Abstract
One hundred one isolates of Macrophomina phaseolina from various hosts and eco-geographical locations were employed for elucidating relationships among genetic diversity and virulence. Highly pathogenic, moderately pathogenic, and hypovirulent cluster bean specific isolates were identified. In order to correlate respective phenotypes of plant pathogenic fungus multiple and complex patterns of dsRNA elements were analyzed. Double-stranded ribonucleic acids (dsRNA) are ubiquitous in all major groups and most of them have vast potential as biological control agents for fungi. Rate of virulence and its further association could ascertain by host plant and their fungal genotypes. Variability of the fungal genotypes decides the link between the complexity of dsRNA with different variants and the change in virulence pattern. Double-stranded RNA was identified in approximately 21.7% of M. phaseolina isolates from charcoal rot infected cluster bean varieties. After recurrent laboratory transfer on culture media, the preponderance of the isolates harboring dsRNAs developed degenerate culture phenotypes and showed reduced virulence (hypovirulence) to cluster bean. Macrophomina has successfully showed diversified and reproducible banding profile in dsRNA containing/free isolates. This is the first report of hypovirulence and detection of dsRNA in Macrophomina phaseolina isolates of cluster bean origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cluster bean (Cyamopsis tetragonoloba) the cash crop contributing 80% shares of its total worldwide production has tremendous application in textile, paper, petroleum, mining, pharmaceuticals, explosives, and food industries. It is widely cultivated in countries such as India, Pakistan, USA, Italy, Morocco, Germany, Greece, and Spain, thus considered as a new crop for western agricultural practices [1]. Owing to its immense importance, there is a strong need for appropriate addressing and well documentation of the germplasm, by biocontrol of phytopathogenic Macrophomina phaseolina. The fungus is a non-specific pathogen of charcoal rot disease and hampers a broad spectrum of economically important crops such as common beans, maize, soybean, sorghum, sesame, cotton, sunflower or cucurbits [2]. M. phaseolina, the only species of the genus, has a wide host range, which is responsible for causing losses in more than 500 variable plant species growing across the world [3]. The fungus produces two anamorphs for various intense examinations; microsclerotial phase (black) of M. phaseolina, profoundly considered more pathogenic as compared to its pycnidial (hyaline) anamorph structures [2]. Mihail [4] also recognizes anchoring diversified synonyms M. phaseolina (Tassi) as M. phaseoli (Maubl.), Macrophoma conchoci (Swada), Sclerotium bataticola (Taub.) and Rhizoctonia bataticola (Taub.). Its morphological and pathogenic variability was widely acknowledged among isolates of different hosts and between isolates of same plant [5]. The most common diagnostic symptom for charcoal rot disease on prematurely dying or dead plants is the sloughing of cortical tissues from the lower stem and tap root and the speckled grey appearance of the infected tissues due to the formation of abundant microsclerotia in vascular, cortical and pith tissues. The fungus population in different parts of the world was characterized based on its pathogenic variability [6], morphological characteristics [5], and molecular characteristics [7–9], but the basis of differences in pathogenicity is still unknown [10].
The term virulence (hypo/moderate/hyper) describes the ability of specific fungal isolate to cause disease. Until now, several studies on plant pathogenic fungi have reported association of the presence of dsRNA with virulence. Double-stranded RNAs (dsRNA) associated with virus-like particles is found prevalent in various fungal species [11–14]. The dsRNA molecule exists as collection of unique and discrete entities that replicates very differently from double stranded DNAs. Effects of dsRNA on plant pathogenic fungal species had studied globally and their hypovirulence to host plant is well documented [15–18]. The classified quoted example of dsRNA-virulence association is Cryphonectria parasitica of chestnut blight. In this, presence of dsRNA is correlated with transmissible hypovirulence in various crops [19, 20]. In contrast, Macrophomina the only species has not investigated extensively among cluster bean specific isolates, and no information exists on the use of hypovirulence, associated with dsRNA, to control Macrophomina-incited disease in cluster bean cultivars. The objectives of the present study were: (1) To detect the occurrence of extrachromosomal elements (dsRNA) amongst cluster bean infecting isolates of M. phaseolina grown under different eco-geographical conditions of North India and to differentiate highly pathogenic, less pathogenic, and moderately pathogenic isolates. (2) To determine if there is an association between the presence of dsRNA and virulence in Macrophomina isolates of cluster bean plants.
