Abstract
This study investigated the effects of the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) on cartilage degradation in an experimental model of osteoarthritis (OA). Thirty-two male New Zealand rabbits underwent unilateral anterior cruciate ligament transection (ACLT) on left knee joints to induce OA and were randomly divided into two groups (n = 16), the TSA group was injected intra-articularly with 0.3 ml TSA [250 ng/ml in the dimethylsulphoxide (DMSO)], the OA group received DSMO since 4 weeks after operation once a week for 5 weeks. Rabbits were killed seven days after the last injection. Left knee cartilage was harvested for morphological, histological and genetic analysis. Another ten rabbits were used for normal control and received no injection. The TSA group showed less cartilage degradation as compared to the OA group assessed by morphological and histological evaluation. Gene expression of matrix metalloproteinase-1 (MMP-1), MMP-3, MMP-13, and interleukin-1 (IL-1) was increased significantly in the OA group compared to the normal group. The elevated expression was reduced by TSA. Our results suggest that TSA could be considered as a potential agent for treatment for OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a degenerative disease characterized by the degradation of articular cartilage. It is believed that proteolytic enzymes were involved in this process. Aggrecanases as well as matrix metalloproteinases (MMPs) have received intensively attentions [1–5]. It is well established that MMPs, as the main proteolytic enzymes play a critical role in the destruction of cartilage via degrading extracellular matrix (ECM) [5, 6]. Among these enzymes, MMP-1 and MMP-13 are of particular importance because they can degrade the components of the cartilage matrix including aggrecans and collagens, especially, collagen type II (Col II) which is the major component of the cartilage matrix [7–9]. MMPs expression was enhanced by the pro-inflammatory cytokine interleukin-1 (IL-1) [10, 11]. It has been well established that IL-1 plays a key role in cartilage destruction. In addition to increasing the expression of MMPs in chondrocytes, IL-1 is also involved in synovial inflammation in arthritis, leading to cartilage degradation. Previous study showed that inhibition the activity of IL-1 resulted in down-regulation of MMPs in chondrocytes [12].
Although nonsteroidal anti-inflammatory drugs (NSAID) and corticosteroid as well as hyaluronan have been used in the treatment of OA, they are failed to reverse cartilage damage. Therefore, investigators are still trying to find an ideal therapeutic agent in treatment for OA [13].
Histone deacetylase (HDAC) inhibitors are emerging as a promising new treatment strategy in cancer [14]. Besides, HDAC inhibitors also act as potent anti-inflammatory agents in animal studies and in cell culture models [15, 16]. Moreover, HDAC inhibitors can repress inflammatory responses in articular chondrocytes, for example, two HDAC inhibitors [trichostatin A (TSA), butyric acid (BA)] suppressed the production of nitric oxide (NO) and prostaglandin E2 (PGE2) in human OA chondrocytes as well as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) at both protein and mRNA level [17]. Furthermore, previous study has shown that HDAC inhibitors (TSA, NaBy) suppressed the gene expression of MMP-1, MMP-13 in chondrocytes [18]. Similar study was reported by other researchers [19].
The inhibition of inflammation and MMPs expression by HDAC inhibitors suggested their potential pharmacological use for arthritis. Animal studies have showed that HDAC inhibitors exhibited beneficial effects on rheumatoid arthritis (RA) mainly by suppressing synovitis [20–22]. However, little is known about the effects of HDAC inhibitors in the experimental OA model. In this study, we evaluated the in vivo effects of a HDAC inhibitor, trichostatin A on cartilage degradation following intra-articular injection in an experimental OA model at the concentration 250 ng/ml, which has been confirmed effective in vitro [17, 18].
Methods and materials
Reagents
TSA (Sigma, St. Louis, MO, USA) was dissolved in DMSO at 250 ng/ml.
Animals
Male New Zealand rabbits weighing 2.0 kg (Animal center of Zhejiang University) were used in this study. Rabbits were housed individually in stainless steel cages, provided standard laboratory food and water, maintained for one week. Thirty-two rabbits underwent unilateral anterior cruciate ligament transection (ACLT) on left knee joints as described in a preceding paper [23]. After surgery, animals were returned to cages and the limbs were not immobilized. The remained ten rabbits were used for normal control. All experiments were conducted with the approval of Zhejiang University Animal Care and Use Committee.
Treatment
Four weeks after surgery, the rabbits underwent ACLT were randomly divided into two groups of 16 animals each as the TSA group and the OA group. The TSA group was given intra-articular injection of 0.3 ml TSA (250 ng/ml) in the left knees once a week for 5 weeks. The OA group was injected with DMSO under the same conditions. Rabbits were killed seven days after the last injection. The left knee joints were harvested. Ten additional rabbits that did not undergo surgery and received no injection were used for normal control.
