.
Colony formation assay
SMMC-7721 and SK-Hep1 cells were transfected with pcDNA3.1a(-) and pcDNA3.1a(-)-SEPT4_i1 separately. 24 h after transfection, cells were replated at a density of 1 × 104 cells/well into 6-well plates in triplicate. G418 (650 μg/ml, Invitrogen) was added into the culture medium 24 h later. Colonies were identified by crystal violet staining after screen for about 14 days. Results are the representatives of three independent experiments.
Cell proliferation assay
24 h after transfection, HepG2 and QGY-7703 cells were plated into 96-well plates at a density of 2500 cells/well with medium containing 10% FBS. For the low serum assay, culture medium was replaced by DMEM supplemented with 0.25% serum-containing media at 8 h after cell plating. During a 4-day culture period, cells were subjected to MTS assay (Promega) every day. Cell growth curve was recorded as OD at 490 nm (n = 6) read by a microtiter reader (Bio-RAD).
Tumor tissues
Fresh surgical tissues of human hepatocellular carcinoma, including tumor tissues and the neighboring pathologically non-tumorous liver tissues, were obtained from Zhongshan Hospital, Shanghai, China. All the samples were immediately frozen in liquid nitrogen after surgery and stored at −80°C for further analysis.
Immunohistochemistry
Each section was sliced into 4 micrometers sections. The sections were deparaffined in xylene and rehydrated in alcohol. Endogenous peroxidase was perished by 3% H2O2 for 10 min. Antigen retrieval was achieved by treatment of microwave in citrate acid buffer (pH 6.0) for two times, and then blocked in 10% goat serum (Boster, China) for 30 min in 37°C for any none specific reaction. After blockade, slices were incubated with primary antibody at 4°C overnight, followed by biotin-labeled secondary antibody incubation. The avidin–biotin complex was finally revealed with diaminobenzidine. The images were quantitatively analyzed by a LEICA DC 500 camera on a microscope equipped with LEICA DMRA2 fluorescent optics (LEICA).
Statistical analysis
All numeric data were presented as mean ± SD. The two-tailed Student’s t test was used to determine statistical significance in this work. We considered P < 0.05 to be different (*) and P < 0.01 to be significant different (**).
Results
Expression pattern of SEPT4_i1 in HCC cell lines
In order to examine the potential biological roles of SEPT4_i1 in HCC, the expression pattern of SEPT4_i1 in different HCC cell lines was first analyzed by western blot. Anti-SEPT4 antibody against the amino acid residues 1–120 of SEPT4_i1 was used and it could only recognize a specific 55-kDa protein in the whole cell lysate. As shown in Fig. 1a, while HepG2, HuH-7 and QGY-7703 exhibited high levels of SEPT4_i1 protein, most of the other HCC cell lines showed fairly low or undetectable SEPT4_i1 expression.
Expression of SEPT4_i1 in HCC cell lines. a SEPT4_i1 expression in nine HCC cell lines. All cell lysates were subjected to anti-SEPT4 immunoblot analysis. β-actin was used as internal control. b Subcellular localization of SEPT4_i1 in SMMC-7721 and SK-Hep1 cells. Cells grown on cover slips were stained with DAPI at 36 h after transfection with pDsRed1-C1 or pDsRed1-C1-SEPT4_i1. The scale bar represents 20 μm (Magnification 400×)
Next, recombinant SEPT4_i1 plasmid was transiently introduced into SMMC-7721 and SK-Hep1 cells separately to observe its subcellular localization. After transfected with pDsRed1-C1 vector or pDsRed1-C1- SEPT4_i1, cells were fixed, stained by DAPI and photographed under fluorescence microscope. In control cells tranfected with vector only, diffusing RFP signals were observed in both cell cytoplasm and nucleus (Fig. 1b, upper panel). These control cells also displayed intact chromatin structure as identified by DAPI staining. By contrast, in cells transiently transfected with the recombinant pDsRed1-C1-SEPT4_i1, SEPT4_i1 specifically localized in cytoplasm with a speckled pattern (Fig. 1b, middle panel). Moreover, while a few cells expressing SEPT4_i1 displayed intact chromatin and normal phenotype (Fig. 1b, middle panel), condensed and separated chromatin was shown in some other SEPT4_i1 positive cells (Fig. 1b, bottom panel), which represented a typical apoptotic phenotype. This finding brought us the first hint that SEPT4_i1 in HCC cells might be involved in cell apoptosis.