Materials and methods
Fungal isolates and culture venue
101 isolates of Macrophomina used in the present study were isolated from various rhizosphere soils/seeds/plants of cluster bean from the four majorly cluster bean growing states, viz., Haryana, Rajasthan, Punjab and Gujarat of North and North-West India (Nagpur, Lyallpur, Kalyani, Almora, Coimbatore, New Delhi, Assam) (Table 1). The isolates collected were used for isolation on Potato dextrose agar (PDA), pH 5.5. Culture plates were incubated in the dark at 28 ± 1°C for 4–5 days. Purified fungal cultures were preserved and maintained routinely on potato dextrose agar (Hi-media) medium (Table 2). The purified isolates were maintained as pure cultures using the single hyphal tip technique [9] for further studies. Dried mycelial disks were stored at −20°C for long-term preservation. For dsRNA extraction, fungal liquid cultures were grown in broth complete medium (PDB) for 2 weeks at 28°C to produce mycelia for the extraction and purification of dsRNA.
Extraction and purification of dsRNA
Mycelial mat was harvested by centrifugation at 8,000 rpm for 10 min from liquid cultures and the mat was washed with 10 ml TES buffer [10], prior to lyophilization process. Fungal dsRNA was extracted according to the method of Morris and Dodds [21] and Valverde et al. [22] with minor amendments. Modified CF11 cellulose packed column chromatography was employed for the extraction of dsRNA elements. Approximately 2 g lyophilized dried mycelium was used for the extraction of dsRNA from each Macrophomina variable. The dsRNA was precipitated and eluted by centrifugation at 10,000 rpm (15 min), and pellets were finally dissolved in 40 μl of sterile distilled water. The dsRNA species was further separated by agarose gel electrophoresis (1%) and the purified dsRNA was stored at −20°C. The presence of the dsRNA was verified by reisolating the hyphae and growing it in pure liquid culture. After several days of growth, the dsRNA was purified, and its presence, quality and relative concentration was checked by electrophoresis on a 1% agarose gel with 1× TAE buffer (40 mM Tris–Acetate, 1 mM EDTA, pH 8.0) at 6 V cm−1 and visualized with a UV light source by ethidium bromide staining. A fairly accurate molecular weight was determined by comparing electrophoretic mobility of the dsRNAs with molecular weight standard, 1-kb DNA ladder (Genei, Bangalore).
Confirmation of dsRNA by enzymatic digestions
Two enzymatic digestions were performed separately using DNase-I and RNase-A as described by Bogo et al. [23]. For DNase digestion, the samples were treated with 30 U of enzyme in 6 mM MgCl2 (Gibco Life Technologies, USA) according to the manufacturer’s instructions. For RNase-A digestion, the enzyme (Invitrogen, USA) was added to 3 mg of total nucleic acids in 0.3 M NaCl, to a final concentration of 4 μg ml−1 and incubated at 37°C for 1 h. The reactions were purified twice using equilibrated phenol:chloroform:isoamyl alcohol (25:24:1), and precipitated by the addition of 5.5 M ammonium acetate (0.6 vol) and 95% ethanol (2 vol). Precipitates were eluted by centrifugation at 10,000 rpm for 20 min and pellets were dissolved in 30 μl of sterile distilled water. Samples were analyzed by electrophoresis on 1.0% agarose gel in 1× TAE buffer, stained with ethidium bromide (100 ng ml−1) and visualized under UV transilluminator. Isolates not hydrolyzed by DNase or high salt concentration were designated as dsRNA-containing isolates (+) while isolates hydrolyzed, by the activity of RNase in low NaCl concentration were designated as dsRNA free (−) isolates [24].
