Abstract
The tetra-membrane-spanning protein, CD9 is a 24–27 kDa cell surface glycoprotein expressed in a wide variety of human cells being involved in a variety of cell processes, including signaling, adhesion, motility, fertilization and tumor cells metastasis. By means of a polyclonal antibody (N1) raised against recombinant swine CD9 protein, we studied the immunohistochemical expression of CD9 on different normal swine tissues. Immunochemistry shows that swine CD9 was distribute in a similar form than in human tissues, being present on epithelial cells of lung, liver, kidney, skin, tonsil, testis (epididymo), gut mucosa, uterus and mama. Furthermore, polyclonal antibody against swine CD9 reacts with white matter from cerebrum and cerebellum, peripheral nerves fibers and Hassal corpuscle from thymus and ovum. Platelets react strongly with our antibody, but monocytes and neutrophils react lightly. These results suggest that CD9 antigen should play a similar functional role in swine and human and therefore studies on CD9 on swine as an animal model would allow new knowledge about its role in adhesion, fertilization and tumor metastasis among other important biomedical processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD9 is a 24-kDa member of the tetraspanin or transmembrane-4 (TM4) superfamily of surface molecules (33 human members) that cross the cellular membrane four times, having both NH2 and COOH termini inside the cell, exhibits two extracellular loops and short cytoplasmic domains [1, 2]. CD9 is widely present on the surface of normal and malignant animal cells [3–6]. Originally, CD9 was identified as a surface antigen on a wide variety of lymphohemopoietic cells [7], however it is also widely expressed in non-lymphohemopoietic tissues including tissues of the central and peripheral nervous system [3, 8, 9]. In fact, CD9 molecule was absent on primitive hematopoietic progenitors and has been proposed as a tool for enrichment of hematopoietic stem cells [10, 11].
CD9 molecule has been involved in a variety of biological functions including cellular growth and development, activation, adhesion, and motility [12–14]. It is well known that CD9 is involved in platelet aggregation together with FCyRII receptor and aIIβ3 integrin [15]. It is also known that CD9 participates in adhesive and migratory events via integrins of the β1 family, especially a4β1 and α5β1, with which it seems to be non-covalently associated in various non hematopoietic cells lines [16, 17]. Furthermore, it has been also known that CD9 can associate with other proteins, like EFGR, or the membrane metalloproteinase MT1-MMP [18, 19], and that CD9 may exhibit its effect as a molecular organizer by modulating the composition of adhesive complexes important in facilitating cell adhesion and matrix assembly [18, 20].
CD9 is also related to cell differentiation, having been observed a drastic reduction in its expression in some metastatic cancers as lung, breast, colon, bladder pancreatic cancer and squamous cell carcinoma, as well as malignant melanoma, when they are compared with the primary cells [21–23]. In fact, the CD9 expression level has been inversely correlated with invasion and metastasis [5, 24], or with the prognosis of patients with different types of cancer [25, 26]. Other recent results suggest that CD9 might have an important role that attenuates EGFR signaling affecting EGF-induced signaling in cancer cells [27]. These results provide insight into the role of CD9 in the presentation of TGF-α in epithelial and carcinoma cells, whose physiology is driven by ligand-induced EGFR activation [28].
On the other hand, CD9 also plays an important role in the gamete membrane interactions that regulate the fertilization in mice. For example, oocytes from CD9 knockout mice were rarely fertilized although both the ovulation and the maturation were normal [29]. Additionally, reduced sperm binding was found in zona-pellucida free mouse oocytes treated with anti-CD9 monoclonal antibodies [30–33]. Recently, has been shown [34] that CD9 is also expressed on pig ovarian tissues, oocytes and spermatozoa and that the fertilization can be blocked by anti-CD9 antibodies.
In domestic animals, CD9 has also been found to be a putative cellular receptor for distemper virus in canine specie [35] and for the immunodeficiency virus in feline species [36].
In a previous investigation we have cloned and sequenced the porcine CD9 gene, and studied the expression pattern of the porcine CD9 mRNA [37] showing that, like its homologs in other species, CD9 transcripts are ubiquitously expressed, although with heterogeneous levels depending on cells and tissues.
However, except for the localization of the CD9 pig protein on oocytes and stem cell populations, scarce information is available for swine CD9 protein expression on cells and tissues, although swine, beside to be a major food-animal group for humans, constitutes an animal model in biomedical research due to the anatomical and physiological similarities with humans [38]. For this, it is an important task in porcine immunology research to study more precisely the expression and distribution of CD9 on swine cells and tissues.