Over-expression of SEPT4_i1 suppressed colony formation
The effect of SEPT4_i1 expression on the behaviors of HCC cells was further studied by flow cytometry analysis. In this experiment, pcDNA3.1a(-) vector or recombinant pcDNA3.1a(-)-SEPT4_i1 was transfected into SMMC-7721 and SK-Hep1 cells respectively, both of which show low levels of SEPT4_i1 protein (Fig. 1a). Expression of SEPT4_i1 was firstly confirmed by western blot at 36 h post-transfection using anti-SEPT4 antibody (Fig. 2a). FACS analysis was carried out at 48 h post-transfection after cells were stained with PI for 15 min. The cell population corresponding to sub-G1 phase was considered to be apoptotic. As shown in Fig. 2b, cells transfected with SEPT4_i1 exhibited a slightly elevated cell apoptotic level compared with control cells transfected with pcDNA3.1vector only (P < 0.05, Student’s t test), indicating that SEPT4_i1 expression might have a slight effect on cell apoptosis under normal conditions.
Over-expression of SEPT4_i1 suppressed colony formation. a Cellular extracts derived from cell transfectants were analyzed by western blot using anti-SEPT4_i1 antibodies. b Over-expression of SEPT4_i1 exhibited a slight pro-apoptotic effect on cell apoptosis. The apoptosis levels of SMMC-7721 and SK-Hep1 transfectants were calculated as the percentage of sub-G1 population compared to total cells through FACS analysis. Values were presented as means ± SD; n = 3. *P < 0.05. c Over-expression of SEPT4_i1 significantly suppressed colony formation. Representative images of colony formation assay on SMMC-7721 and SK-Hep1 were shown. Cells were transiently transfected, split and cultured in the presence of G418 before the colonies were identified by crystal violet staining. Colony number formed by pcDNA control-transfected cells was arbitrarily set as 100%. Values were given in mean ± SD; n = 3. **P < 0.01
To explore the function of SEPT4_i1 during long-time cell growth, colony formation assay was also performed. SMMC-7721 and SK-Hep1 cells were subjected to G418 screen for about 2 weeks after transiently transfected with pcDNA or SEPT4_i1 separately. In the end of the screen, colonies were identified by subjected to crystal violet staining. Both SMMC-7721 and SK-Hep1 cells transfected with SEPT4_i1 formed much fewer and smaller colonies than control cells (Fig. 2c). This finding raised a hypothesis that the pro-apoptotic function of SEPT4_i1 might become stronger under cell growth stress.
SEPT4_i1 enhanced the sensitivity of HCC cells to serum starvation-induced apoptosis
It is known that withdrawal of trophic factors (serum starvation) can induce cellular apoptosis. Thus, serum starvation was chosen as a growth stress to investigate the function of SEPT4_i1 under apoptosis conditions. In this case, at 18 h post-transfection of pcDNA3.1a(-)-SEPT4_i1 or pcDNA3.1a(-) into SMMC-7721 and SK-Hep1 cells, cells were deprived of serum for another 36 h, followed by flow cytometry analysis. Representative Figures and statistical results were shown in Fig. 3a. The percentage of sub-G1 population was significantly higher in cells expressing SEPT4_i1 than that in control cells (P < 0.01, Student’s t test). By comparsion with the data in Fig. 2b, results from starvation assay revealed that SEPT4_i1 probably exhibits a much stronger pro-apoptotic effect on HCC cells upon serum starvation.
Over-expression of SEPT4_i1 sensitized HCC cells to serum starvation-induced apoptosis. a Over-expression of SEPT4_i1 significantly promoted cell apoptosis under serum starvation conditions. SMMC-7721 and SK-Hep1 transfectants were cultured in 0.25% serum-containing media for 36 h before sub-G1 analysis. Values were given in mean ± SD; n = 3. **P < 0.01. b Annexin-V analysis showed that SEPT4_i1 exerted pro-apoptotic effect under serum-starvation conditions. Cell apoptosis was calculated as the percentage of Annexin-V positive and 7-AAD negative population compared to total cells and indicated as mean ± SD; n = 3
In addition, another standard cell apoptosis assay by staining cells with Annexin V was carried out to further demonstrate our finding. Both SMMC-7721 and SK-Hep1 cells were transfected, starved and harvested as previously described before subjected to Annexin V analysis. In Fig. 3b, the fraction of Annexin V positive and 7-AAD negative cells was indicated, representing the apoptotic cell population. A remarkable increasing in the population of apoptotic cells was observed in cells expressing SEPT4_i1 compared to that in control cells, demonstrating the strong pro-apoptotic function of SEPT4_i1 expression under serum starvation. All these findings suggest that SEPT4_i1 expression could sensitize HCC cells to serum starvation-induced apoptosis.