Virulence of dsRNA
Four dsRNA-containing M. phaseolina isolates (+) and four dsRNA free (−) isolates were arbitrarily selected and tested against superior varieties of cluster bean for its virulence. The isolates tested that have dsRNA were isolates CB-11 (+), CB-17 (+), CB-28 (+), GH-57 (+), while those tested that did not had dsRNA were isolates CB-14 (−), CB-38 (−), CB-62 (−) and CB-71 (−) (Table 1). Only single isolate originating from each state of North India were selected for field studies, as probably the isolates would be tolerant to the drought and heat stress commonly encountered with no significant geographic barrier in their location environment. These isolates were also selected according to their dense and feathery physiology in an attempt to minimize concerns associated with the prologue of new fungal isolates into areas where cluster bean is cultivated commercially.
Inoculation of seeds with virulent isolates of M. phaseolina
Pathogenicity of M. phaseolina isolates was evaluated using seeds of two superior cluster bean differential cultivars [25]. Seeds were surface sterilized with 2% sodium hypochlorite for 2 min, rinsed in sterile tap water. Isolates of M. phaseolina were cultured in 12 cm petri dishes at 28 ± 2°C in the dark. When PDA of the dishes was completely colonized ten seeds of each cultivar were placed on the colony of each of the M. phaseolina isolates. Petri dishes were incubated at 28 ± 2°C in the dark. After 5 days, virulence index and disease severity of seeds was evaluated (Table 3) based on symptoms caused by the pathogen using 0–5 scale. Where 0 = healthy seed; 1 = discoloration of a portion of the seedling in contact with the mycelium; 2 = seed teguments invaded by mycelium and sclerotia but healthy seedling; 3 = seed teguments free from the fungus, but seedling infected; 4 = seed tegument and seedling infected; 5 = seed infected and not germinated [26]. On the basis, of their virulence index and disease causing severity, isolates were eventually characterized and categorized as highly pathogenic, moderately pathogenic or hypovirulent.
Pathogenicity of the dsRNA (+) and dsRNA (−) M. phaseolina isolates
Pathogenicity tests were conducted on two susceptible cluster bean cultivars namely, PNB and IC-102827 to charcoal rot disease. Seeds of the tested cultivars were acquired from Forage Section, CCS Haryana Agriculture University, Hisar, Haryana. Seeds were surface sterilized with 2% sodium hypochlorite, rinsed in sterile distilled water and sown in 25 cm plastic pots filled with sand under green house conditions. Four replicate pots (each) were sown with two seeds of cluster bean for each tested isolate. Plants were watered as needed and treated according to the recommended agricultural practices. The 30-day-old plants were stem inoculated with the tested isolates CB-11 (+), CB-17 (+), CB-28 (+), GH-57 (+), CB-14 (−), CB-38 (−), CB-62 (−) and CB-71 (−) in the second internode using the stem tape inoculation technique [27]. Ten days after inoculation, developed lesions were measured in cm that may appear as the longitudinal bark necrosis at the site of inoculation (Fig. 1). Percentage of plant death was determined 21 days after inoculation [28]. Repeated reisolation was conceded to ensure the association of the tested isolates with the developed disease.
Schematic representation of stem tape inoculation to check the lesion length and plant death rate under green house controlled conditions [a 96 h old strain of M. phaseolina on culture media. b Mycelial plugs (5 mm) cut from the mat of the colony, using a metallic cork borer. c and d A mature cluster bean plant superficially wounded and the mycelial plug was induced against the wound. e Brown colored wound sealed with cellophane tape. f Positive control of plant inoculated with sterile PDA plug, sealed with cellophane tape]
Statistical analysis
For ascertaining virulence index and pathogenicity analysis, the mean values of virulence index and pathogenicity caused by each isolate was calculated. Mean values were subjected to analysis of variance (ANOVA) using a completely randomized design where treatments were each state of origin of isolates and the replications of the isolates. Data were subjected to one-way analysis of variance, with P < 0.05 considered significant. Statistical analysis was performed by employing SPSS software version 1.0.