On the other hand, although, as an exception, it has been used a cross reactive anti-human CD9 monoclonal antibody to localize CD9 in porcine oocytes [34], porcine CD9 molecule is not usually recognized by human antibodies against it [39]. Therefore, to detect the porcine CD9 it is more effective to produce specific anti-porcine CD9 antibodies.
With the aim of defining the detailed expression of the porcine CD9 protein, we first produced a polyclonal antibody (N1) against a recombinant protein of the porcine CD9 molecule and then we used it to test its immunochemical distribution in a broad variety of healthy swine cells and tissues.
Materials and methods
Tissues and cells
All tissues (skin, gut, kidney, liver, pancreas, brain -cerebral and cerebellum cortex-, tonsil, ovary, mama, testis -epididymo-, peripheral nerve, thymus, spleen and lymph node) were obtained fresh from the local abattoir from 6 to 8 months old swine. Peripheral blood mononuclear cells (PBMCs) and granulocytes were isolated from pig blood by density gradient centrifugation on Ficoll-Paque (Pharmacia). Porcine platelet-rich plasma (PRP) was obtained by low centrifugation of blood anticoagulated with trisodium citrate. Platelets were pelleted from PRP by centrifugation at 2,200g for 7 min and washed three times with PBS containing 5 mM EDTA.
Recombinant CD9 protein (rpCD9)
cDNA sequence covering nucleotides 426–674 that encodes the third TM domain and most part of the second extracellular domain, EC2, of the porcine CD9 protein [see 37] was amplified by PCR from plasmid template. Primers used for amplification contained restriction sites (indicated in bold) enabling ligation into the expression vector PGEX-4T-1 (Amersham Biosciencies) following digestion of the PCR product and the vector with EcoRI. Primers were as follows: F426-EcoRI 5′-GGAGAATCCCACAAGGATCAGGTGATCAAA-3′ (sense) and R674-EcoRI 5′-GGCGAATTCGATGTGTAACTTGTTTTGGAA-3′ (antisense). A 249 bp PCR product was ligated into the expression vector pET28b and used to transform Escherichia coli strain BL21 (DE3) (Novagen). Protein expression was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 37°C. Cells were collected by centrifugation at 4,000g, 20 min, 4°C, and suspended in PBS buffer, then sonicated 15 times at intervals of 60 s on ice followed by incubation in lyses buffer 1% Triton X-100, 1 mg/ml lysozyme) for 1 h, 4°C. After centrifugation at 1,000g, 30 min, 4°C, an about 8 kDa recombinant EC2-CD9 protein was purified from the supernatant by affinity chromatography using a Glutatione Sepharose 4B Matrix (Amersham Biosciences) according to the manufacturer’s instructions. Purified recombinant protein was dialyzed against PBS containing 5 M urea and adjusted to a final concentration of 1 mg/ml. Purity of the recombinant protein was assessed by SDS-PAGE, followed by Coomassie blue staining. Protein identification was carried out by MALDI-TOF mass spectrometry analysis of the tryptic peptides.
Polyclonal antibody production
New Zealand rabbits, obtained from the animal facilities at University of Córdoba, Spain, were intradermally immunized with rpEC2-CD9 (50 mg) in an equal volume of complete Freund’s adjuvant, and then were boosted five times with an emulsion of recombinant protein and incomplete Freund’s adjuvant at 1-week intervals. Two week after the last booster, rabbit serum was collected and titers of antibodies specific for rpEC2-CD9 assayed using an antibody capture ELISA.
Western blot
Purified porcine rpEC2-CD9 was mixed with the sample buffer and run on a 5–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions. The separated protein were transferred to an Immobilon membrane (Millipore, Bedford, MA, USA) using a semi-dry transfer cell (Millipore). Immobilon free binding sites were blocked with PBS-2% BSA. Strips were then cut and incubated with the polyclonal antibody for 2 h at room temperature, followed by 1 h incubation with a peroxidase-conjugated rabbit anti-mouse Ig (Sigma). Peroxidase activity was visualized using the ECL detection method (Amersham).
Immunohistochemistry
All tissue specimens were fixed in Bouin liquid for 16 h. Tissues were dehydrated in ascending concentrations of ethanol and xylene and embedded in paraffin. Sections of 5 µm were prepared from selected tissue blocks and consecutive sections were placed on slides coated with Vectabound (Vector Laboratories, Inc.). The tissue slides were kept at 55°C for 45 min in an oven to improve the adherence of sections to glass.