Knockdown of SEPT4_i1 in HCC cell lines protected cells from serum starvation induced cell apoptosis and stimulated cell growth
To further examine the role of endogenous SEPT4_i1, two specific siRNAs targeting to SEPT4 (SEPT4 siRNA-1 or SEPT4 siRNA-2) were introduced into HepG2 and QGY-7703 cells, both of which express relative high levels of SEPT4_i1 protein (Fig. 1a). SiRNAs were used at very low concentration (80 pmol) to avoid unwanted off-target effects. At 48 h post siRNA transfection, whole cell lysate was subjected to western blot to evaluate the knockdown effect (Fig. 4a). By comparison with non-related control siRNA, both SEPT4 siRNA-1 and SEPT4 siRNA-2 effectively reduced the endogenous protein levels of SEPT4_i1. In order to test whether SEPT4_i1 silencing was able to protect HCC cells against serum starvation-induced apoptosis, cells transiently transfected with SEPT4 siRNA-1, SEPT4 siRNA-2 or control siRNA were cultured in 0.25% serum-containing medium at 18 h post transfection for another 36 h before apoptosis analysis. As shown in Fig. 4b, a significant decreasing of cell population corresponding to sub-G1 phase was shown in cells transfected with SEPT4 siRNA-1 or SEPT4 siRNA-2 in comparison with control group (P < 0.01, Student’s t test). Additionally, we also observed that knocking down of SEPT4_i1 might promote cell growth. To demonstrate this hypothesis, we recorded cell growth curves to assess the function of SEPT4_i1 on cell growth. At 24 h post-transfection with SEPT4 siRNA-1, SEPT4 siRNA-2 and control siRNA separately, HepG2 and QGY-7703 cells were replated into 96-well plates. Culture medium was replaced with 0.25% serum-containing medium at 8 h after cell plating. Then, the two 4-day growth curves were calculated using MTS assay. As shown in Fig. 4c, when cells were cultured in the absence of serum, significant cell growth advantage was induced in cells transfected with both SEPT4 siRNAs (P < 0.01, Student’s t test) (Fig. 4c). Therefore, these data all together highlighted a role of SEPT4_i1 on HCC cells in the acquisition of malignant features under growth stress.
Knockdown of SEPT4_i1 protected HCC cells from serum starvation-induced cell apoptosis and promoted cell growth. a The SEPT4_i1 protein level was reduced in HepG2 and QGY-7703 cells after RNA interference. The knockdown effect of SPET4_i1 was achieved by transfection with SEPT4 siRNA-1 or SEPT4 siRNA-2, but not control siRNA. The SEPT4_i1 level was examined by western blot with anti-SEPT4 antibody. β-actin was used as internal control. b Knockdown of SEPT4_i1 suppressed cell apoptosis induced by serum deprivation. Cell transfectants were cultured in 0.25% serum-containing medium for 36 h before sub-G1 analysis. Values were given in mean ± SD; n = 3. **P < 0.01. c Knockdown of SEPT4_i1 could promote cell growth as demonstrated by cell proliferation assay. Cells transfected with control siRNA (filled diamond), SEPT4 siRNA-1 (filled square), SEPT4 siRNA-2 (filled triangle) separately were seeded in 96-well plates and cultured under the serum starvation condition. Cell number was recorded with the MTS kit and the cell growth curve was expressed as the absorbance at 490 nm with a microtiter reader (mean ± SD; n = 6). *P < 0.05, **P < 0.01
SEPT4_i1 were significantly down-regulated in human HCC tissues
The balance between cell survival and apoptosis is essential for the progression of cancer. The pro-apoptotic role of SEPT4_i1 on HCC cells indicated the involvement of SEPT4_i1 in HCC. Therefore, by utilizing paired HCC tissue samples, we investigated the expression pattern of SEPT4_i1 in tumor. To this end, western blot analysis of lysates from twelve HCC tissues and their corresponding adjacent non-tumorous tissues was performed. Anti-SEPT4 antibody recognized a specific 55-kDa band corresponding to SEPT4_i1 in these tissue samples and the protein level of SEPT4_i1 was remarkably down-regulated in all the tumor samples by comparison with that in their corresponding normal liver tissues (Fig. 5a).