Results
Detection of dsRNA in M. phaseolina
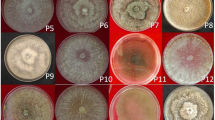
The presence of dsRNA among the isolates of M. phaseolina was detected in 22 of the 101 isolates (21.7%) including 13 isolates from Rajasthan, 6 isolates from Haryana, 2 isolates from Gujarat and 1 from Punjab as shown in Table 1. The 22 isolates were majorly obtained from cluster bean showing dense phenotype. M. phaseolina segregates were further characterized on the derivation of their electrophoretic profile. Without DNase treatment, DNA was found contaminating dsRNA, suggesting that it may compete with dsRNA for adsorption to cellulose. Low salt concentration was found vivacious in reducing DNA recovery by phenol extraction. dsRNA capitulate was also found erratic with tissue extraction. Purified dsRNA fraction collected after the phenol–chloroform treatment, and precipitated with ethanol. The size range of dsRNA varies between ~800 bp and 12 Kb (Fig. 2). The dsRNA panoramas of these molecules were further entrenched by optimizing cycles of Cellulose CF-11 chromatography and DNase and RNase treatments. Repeated sub-culturing until three cycles confirms the presence of dsRNA in isolates obtained from various eco-geographical locations, showing its stable nature. Resistance to DNase, but sensitivity to RNase-A confirmed that these bands were dsRNA. This property of dsRNA stability was used to our advantage for reducing the amount of fungal cellular RNA contamination. Two trials on each isolate were done on dsRNA and results were the same for each trial of M. phaseolina isolates. No significant differences were observed between dsRNA-containing and dsRNA-free isolates for colony morphology, mycelial growth and sporulation ability in this study (Table 1) concomitantly dsRNA was found invariably allied with virulence attributes. The present study on dsRNA stems on detection of “virus-host relationship”.
Response of cluster bean seeds with virulent isolates of M. phaseolina
Disease severity was found prevalent in all the eight M. phaseolina isolates in which the seeds were randomly dispersed, its mean ranged from 0.510 to 2.06 (+) and 2.62 to 3.04 (−) in the randomly chosen germinated sections, however not much difference was observed in the promptness of necrosis symptoms in cotyledon, hypocotyls or the root parts of cluster bean. Virulence rating was generated based on mean virulence index that ranged from minimum 1.83 (+) to maximum 5.31 (−) in cluster bean seeds infected with M. phaseolina (Table 3).
Pathogenicity tests of the dsRNA (+) and dsRNA (−) M. phaseolina isolates
All the recovered M. phaseolina isolates tested were pathogenic to both the cluster bean varieties PNB and IC-102827, as incited total mean stem lesions ranged between 0.4 and 4.4 cm in the pathogenicity test (Table 4). The mean lesion length range varied from 0.4–2.8 cm (+) to 2.6–4.4 cm (−). Data revealed that, isolates were variable in their ability to cause plant death which was in the range of 0–100% of the control on the tested cultivars with total means of 31 and 74% for the Macrophomina isolates, respectively. Almost means of 68–74% of the total M. phaseolina recovered isolates were low pathogenic and classified as hypovirulent to cause plant death on the tested cluster bean cultivars in which double stranded RNA was present (Table 4). On the other hand, with means of 31–90% of the isolates were highly pathogenic in which dsRNA was totally absent.
Discussion
Per se, presence of dsRNA has reported for a number of plant pathogenic fungi; however, this is the first report dsRNA in Macrophomina isolates of cluster. By numerous attempts with isolates of many different geographic origins, we were able to reveal that the presence of dsRNA is widely distributed among isolates of the fungus. The occurrence of dsRNA in isolates of M. phaseolina probably implies the presence of viruses [29, 30]. Sucrose gradient centrifugation to isolate and purify viruses revealed unsuccessful results [31]. Multiple dsRNA wreckage accentuates infection of virus with a segmented genome. It confirms its wide heterogeneity among the isolates as similar pro-founding’s was confirmed earlier in C. elegans [31]; R. solani [32] and M. phaseolina isolates of various crop cultivars [10] which further confirms our findings. However, further studies are still required to elucidate the origin of dsRNA elements in M. phaseolina. The dsRNA profile revealed (Fig. 2) that both the number and size of the molecules in different isolates of M. phaseolina were heterogeneous in nature. The heterogenic pattern of dsRNA elements among the isolates was not unique, as it have been observed for several other fungal species, including P. oryzae, C. elegans, C. parasitica, S. homoeocarpa, C. ulmi and R. solani [17, 30–35]. A dsRNA molecule of ~10 kb was present in the isolates from Haryana, Rajasthan and Gujarat (Fig. 2).