Gross morphology
The femoral condyles were examined. Gross morphological changes were evaluated by two independent researchers in a blind fashion after applying India ink. Criteria for grading were used as follows: Grade 1 (intact surface), grade 2 (minimal fibrillation), grade 3 (overt fibrillation), grade 4 (erosion) [24]. Condyles were prepared for histologic evaluation and gene expression analysis after morphologic grading.
Histological examination
Ten samples from each of the OA group and TSA group were fixed in 10% neutral buffered formalin, decalcified with ethylenediaminetetraacetate (EDTA) buffered at pH 7.4, dehydrated in a series of ethanol solutions and embedded in paraffin, cut into 5 μm sections and stained with Safranin O-fast green. The samples were scored for the degree of histological change using Mankin Score system [25]. Two independent researchers assessed the extent of histological cartilage damage in a blind manner.
Gene analysis by quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from the TSA treated group (n = 6), the OA group (n = 6) and the normal group (n = 6) using TRIzol (Invitrogen) reagent. First strand cDNA was synthesized from total RNA using Reverse Transcriptase cDNA synthesis kit (Promega) according to the manufacturer’s recommendation. Quantitative real-time PCR was performed with SYBR green detection using the iCycler (Bio-Rad, Hercules, CA, USA). Gene specific primer sequences were as follows: for MMP-1, forward AGGAGCCTTCCCAAGAGGAA; reverse CTTGTCTCTTGCATATCAGGATGATG, for MMP-3, forward ACACCGGATCTGCCAAGAGA; reverse ACACCGGATCTGCCAAGAGA, for MMP-13, forward ACACCGGATCTGCCAAGAGA; reverse CTGGAGAACGTGATTGGAGTCA, for IL-1, forward CGCATCTCCTGCCAACCCTACA; reverse GCTTCTCCAGAGCCACAACGACT, for 18S rRNA, forward GACGGACCAGAGCGAAAGC; reverse CGCCAGTCGGCATCGTTTATG. Specificity of the expected products was demonstrated by melting curves analysis. 18S rRNA was used as an internal control. PCR reactions for each sample were done in triplicate. Relative quantification of mRNA expression was calculated by the comparative Ct method described by the manufacturer. The relative quantification value of the target gene was expressed as 2-(△ct target gene −△ct18s rRNA).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis of the gross morphology data was performed by nonparametric Mann–Whitney U test, histological and gene expression data was analyzed by a paired t test. Differences were considered significant when P was less 0.05.
Results
Gross morphology evaluation
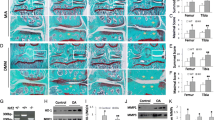
Rabbits in TSA group and OA group exhibited complete transection of the ACL and various degree of cartilage degradation. One condyle in the TSA group and none in the OA group showed Grade 1 damage. Five condyles in the TSA group and three in the OA group showed Grade 2 damage. Six of the TSA group and seven of the OA group showed Grade 3 damage. Four of the TSA group and six of the OA group showed Grade 4 damage. (Data are shown in Table 1 in supplementary data) Cartilage degradation in the TSA group was less severe than in the OA group, but this difference was not significant (P > 0.05). Typical photographs were showed in Fig. 1 .
Macroscopic analysis of the femoral condyles from normal group (a) TSA treated group (b) and OA group (c) in the 9 weeks after ACLT. Cartilage from normal group showed no lesions in surface. Arrowheads show complete loss of articular cartilage and exposure of the underlying bone in OA group and moderate velvety fibrillation, staining as black patches in TSA group. OA osteoarthritis, TSA trichostatin A, ACLT anterior cruciate ligament transection. M medial, L lateral
Histological analyses
Histological analyses showed the similar results. Cartilage from the OA group exhibited more severe degenerative changes including hypocellularity as well as loss of proteoglycans as compared to that treated with TSA (Fig. 2). When evaluated with Mankin score system, cartilage from the OA group had average values of 10.99 ± 4.21 while the TSA-treated cartilage showed significantly lower values of 8.52 ± 2.87 (P < 0.05). (Data are shown in Table 2 in supplementary data).
Representative Safranin O-stained sections of articular cartilage. Normal cartilage showed no loss of Safranin O staining (a). Arrowhead in cartilage from TSA-treated group indicate the loss of Safranin O staining in the superficial zones (b). As shown with arrowhead, loss of Safranin O staining was more serious in OA group (c) compared to the TSA-treated group (Original magnification ×50)
Expression of MMP-1, MMP-3, MMP-13, and IL-1 in Cartilage.
Quantitative real-time PCR was performed in triplicate and yielded almost identical results. The mRNA expression of MMP-1, MMP-3, MMP-13, and IL-1 was increased significantly in the OA group compared with the normal group and the up-regulated gene expression was suppressed by TSA (Fig. 3).