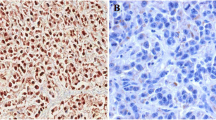
SEPT4_i1 protein levels were significantly reduced in human HCC tissues. a Down-regulated expression pattern of SEPT4_i1 in HCC. Twelve paired HCC specimens (T tumor, N non-tumorous liver tissue) were subjected to anti-SEPT4 immunoblot analysis. β-actin was used as internal control. b Immunohistochemical staining analysis shows a reduction of SEPT4_i1 expression in tumor cells by contrast to neighboring normal hepatocytes on a representative human liver specimen containing both tumor (T) and non-tumorous (N) parts. a Sections stained with anti-SEPT4 antibody. b Sections stained with rabbit IgG. The scale bar represents 40 μm (Magnification 200×)
This expression alteration of SEPT4_i1 protein in HCC was further demonstrated by immunohistochemistry (IHC) study. In Fig. 5b, no or very weak staining of SEPT4_i1 was observed in HCC tissues (left section), while normal adjacent liver tissues exhibited strong nuclear staining and moderate to strong cytoplasmic staining (right section). This result provided additional evidence that SEPT4_i1 was specifically down-regulated in HCC.
Discussion
The involvement of SEPT4 in cell apoptosis has been widely studied. Hallstrom et al. found that SEPT4 was up-regulated in serum-starved REF52 cells through activation of E2F1 and it participated in E2F1-induced cell apoptosis. However, under normal conditions, SEPT4 expression was repressed by PI3 K/Akt pathway activated by serum [21]. ARTS, another transcript of SEPT4, was able to induce cell apoptosis through translocating from mitochondria to nucleus after cells were exposed to apoptotic agents [12]. Under the condition of apoptosis, ARTS released from mitochondria co-localizes with XIAP (X-linked inhibitor of apoptosis) in the cytosol, and then promotes cell apoptosis by antagonizing XIAP which subsequently activates downstream caspases [12, 22]. The C-terminal domain of ARTS is critical for its pro-apoptotic activity. Particularly, it has been previously reported that SEPT4_i1 can also be transferred from cytoskeleton to cytosol upon removal of serum [23]. Our present work provides further evidence that SEPT4_i1 has an important role in regulating HCC cell apoptosis. Over-expression of SEPT4_i1 in HCC cells sensitized cells to apoptosis when serum was deprived, while knockdown of SEPT4_i1 played an opposite role. Our further finding that SEPT4_i1 protein expression level was remarkably down-regulated in HCC specimens compared to their corresponding adjacent non-tumorous tissues suggested that the reduced expression of SEPT4_i1 might be one mechanism by which cells escaped from apoptosis stimulus in tumor microenvironment and developed to be a tumor tissue. The detailed mechanism through which SEPT4_i1 participates in cell apoptosis remains to be experimentally assessed.
Recently, Iwaisako et al. have reported two other novel transcript variants of SEPT4 (43- and 40-kDa) specifically expressed in human hepatic stellate cells and in which loss of SEPT4 exacerbates liver fibrosis [24]. In our work, anti-SEPT4 antibody could only recognize a specific 55-kDa band in samples from both HCC tissues and cell lines (Figs. 1a, 5a), indicating the existence of SEPT4_i1 isoform in liver. And immunohistochemistry studies further demonstrated that SEPT4_i1 is mainly expressed in hepatocytes (Fig. 5b). The discrepancies between the findings from Iwaisako and ours appear to be largely dependent on the very variable experimental conditions, which are complicated by samples collected from different tissues, the use of different cell systems and culture conditions.