We interestingly found stable dsRNA even after seven cycles of successive sub-culturing from diverse geographic locations. These results are quite analogous to those formerly recorded from stable dsRNA of C. parasitica [24] and R. solani [36], over several successions of subculture. Earlier, variation in virulence among isolates of M. phaseolina have been reported [37], but until now the basis for these differences is unknown. In this study, the virulence of isolates was evaluated using a commercial guar-gum producing cluster bean cultivar grown under agro-field conditions in different locations of North India.
A rain-fed kharif crop; cluster bean is primarily sown in June–July and harvested in the month of October–November. Rajasthan being a dominant shareholder of 80% is the largest guar or cluster bean producing state in the India. However, states like Haryana and Gujarat just adds-on 12 and 11%, respectively to the guar production. Guar gum exports during the 2009–2010 were around 2.2 lakh ton, which estimated to reach up to 2.5 lakh ton in 2011 year ahead. The important feature of this commodity is, it is less perishable as compared to other agro commodities and its seed can be stored for more than 3 years, which makes it most preferred goods for the stockiest and traders. According to the recent report, the Northwest province of India, a key guar-producing region received 12% above normal rainfall in the monsoon season annually. As a result, its seed cultivation area in Rajasthan, the largest producing state in India tremendously rose 10% Y/Y to 2.84 lakh ha. The trade sources reports incessant rains in late September and in early October, which causes around 20% of crop damage (http://www.karvycomtrade.com). It is a drought-tolerant annual legume crop majorly grown in arid zones of west and North West India and parts of Pakistan. So, growers that cultivate cluster bean under one cycle were exposed to a greater risk of total crop loss due to drought and high temperatures than are encountered during the spring cycle. However, not much significant difference in lesion length between locations were observed in this study, the average mean temperature at the N. Indian field locations goes approximately from 30 to 35°C. Punia et al. [38] has reported variation in some elite varieties of cluster bean based on their peculiar morpho-physiological characteristics. We had chosen the two diversified cultivars from the elite list for studying the effectual virulence of Macrophomina.
The fungus produces distinctive blackish stem lesions on infected plants that can be used for quantitative evaluation of pathogenicity. Isolates with dsRNA elements were significantly less virulent than dsRNA-free isolates in both the elite cultivars (Table 3). The combined analysis of the isolates based on virulence index, disease severity and mean lesion length for both the elite cultivars indicated that the three most virulent were free of dsRNA (Table 3, 4). These results strongly suggest a strong association between the presence of dsRNA and decline in both mycelial growth rates and virulence. Similar reports of dsRNA have been observed for isolates of C. parasitica [21] and S. sclerotiorum [18].
In recent years, dsRNA-associated novel host–parasite interaction for the regulation of virulence has engrossed significant interest in plant pathogenic fungal diseases as eventual biological control agent for diseases. Transmission of dsRNA viruses occur either through cell division followed by spore or conidial production i.e. vertical transmission [39, 40] or by cell fusion between genetically compatible strains i.e. horizontal transmission [11, 13]. Because of the double-stranded nature of the dsRNAs, they are resistant to RNase treatment under high salt conditions [21]. Further studies with large number of strains, and including broad range of host plants could yield more information. The occurrence of dsRNA in Indian M. phaseolina isolates provides an additional means to distinguish Indian populations from those in other regions of the world. dsRNA may thus serve as another useful character for monitoring the migration of Indian isolates to other parts of the world. Confirmation of unique viral genetic elements and their genetic relatedness can be done by hybridization analysis in varying dsRNA patterns observed in the isolates in future by further following molecular ecology studies of dsRNA.
Conclusion
The data presented here will make it possible to characterize the individual viral effects in more detail in future. This could provide a viable approach for introducing phenotypic characteristics into the given fungus that would be useful for biological control of the pathogen. Further studies are required to elucidate the acquisition mechanisms of infectious elements and other factors of the host fungus to evolve additional dsRNA elements. A much better understanding of the basic population ecology of fungi is necessary before predictions of the potential transfer and escape of engineered candidate genes could be made. The present study provides information about characterization of various fungal isolates using dsRNA as potential epidemiological tool associated with virulence and pathogenicity traits.