The sections were deparaffinized and rehydrated in xylene and descending concentrations of ethanol, respectively. Endogenous peroxidase activity was inhibited by treatment with 3% hydrogen peroxidase in distilled water for 30 min at room temperature. After washing with PBS, the sections were incubated with normal goat serum (1:10 dilution in PBS) (Vector) for 30 min at room temperature. After removing the serum, polyclonal antibody (1/3,000 dilution in PBS) or normal rabbit serum (as negative control) were added for 18 h at 4°C in a wet chamber. The sections were washed in PBS and incubated with biotinylated anti-mouse Ig (Dako) diluted 1/50 in PBS for 30 min at room temperature. After washing again in PBS, tissue sections were covered with avidin–biotin peroxidase complex (Sigma) diluted 1/50 with PBS for 1 h in a wet chamber at room temperature, washed and then developed with 3, 3′-diaminobenzidine (Sigma) (5 µg in 10 ml PBS). Sections were counterstained with Mayer hematoxylin and mounted with Eukitt. The sections of lymph node were covered with biotin–streptavidin–alkaline phosphatase complex (Biogenex). The chromogen substrate for alkaline phosphatase was naphthol and fast red (20% w/v). After incubation, slides were washed and mounted in aqueous medium of Shandon Immuno-Mount (Sigma).
Results
Purification of recombinant CD9 protein (rpCD9)
A swine recombinant CD9 protein (rpCD9) encoded by cDNA sequence covering nucleotides 426–674 that includes the third TM domain and most part of the second extracellular domain, EC2, was purified after the expression of the cDNA PCR sequence into pGEX-4T-1 expression vector. Figure 1a shows three fractions of the purified rpCD9 protein showing a molecular weight of about 8 kDa, as well as the glutathione-S-transferase (GST) tail (about 30 kDa) released from the fusion protein after thrombin digestion. After rabbit immunization with the purified 8 kDa rpCD9 protein the N1 polyclonal antibody was obtained. Figure 1b shows an immunoblotting of the N1 antibody detecting the rpCD9 protein.
Tissue distribution of the swine CD9 molecule
The precise localization of the protein coded by CD9 gene was studied by immunohistochemistry with N1 polyclonal antibody obtained using a porcine CD9 recombinant protein. The antibody was raised against a recombinant protein corresponding to a 243 amino acid polypeptide fragment located in the EC2 extracellular domain of the porcine CD9 molecule, the most variable domain among the species compared [37]. The reactivity of this polyclonal antibody was tested on a broad panel of healthy porcine cell and tissues: PBMCs, granulocytes, platelets, skin, gut, kidney, liver, pancreas, brain -cerebral and cerebellum cortex-, tonsil, ovary, mama, testis -epididymo-, peripheral nerve, thymus, spleen and lymph node. The representative results of this immunohistochemical analyses are shown in Fig. 2.
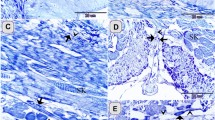
Immunohistochemical staining of formalin-fixed, paraffin-embedded sections of different porcine tissues with N1 polyclonal antibody. a Skin (×20), b lung (×40), c gut (×10), d kidney (×20) and detail of tubules (×40), e liver (×40), f pancreas (×20), g1 cerebron (×4), g2 cerebellum (×10), h tonsila (×10), i uterus (×20), j ovary (×10) and ovum detail (×40), k mama (×20), l testis, epididymo (×40), m peripheral nerve (×40), n thymus (×20)
In skin, the anti-CD9 polyclonal produced an intense staining of the he epidermis, especially in the membrane of all keratinocytes. However it did not stain the dermis (Fig. 2a).
In lung, the reaction was positive within the parenchyma, in the smooth muscle associated with bronchi, in epithelium of bronchi and bronchioles, in blood vessels, and in alveolar pneumocytes. Macrophages inside alveolar sacs also showed some staining (Fig. 2b).
In gut, CD9 was detected on the crypts and on the bases of the villi. Smooth muscle was also stained. Staining of some intraepithelial lymphocytes was also observed (Fig. 2c).
In kidney, the anti-CD9 strongly stained renal corpuscules and epithelial cells of collecting ducts, detecting a weaker staining on glomerular endothelium and Bowman capsule (Fig. 2d).