In summary, the present findings showed that over-expression of SEPT4_i1 enhanced the sensitivity of HCC cell lines to serum starvation-induced apoptosis and knockdown of SEPT4_i1 rescued cells from serum starvation-induced apoptosis and promoted cell growth. These results well correlated with the down-regulated expression pattern of SEPT4_i1 protein in HCC tissues. It is necessary and valuable to further explore the mechanisms by which SEPT4_i1 regulates cell apoptosis in the future. Collectively, SEPT4_i1 is likely to be a candidate tumor suppressor in HCC, as well as a potential therapeutic target in cancer therapy.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- KD:
-
Kilodalton
References
Kartmann B, Roth D (2001) Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci 114(Pt 5):839–844
Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ (2002) Self- and actin-templated assembly of Mammalian septins. Dev Cell 3(6):791–802
Faty M, Fink M, Barral Y (2002) Septins: a ring to part mother and daughter. Curr Genet 41(3):123–131
Hartwell LH (1971) Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res 69(2):265–276
Spiliotis ET, Kinoshita M, Nelson WJ (2005) A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science 307(5716):1781–1785
Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M (1997) Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev 11(12):1535–1547
Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH (1998) Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 20(6):1111–1122
Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H (2003) Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem 278(20):18538–18543
Kremer BE, Adang LA, Macara IG (2007) Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell 130(5):837–850
Kim DS, Hubbard SL, Peraud A, Salhia B, Sakai K, Rutka JT (2004) Analysis of mammalian septin expression in human malignant brain tumors. Neoplasia 6(2):168–178
Jia ZF, Huang Q, Kang CS, Yang WD, Wang GX, Yu SZ, Jiang H, Pu PY (2010) Overexpression of septin 7 suppresses glioma cell growth. J Neurooncol 98(3):329–340
Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D (2000) A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2(12):915–921
Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O (2005) Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell 8(3):343–352
Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H (2005) The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell 8(3):353–364
Blaser S, Horn J, Wurmell P, Bauer H, Strumpell S, Nurden P, Pagenstecher A, Busse A, Wunderle D, Hainmann I (2004) The novel human platelet septin SEPT8 is an interaction partner of SEPT4. Thromb Haemost 91(5):959–966
Kinoshita A, Kinoshita M, Akiyama H, Tomimoto H, Akiguchi I, Kumar S, Noda M, Kimura J (1998) Identification of septins in neurofibrillary tangles in Alzheimer’s disease. Am J Pathol 153(5):1551–1560
Ihara M, Tomimoto H, Kitayama H, Morioka Y, Akiguchi I, Shibasaki H, Noda M, Kinoshita M (2003) Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson’s disease and other synucleinopathies. J Biol Chem 278(26):24095–24102
Shehadeh L, Mitsi G, Adi N, Bishopric N, Papapetropoulos S (2009) Expression of Lewy body protein septin 4 in postmortem brain of Parkinson’s disease and control subjects. Mov Disord 24(2):204–210
Tanaka M, Tanaka T, Kijima H, Itoh J, Matsuda T, Hori S, Yamamoto M (2001) Characterization of tissue- and cell-type-specific expression of a novel human septin family gene, Bradeion. Biochem Biophys Res Commun 286(3):547–553
Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI (2001) Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA 98(24):13784–13789
Hallstrom TC, Mori S, Nevins JR (2008) An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell 13(1):11–22
Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S (2004) The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J 23(7):1627–1635
Xie H, Surka M, Howard J, Trimble WS (1999) Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other septin proteins. Cell Motil Cytoskeleton 43(1):52–62
Iwaisako K, Hatano E, Taura K, Nakajima A, Tada M, Seo S, Tamaki N, Sato F, Ikai I, Uemoto S (2008) Loss of Sept4 exacerbates liver fibrosis through the dysregulation of hepatic stellate cells. J Hepatol 49(5):768–778
Acknowledgments
We thank Dr. Makoto Kinoshita (Kyoto University, Graduate School of Medicine, Japan) for providing pFLAG–SEPT4_i1 plasmid. We also thank Dr. Jinhu Guo (Sun Yat-Sen University) for helpful discussion. This project was supported by 863 projects of China (2006AA020501), the Project of the Shanghai Municipal Science and Technology Commission (03dz14086) and the National Natural Science foundation of China (30024001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, S., Liu, M., Wu, Y. et al. Involvement of SEPT4_i1 in hepatocellular carcinoma: SEPT4_i1 regulates susceptibility to apoptosis in hepatocellular carcinoma cells. Mol Biol Rep 39, 4519–4526 (2012). https://doi.org/10.1007/s11033-011-1242-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1242-z