References
Hymowitz T, Matlock RS (1963) Guar in the United States. Okla Agric Exp Stn Bull B 611:1–34
Dhingra OD, Sinclair JB (1978) Biology and pathology of Macrophomina phaseolina. Viscosa, Minosa
Indera K, Singh T, Machado CC, Sinclair JB (1986) Histopathology of soybean seed infection by Macrophomina phaseolina. Phytopathology 76:532–535
Mihail JD (1992) Macrophomina. In: Singleton L, Mihail J, Rush C (eds) Methods for research on soil-borne phytopathogenic fungi. American Phytopathology Society, St. Paul, pp 134–136
Fernandez RB, De Santiago A, Delgado SH, Perez NM (2006) Characterization of Mexican and non-Mexican isolates of Macrophomina phaseolina based on morphological characteristics, pathogenicity on bean seeds and endoglucanase gene. J Plant Pathol 88:53–60
Karunanithi KM, Muthusamy Seetharaman K (1999) Cultural and pathogenic variability among the isolates of Macrophomina phaseolina causing root rot of sesame. Plant Dis Res 14:113–117
Almeida A, Abdelnoor R, Arias C, Carvalho V, Filho D, Marin S, Benato S, Pinto M, Carvalho C (2003) Genotypic diversity among Brazilian isolates of Macrophomina phaseolina revealed by RAPD. Fitopatol Bras 28:279–285
Jana TK, Sharma TR, Prasad RD, Arora DK (2003) Molecular characterization of Macrophomina phaseolina and Fusarium species by using a single primer RAPD technique. Microbiol Res 158:249–257
Purkayastha S, Kaur B, Dilbaghi N, Chaudhury A (2006) Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR based molecular markers. Plant Pathol 55:106–116
Pecina V (2000) Detection of dsRNA in Macrophomina phaseolina. Mycology 92(5):900–907
Bidochka MJ, Melzer MJ (2000) Genetic polymorphisms in three subtilisin-like protease isoforms (Pr1A, Pr1B, and Pr1C) from Metarhizium strains. Can J Microbiol 46(12):1138–1144
Leal SCM, Bertioli DJ, Ball BV, Butt TM (1994) Presence of double stranded RNA and virus-like articles in the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Tech 4:89–94
Liu YC, Milgroom MG (1996) Correlation between hypovirus transmission and the number of vegetative incompatibility (vic) genes different among isolates from a natural population of Cryphonectria parasitica. Phytopathology 86:79–86
Martins MK, Furlaneto MC, Sosa-Gomez DR, Faria MR, Fungaro MHP (1999) Double-strand RNA in the entomopathogenic fungus Metarhizium flavoviride. Curr Genet 36:94–97
Castanho B, Butler EE, Shepherd RJ (1978) The association of double stranded RNA with Rhizoctonia decline. Phytopathology 68:1515–1519
Anagnostakis SL, Day PR (1979) Hypovirulence conversion in Endothia parasitica. Phytopathology 69:1226–1229
Pusey PL, Wilson C (1982) Detection of double stranded RNA viruses in Ceratocystis ulmi. Phytopathology 73:470–474
Boland GJ (1992) Hypovirulence and double-stranded RNA in Sclerotinia sclerotiorum. Can J Plant Pathol 14:10–17
MacDonald WL, Fulbright DW (1991) Biological control of chestnut blight: use and limitations of transmissible hypovirulence. Plant Dis 75:656–661
Robin C, Anziani C, Cortesi P (2000) Relationship between biological control, incidence of hypovirulence, and diversity of vegetative compatibility types of Cryphonectria parasitica in France. Phytopathology 90:730–737
Morris TJ, Dodds JA (1979) Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 69:854–858
Valverde RA, Fontenot JF (1991) Variation in double-stranded ribonucleic acid (dsRNA) among pepper cultivars. J Am Soc Hortic Sci 116:903–905
Bogo MR, Queiroz MV, Silva DM, Gimenez MP, Azevedo JL, Schrank A (1996) Double-stranded RNA and isometric virus like particles in the entomopathogenic Metarhizium anisopliae. Mycol Res 100:1468–1472