In liver, the endothelium in hepatic sinusoids was weakly stained. The cell membranes of hepatocytes also showed a weak staining (Fig. 2e).
In pancreas, epithelial cells of excretory conducts are stained but acinar cells and pancreatic islets were either unstained or lightly stained for CD9 (Fig. 2f).
In brain, cerebral and cerebellum cortex, the anti-CD9 polyclonal stained the white matter. However, the cytoplasm of the cells gray matter showed a strong diffuse staining, and the Purkinje cells were not stained (Fig. 2g).
In tonsil, squamous epithelium, germinal centers, and high endothelial venules (HEVs) were stained by polyclonal ant-CD9. A strong staining for CD9 was observed on cell membranes throughout the epithelium (Fig. 2h).
In uterus, the epithelial cells covering both the endometrio and the endometrial glandules were stained. Reaction was only observed in membrane at the basal zones of the cellules (Fig. 2i).
In ovary, the cytoplasm of oocytes were strongly stained (Fig. 2j).
In mama, two clearly differentiate zones were detected: the epithelial cells of the glandule conducts were strongly stained, and the epidermis of the nipple showed a weakly staining (Fig. 2k).
In testis, the epididymo epithelial cells were strongly stained, however this stain was not homogeneous, being concentrated on the basal zone (Fig. 2l).
In peripheral nerves, the fibbers were stained within transverse and longitudinal sections of skeletal muscle. The perineurium of peripheral nerve fascicles was strongly positive for CD9 (Fig. 2m).
In thymus, the Hassall’s corpuscles were stained by N1 antibody, and some infiltrate macrophages were also lightly stained (Fig. 2n).
In spleen and lymph node, a negative staining was obtained (data not shown).
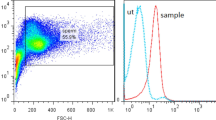
In peripheral blood the polyclonal against CD9 stained platelets (Fig. 3a) and several other hemopoietic cell types, including neutrophils, macrophages and monocytes (Fig. 3b) and granulocytes (Fig. 3c).
Discussion
A specific polyclonal antibody against swine CD9 molecule have been produced and used to study the expression of CD9 molecule in a broad group of swine cells and tissues. A summary of differences and coincidences on the expression of swine and human CD9 can be observed in Table 1 where data obtained by Sincock et al. [40] and Nakamura et al. [41] were compared with our own data. The following conclusions can be remarked from our study:
The peripheral blood cells distribution of CD9, as described in the present study, agrees with previous studies in humans [8, 40, 42].
CD9 expression on swine ovum cytoplasm is in according to data about the importance of CD9 in fertilization of mice and swine offered by Miyado et al. [43] and Li et al. [34], respectively, and supports that CD9 might have an important role in the fusion of sperm and oocytes.
Expression of CD9 in the human brain [44] has also been confirmed in swine with the positive stain of the white matter of cerebellum and cerebrum cortex.
The general distribution of CD9 in epithelial cells supports that this molecule is involved in multiple cellular interactions and possibly in intercellular adhesion.
The expression of CD9 by gut epithelium was restricted to the bases of the villi. Therefore, CD9 function may be limited to interactions with specific extracellular matrix components in the crypt microenvironment.
The widespread expression of these antigens throughout connective tissue probably reflects their interaction with components of the extracellular matrix such as collagen, laminin and fibronectin.
Discrepancies have found especially in spleen, pancreas and some peripheral blood cells like neutrophils may arise because different anti-CD9 antibodies may not necessarily be equivalent in their recognition of CD9. For example, the glycosylation state of CD9 in different cell types may vary, thus modifying antigen recognition by certain antibodies, especially if these are monoclonal antibodies [40, 41].
In summary, this study has shown by the first time the tissue distribution of swine CD9. Evidence about the similar distribution of CD9 between human and swine cells and tissues have been presented. All these evidences open the possibility to use swine as animal model in studying human biological processes, as adhesion, motility, fertilization or tumor metastasis, in which CD9 molecule is or could be involved.