Discussion
In this study, we examined the effects of TSA on cartilage degradation in a widely used OA model induced by ACLT. Our data showed that severity of articular cartilage degradation was reduced and the histological scores of cartilage were improved in the TSA group as compared to that in the OA group, gene expression of MMP-1, MMP-3, MMP-13, and IL-1 was significantly suppressed by TSA in vivo.
We administered TSA by intra-articular injection at a concentration of 250 ng/ml which has been confirmed effective in vitro [17, 18]. Our results showed that TSA at this dose reduced the severity of cartilage damage in experimental OA as evaluated by morphological, histological and gene analysis. In previous studies, HDAC inhibitors were used at higher doses by intra-gastric administration, intra-venous or subcutaneous injection in RA model [21, 22].
MMPs are implicated in cartilage degradation via degrading collagens and aggrecans. In this study, we confirmed that expression of MMP-1, MMP-3, and MMP-13 was increased in arthritic cartilage. Our data are consistent with the findings of other studies that noted elevated MMP level in arthritic cartilage [26]. In the present study, genic analysis showed the augmented MMP-1,MMP-3, and MMP-13 expression in OA cartilage in vivo were suppressed by TSA. Our results indicate that TSA may inhibit MMPs expression and consequently reduce cartilage degradation in experimental OA rabbits. The results are in a line with the findings of other studies in vitro including those by Young et al. and Wang et al. [18, 19]. They reported that HDAC inhibitors repressed the expression of MMPs in human chondrocytes. Interestingly, the results are not consistent. Young et al. found that MMP-3 was not repressed by TSA in human chondrocytes while Wang et al. showed gene expression of MMP-3 was repressed by TSA in human chondrocytes. Nasu et al. [22] noted that TSA repressed MMP-13 expression in human chondrocytes but not in ATDC5 chondrogenic cell. MMP-2 expression affected by TSA also showed the different results in different cells [27]. These results indicate that HDAC inhibitors can suppress the expression of MMPs and the effects may depend on cell types, exposure time and stimulation conditions and so on.
In this study, we confirmed that gene expression of IL-1 was increased in OA cartilage. It is well known that cytokines secreted by synovial cells and chondrocytes are implicated in cartilage degradation. Among these cytokines, IL-1 is believed to play pivotal roles [28]. IL-1 contributes to cartilage degradation through increasing MMPs gene expression, reducing the production of TIMPs, up-regulating other cytokines and so on [29]. Thus, the inhibition of IL-1 may be effective in the treatment of OA. IL-1 receptor antagonist has been found to inhibit the Col II cleavage and the glycosaminoglycan (GAG) release in OA cartilage [12]. In the present study, TSA inhibited the expression of IL-1, it may partly due to the anti-inflammatory effects of TSA. It is well known that HDAC inhibitors has anti-inflammatory property, Leoni F et al. [30, 31] reported that ITF2357 and suberoylanilide hydroxamic acid (SAHA), two HDAC inhibitors, reduced the release of IL-1 in human peripheral blood mononuclear cells, respectively. In the present study, we examined the effects of TSA on IL-1 in cartilage in vivo. Our results confirmed the anti-inflammatory effects of HDAC inhibitors. The results may partly explain the protective effects of TSA on cartilage.
It is well known that NFκB plays critical role in the induction of pro-inflammatory cytokines such as IL-1 and catabolic factors including MMPs [32,33]. Therefore, inhibition of NF-κB activity may result in down-regulation of expression of these NF-κB dependant genes. Inhibition of DNA-binding activity of NF-kB by TSA and SAHA was reported in A549 cells, similar results were also noted in human colon cell lines [34, 35]. The inhibition of NF-κB activity may partly account for the depressant effects of TSA on gene expression of MMP-1, MMP-3,MMP-13, and IL-1. However, Chabane et al.[17] found that TSA and BA did not affect the DNA-binding activity of NF-κB in IL-1 stimulated human chondrocytes, thus, they speculated that down-stream of DNA-binding also may be influenced by HDAC inhibitors in chondrocytes.
It is well known that HDAC are related to transcriptional regulation [36]. In generally, HDAC inhibitors cause acetylation of histone, leading to a loosen nucleosomal structures and resulting in an increased transcription, on the other hand, HDAC inhibitors also down-regulate some expression [37, 38]. As found in the present study, TSA showed the inhibitory effects on gene expression. The explanation may be that the influence of acetylation of histone on target promoters depend on the cell type as well as promoter context. In addition to histone, signal transduction molecules as well as transcription factors which are involved in gene expression may also be affected by acetylation and deacetylation, thus can be modulated by HDAC inhibitors. However, the mechanism is still unclear.
In conclusion, our results demonstrate that intra-articular injection of TSA plays a protective role against cartilage degradation during OA development in an experimental model. To our knowledge, this study is the first to demonstrate the cartilage protective effect of HDAC inhibitor in an induced OA model. The results of this study suggest that TSA can be considered as a therapeutic agent for human OA.