Anagnostakis SL (1981) Stability of double-stranded RNA components of Endothia parasitica through transfer and subculture. Exp Mycol 5:236–242
Mayek-Pérez N, López-Castañeda C, González-Chavira M, García-Espinosa R, Acosta-Gallegos JA, Martínez-De la Vega O, Simpson J (2001) Variability of Mexican isolates of Macrophomina phaseolina on basis of pathogenesis and AFLP genotype. Physiol Mol Plant Pathol 59:257–264
Manici LM, Caputo F, Castro C (1995) Temperature responses of isolates of Macrophomina phaseolina from different climatic regions of sunflower production in Italy. Plant Dis 79:838–839
Zazzerini A, Tosi L (1989) Chlorate sensitivity of Sclerotium bataticola isolates from different hosts. J Phytopathol 126:219–224
El-Deeb A, Mohamed H, Hilal A (1985) Studies on charcoal rot disease of sunflower in Egypt. The First National Conference Pests and Diseases of Vegetables and Field Crops in Egypt. Ismailia University, Ismailia, pp 999–1012
Buck KW (1986) Fungal virology-an overview. In: Buck KW (ed) Fungal virology. CRC Press, Boca Raton, pp 1–84
Nuss DL, Koltin Y (1990) Significance of dsRNA genetic elements in plant pathogenic fungi. Annu Rev Phytopathol 28:37–58
Bottacin AM, Levesque CA, Punja ZK (1994) Characterization of dsRNA in Chalara elegans and effects on growth and virulence. Phytopathology 84:303–312
Brarathan N, Tavantzis SM (1990) Genetic diversity of double-stranded RNA from Rhizoctonia solani. Phytopathology 80:631–635
Hunst PL, Latterell FM, Rossi AE (1986) Variation in double-stranded RNA from isolates of Pyricularia oryzae. Phytopathology 76:674–678
Enebak SA, MacDonald WL, Hillman BI (1994) Effect of dsRNA associated with isolates of Cryphonectrla parasitica from the Central Appalachians and their relatedness to other dsRNAs from North American and Europe. Phytopathology 84:528–534
Zhou T, Boland GJ (1997) Hypovirulence and double-stranded RNA in Sclerotinia homoeocarpa. Phytopathology 87:147–153
Kousik CS, Snow JP, L’alverde RA (1994) Comparison of double-stranded RNA components and virulence among isolates of Rhizoctonia solani AG1 1A and AGI1B. Phytopathology 84:44–49
Diourte M, Starr JL, Jeger MJ, Stack JT, Rosenow DT (1995) Charcoal rot resistance and the effects of water stress on disease development in sorghum. Plant Pathol 44:196–202
Punia A, Yadav R, Arora P, Chaudhury A (2009) Molecular and morphophysiological characterization of superior cluster bean (Cymopsis tetragonoloba) varieties. J Crop Sci Biotechnol 12(3):143–148
Elias KS, Cotty PJ (1996) Incidence and stability of infection by double-stranded RNA genetic elements in Aspergillus section flavi and effects on aflatoxigenicity. Can J Bot 74:716–725
Melzer MS, Dunn M, Zhou T, Boland GJ (1997) Assessment of hypovirulent isolates of Cryphonectria parasitica for potential in biological control of chestnut blight. Can J Plant Pathol 19:69–77
Acknowledgments
Ms. Pooja Arora thanks Council of Scientific & Industrial Research for Senior Research Fellowship. Transgenic Green House facility established under FIST program, Department of Science & Technology, Ministry of Science & Technology, Government of India, New Delhi is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arora, P., Dilbaghi, N. & Chaudhury, A. Detection of double stranded RNA in phytopathogenic Macrophomina phaseolina causing charcoal rot in Cyamopsis tetragonoloba . Mol Biol Rep 39, 3047–3054 (2012). https://doi.org/10.1007/s11033-011-1067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1067-9