Abbreviations
- BSA:
-
Bovine serum albumin
- DIG:
-
Digoxigenin
- DMSO:
-
Dimethylsulphoxide
- EGFR:
-
Epidermal growth factor receptor
- MT1-MMP:
-
Membrane type-1 matrix metalloproteinase
- PBL:
-
Peripheral blood lymphocyte
- PBMC:
-
Peripheral blood mononuclear cell
- PBS:
-
Phosphate buffered saline
- PRP:
-
Porcine platelet-rich plasma
- SDS:
-
Sodium dodecylsulfate
- TGF:
-
Transforming growth factor
References
Lanza F, Wolf D, Fox CF et al (1991) cDNA cloning and expression of platelet p25/CD29. J Biol Chem 266:10638–10645
Hemler ME (2003) Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Develop Biol 19:397–422
Maecker HT, Todd SC, Levy S (1997) Tetraspanin superfamily: molecular facilitators. FASEB J 11:428–442
Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114:4143–4151
Boucheix C, Rubinstein E (2001) Tetraspanins. Cell Mol Life Sci 58:1189–1205
Hemler ME (2001) Specific tetraspanin functions. J Cell Biol 155:1103–1107
Kersey JH, Lebein T, Abramson CS et al (1981) p24 a human leukemia associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody Ba2. J Exp Med 153:726–731
Boucheix C, Benoit P (1988) CD9 antigen: will platelet physiology help to explain the function of a surface molecule during hemopoietic differentiation. Nouv Rev Fr Hematol 30:201–202
Nakamura Y, Iwamoto R, Mekada E (1996) Expression and distribution of CD9 in myelin of the central and peripheral nervous systems. Am J Pathol 149:575–583
Ash RC, Jansen J, Kersey JH, LeBien TW, Zanjani ED (1982) Normal human pluripotential and committed hematopoietic progenitors do not express the p24 antigen detected by monoclonal antibody BA-2: implications for immunotherapy of lymphocytic leukemia. Blood 60:1310
Heinz M, Huang CA, Emery DW, Giovino MA, LeGuern A, Kurilla-Mahon B, Theodore P, Arn JS, Sykes M, Mulligan R, Down JD, Sachs DH, Goodell MA (2002) Use of CD9 expression to enrich for porcine hematopoietic progenitors. Exp Hematol 30(7):809–815
García-López MA, Barreiro O, García-Díez A, Sánchez-Madrid F, Penas PF (2005) Role of tetraspanins CD29 and CD151 in primary melanocyte motility. J Invest Dermatol 125:1001–1009
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6:801–811
Longhurts CM, Jacobs JD, White MM et al (2002) Chinese hamster ovary cell motility to fibronectin is modulated by the second extracellular loop of CD9: identification of a putative fibronectin binding site. J Biol Chem 277:32445–32552
Jennings LK, Fox CF, Kouns WC et al (1990) The activation of human platelets mediated by anti-human platelet p24/CD9 monoclonal antibodies. J Biol Chem 265(7):3815–3822
Rubinstein F, Le Nanour F, Billar M, Prenant M, Boucheix C (1994) CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol 24:3005–3013
Kotha J, Longhurst C, Appling W, Jennings LK (2008) Tetraspanin CD9 regulates b1 integrin activation and enhances motility to fibronectin via a PI-3 kinase-dependent pathway. Exp Cell Res 314:1811–1822
Cook GA, Longhurst CM, Grgurevich S, Crossno JT, Jennings LK (2002) Identification of CD9 extracellular domains important in regulation of CHO cell adhesion to fibronectin and fibronectin pericellular matrix assembly. Blood 100(13):4502–4511
Lafleur MA, Xu D, Helmer ME (2009) Tetraspanin protein regulates membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol Biol Cell 20:2030–2040
Stipp CS, Kolesnikova TV, Helmer ME (2003) Functional domains in tetraspanin proteins. Trends Biochem Sci 28:106–112
Cajot JF, Sordat I, Sylvester T, Sordat B (1997) Differential display cloning identifies motility-related protein (MRP1/CD9) as highly expressed in primary compared to metastatic human colon cancer cells. Cancer Res 57:2593–2597
Sauer G, Windisch J, Kurzeder C, Heilmann V, Kreinberg R, Deisller H (2003) Progression of cervical carcinoma is associated with down-regulation of CD9 but strong local re-expression at sites of transendothelial invasion. Clin Cancer Res 9:6426–6431
Hori H, Yano S, Koufuji K, Takeda J, Shirouzu K (2004) CD9 expression in gastric cancer and its significance. J Surg Res 117:208–215
Takeda T, Hattori N, Tokuhara T, Nishimura Y, Yokoyama M, Miyake M (2007) Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res 67:1744–1749