References
Thirunavukkarasu K, Pei Y, Wei T (2007) Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol Biol Rep 34:225–231
Mizui Y, Yamazaki K, Kuboi Y et al (2000) Characterization of 5′-flanking region of human aggrecanase-1 (ADAMTS4) gene. Mol Biol Rep 27:167–173
Huang K, Wu LD (2010) Suppression of aggrecanase: a novel protective mechanism of dehydroepiandrosterone in osteoarthritis? Mol Biol Rep 37:1241–1245
Caterina JJ, Shi J, Kozak CA, Engler JA, Birkedal-Hansen H (2000) Characterization, expression analysis and chromosomal mapping of mouse matrix metalloproteinase-19 (MMP-19). Mol Biol Rep 27:73–79
Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543
Rengel Y, Ospelt C, Gay S (2007) Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther 9:221
Martel-Pelletier J, Welsch DJ, Pelletier JP (2001) Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol 15:805–829
Neuhold LA, Killar L, Zhao W et al (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest 107:35–44
Dahlberg L, Billinghurst RC, Manner P et al (2000) Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum 43:673–682
Tetlow LC, Adlam DJ, Woolley DE (2001) Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 44:585–594
Goldring MB (2000) The role of the chondrocyte in osteoarthritis. Arthritis Rheum 43:1916–1926
Kobayashi M, Squires GR, Mousa A et al (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52:128–135
Bao JP, Chen WP, Wu LD (2010) Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep. doi:10.1007/s11033-010-9949-9
Glauben R, Sonnenberg E, Zeitz M et al (2009) HDAC inhibitors in models of inflammation-relatedtumorigenesis. Cancer Lett 280:154–159
Glauben R, Batra A, Fedke I et al (2006) Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol 176:5015–5022
Leoni F, Zaliani A, Bertolini G et al (2002) The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits anti-inflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA 99:2995–3000
Chabane N, Zayed N, Afif H et al (2008) Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthr Cartil 16:1267–1274
Young DA, Lakey RL, Pennington CJ et al (2005) Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther 7:R503–R512
Wang X, Song Y, Jacobi JL et al (2009) Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes. Growth Factors 27:40–49
Nishida K, Komiyama T, Miyazawa S et al (2004) Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum 50:3365–3376
Lin HS, Hu CY, Chan HY et al (2007) Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol 150:862–872
Nasu Y, Nishida K, Miyazawa S et al (2008) Trichostatin A, a histone deacetylase inhibitor, suppresses synovial inflammation and subsequent cartilage destruction in a collagen antibody-induced arthritis mouse model. Osteoarthr Cartil 16:723–732
Wu LD, Yu HC, Xiong Y et al (2006) Effect of dehydroepiandrosterone on cartilage and synovium of knee joints with osteoarthritis in rabbits. Rheumatol Int 27:79–85
Shikhman AR, Amiel D, D’Lima D et al (2005) Chondroprotective activity of N-acetylglucosamine in rabbits with experimental osteoarthritis. Ann Rheum Dis 64:89–94
Mankin HJ, Dorfman H, Lippiello L et al (1971) Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53:523–537
Bau B, Gebhard PM, Haag J et al (2002) Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 46(10):2648–2657
Ailenberg M, Silverman M (2003) Differential effects of trichostatin A on gelatinase A expression in 3T3 fibroblasts and HT-1080 fibrosarcoma cells: implications for use of TSA in cancer therapy. Biochem Biophys Res Commun 302:181–185
Daheshia M, Yao JQ (2008) The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol 35:2306–2312
Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39:237–246
Leoni F, Zaliani A, Bertolini G et al (2002) The an-titumor histone deacetylase inhibitor suberoylanilide hydroxamic acidexhibits anti-inflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA 99:2995–3000
Leoni F, Fossati G, Lewis EC et al (2005) The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med 11:11–15
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26
Ahn KS, Aggarwal BB (2005) Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci 1056:218–233
Imre G, Gekeler V, Leja A et al (2006) Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappa B by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res 66:5409–5418
Yin L, Laevsky G, Giardina C (2001) Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem 276:44641–44646
Davie JR (1997) Nuclear matrix, dynamic histone acetylation and transcriptionally active chromatin. Mol Biol Rep 24:197–207
Marks P, Rifkind RA, Richon VM et al (2001) Histone deacetylases and cancer: causes and therapies, Nat. Rev Cancer 3:194–202
Johnstone RW (2002) Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1:287–299
Acknowledgments
This study was supported by grants from Health Bureau of Zhejiang Province (2006A055).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WP., Bao, JP., Hu, PF. et al. Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep 37, 3967–3972 (2010). https://doi.org/10.1007/s11033-010-0055-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0055-9