Miyake M, Nakano K, Itoi SI, Koh T, Taki T (1996) Motility related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res 56:1244–1249
Sakamoto K, Nakamura Y, Nakashima (2004) Immunohistochemical distribution of CD9 in parotid gland tumors. Auris Nasus Larynx 31(1):49–55
Murayama Y, Shinomura Y, Oritani K et al (2008) The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol 216(1):135–143
Imhoff I, Gasper WJ, Derynk R (2008) Association of tetraspanin CD9 with transmembrane TGFa and cytoskeletal organization. J Cell Sci 121:2265–2274
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A (2000) The gamete fusion process is defective in eggs of CD9-deficient mice. Nat Genet 24:279–282
Chen MS, Tung KSK, Coonrod SA, Takahashi Y, Bigler D, Chang A, Tamashita Y, Kincade PW, Herr JC, White JM (1999) Role of the integrin associated protein CD9 in binding between sperm ADAM 2 and the egg integrin a6b1: implications for murine fertilization. Proc Natl Acad Sci USA 96:11830–11835
Takhashi Y, Bigler D, Ito Y, White JM (2001) Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of the b1 integrin-associated proteins CD9, CD81, and CD98. Mol Biol Cell 12:809–820
Wong GE, Zhu X, Prater CE, Oh E, Evans JP (2001) Analysis of fertilin (ADAM 1)-mediated sperm–egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J Biol Chem 276:24937–24945
Zhu X, Evans JP (2002) Analysis of the roles of RGD-binding integrins, a4/a9 integrins, α6 integrins, and CD9 in the interaction of the fertilin b (ADAM) disintegrin domain with the mouse egg membrane. Biol Reprod 66:1193–1202
Li Y-H, Hou Yi, Ma Wei, Yuan Jin-Xiang, Zhang Dong, Sun Qing-Yuan, Wang Wei-Hua (2004) Localization of CD9 in pig oocytes and its effects on sperm–egg interaction. Reproduction 127:151–157
Willett BJ, Hosie MJ, Jarrett O, Neil JC (1994) Identification of a putative cellular receptor for feline immunodeficiency virus as the feline homologue of CD9. Immunology 81:228–233
Hosie M, Willeft BJ, Dunsford TH, Jarrett O, Neil JC (1993) A monoclonal antibody which blocks infection with feline immunodeficiency virus identifies a possible non-CD4 receptor. J Virol 1667–1671
Yubero N, Jiménez-Marín A, Yerle M, Morera L, Barbancho M, Llanes D, Garrido JJ (2003) Molecular cloning, expression pattern and chromosomal mapping of pig CD9 antigen. Cytogenet Genome Res 101:143–146
Tanaka H, Kobayashi E (2006) Education and research using experimental pigs in a medical school. J Artif Organs 9:136–143
Serebruany VL, Ordonez JV, Yurovsky VV, Gurbel PA (1998) The crossreactivity of human vs swine platelet surface antigens: similarity of glycoproteins Ib and IIIa, but not IIb/IIIa complex. J Thromb Thrombolysis 5:37–41
Sincock PM, Mayrhofer G, Asham LK (1997) Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, Cd63 and α5β1 integrin. J Histochem Cytochem 45:515–525
Nakamura Y, Handa K, Iwamoto R, Tsukamoto T, Takahasi M, Mekada E (2001) Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin α3β1 in normal human tissues. J Histochem Cytochem 49(4):439–444
Jones NH, Borowitz MJ, Metzgar RS (1982) Characterization and distribution of a 24, 000-molecular weight antigen defined by a monoclonal antibody (DU-ALL-1) elicited to common acute lymphoblastic leukaemia (cALL) cells. Leuk Res 4:449–464
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of CD9 on the egg plasma membrane for fertilization. Science 287:321–324
Rossler K, Neuchrist C, Kitz K, Scheiner O, Kraft D, Lassman H (1992) Expression of leukocyte adhesion molecules at the human blood brain barrier (BBB). J Neurosci 31:365–374
Acknowledgments
This work has been founded by the National R&D Program Grant of the Spanish Ministry of Education and Science (AGL2002-00529 and AGL2005-01561). Noemi Yubero was a postgraduate scholar of the Spanish Research Programme. Juan J. Garrido was a recipient of a “Ramón y Cajal” Grant of the Spanish Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yubero, N., Jiménez-Marín, Á., Lucena, C. et al. Immunohistochemical distribution of the tetraspanin CD9 in normal porcine tissues. Mol Biol Rep 38, 1021–1028 (2011). https://doi.org/10.1007/s11033-010-0198-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0198-8