Abstract
Cellobiohydrolase genes cbhI and cbhII were isolated from Trichoderma viride AS3.3711 and T. viride CICC 13038, respectively, using RT-PCR technique. The cbhI gene from T. viride AS3.3711 contains 1,542 nucleotides and encodes a 514-amino acid protein with a molecular weight of approximately 53.96 kDa. The cbhII gene from T. viride CICC 13038 was 1,413 bp in length encoding 471 amino acid residues with a molecular weight of approximately 49.55 kDa. The CBHI protein showed high homology with enzymes belonging to glycoside hydrolase family 7 and CBHII is a member of Glycoside hydrolase family 6. CBHI and CBHII play a role in the conversion of cellulose to glucose by cutting the disaccharide cellobiose from the non-reducing end of the cellulose polymer chain. The two cellobiohydrolase (CBHI, CBHII) genes were successfully expressed in Saccharomyces cerevisiae H158. Maximal activities of transformants Sc-cbhI and Sc-cbhII were 0.03 and 0.089 units ml−1 under galactose induction, respectively. The optimal temperatures of the recombinant enzymes (CBHI, CBHII) were 60 and 70°C, respectively. The optimal pHs of recombinant enzymes CBHI and CBHII were at pH 5.8 and 5.0, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing shortage of crude oil, methods of producing fuel ethanol are gaining more attention worldwide. Cellulose is the most abundant and renewable biomass and thus it has been recognized as a candidate for an alternative fuel source [1]. For ethanol production, cellulose needs to be degraded to glucose before fermentation. A promising strategy to meet this challenge involves the production of cellulolytic enzymes, hydrolysis of biomass, and fermentation of resulting sugars to desired products in a single process step via a cellulolytic microorganism or consortium of organisms [2]. Microorganisms with properties required for this process are not currently available, but can likely be developed with sufficient research [3]. One strategy for such developments involves engineering noncellulolytic organisms that exhibit high product yields and titers so that they express a heterologous cellulase system enabling cellulose utilization. The yeast Saccharomyces cerevisiae is an attractive host organism for this strategy given that it is a proven ethanol-producer [4].
Cellulose, the major polysaccharide compound of plant cell walls consisting of 100–10,000 glucose units linked by 1,4-glycosidic bonds, is the most abundant organic material on earth [5]. Cellulose is hydrolyzed by the cellulolytic enzyme systems: endoglucanase (EC 3.2.1.4, endo-1,4-d-glucan 4-glucanohydrolase), cellobiohydrolase (EC 3.2.1.91, 1, 4-d-glucan cellobiohydrolase, exoglucanase) and-glucosidase (EC 3.2.1.21, d-glucoside glucohydrolase). These three types of cellulases act synergistically to degrade crystalline cellulose [6]. During degradation of cellulose, these different types of enzymes act synergistically and efficiently hydrolyze cellulose polymers into glucose. Scale production of these enzymes is important for more widespread utilization of this material. Recently, genetic engineering approaches have been investigated to improve ethanol productivity from cellulose and decrease the cost of cellulases. The genes for cellulolytic enzymes were cloned, expressed in microorganisms [7, 8], and used for ethanol production from cellulosic materials.
Cellobiohydrolases (CBH) are key components in the multienzyme cellulase complexes, which are responsible for deep conversion of cellulose to soluble sugars [9]. They display an exo-type of attack on polymeric substrates, and the major product of their action on cellulose is cellobiose. Being the major cellulase enzymes, cellobiohydrolases will have very high biotechnological importance as soon as large-scale processes of lignocellulose bioconversion to liquid fuels and other useful products will be realized [10]. To increase the production of the enzyme, two cellobiohydrolase genes were isolated and heterologously expressed in the yeast S. cerevisiae. Biological characteristics of the recombinant CBHI and CBHII are shown in this paper.
Materials and methods
Strains, culture conditions and plasmid
Trichoderma viride AS3.3711 from Institute of Microbiology Chinese Academy of Science and T. viride CICC 13038 from China Center of Industrial Culture Collection were cultured aerobically in 5% wheat bran liquid media at 25°C for 5 days. Fungal mycelia were harvested by filtration through a 0.5 mm diameter mesh. Escherichia coli DH5α was used as a host for plasmid propagation. S. cerevisiae H158 was used as a host for expression of the recombinant cellobiohydrolases genes (cbhI and cbhII). It was cultured in YEPD medium [2% (w/v) peptone, 2% (w/v) glucose and 1% (w/v) yeast extract]. The plasmid pYES2 was used as a vector to carry cellobiohydrolases genes (cbhI and cbhII) into yeast S. cerevisiae H158.
Cloning of the two cellobiohydrolase genes
The cbhI gene was cloned from the strain T. viride AS3.3711 using primers P1 (5′-TGAGGCACAGAAACCCAAT-3′) and P2 (5′-GCCGCATCTCCAGTGAAA-3′) and cbhII gene was cloned from the strain T. viride CICC13038 using primers R1 (5′-GGGCTTTATCCTGTGCTC-3′) and R2 (5′-AGTTGCTCATTTGCGGTC-3′). Cloning primers were designed according to the nucleotide sequence of cbhI (E00389) from T. reesei and cbhII (M55080) from T. reesei QM9414.
The mycelia were harvested and then grounded into fine powder in liquid nitrogen with a mortar and pestle. The mycelia were then lysed and total RNA was isolated using TRI-Reagent (Molecular Research Center, USA), according to the manufacturer’s instruction.
The cellobiohydrolase coding sequence was amplified using 50–100 ng of total RNA by reverse transcriptase polymerase chain reaction (RT-PCR) using the OneStep RT-PCR kit (Qiagen, Germany) according to the manufacturer’s instruction. The cDNA was amplified using the primers. The PCR product (1.8 kb) was purified, cloned into pMD18-T vector (TaKaRa, Japan) and transformed into E. coli DH5α competent cells. Transformants were selected on LB agar plates containing ampicillin and X-Gal. Plasmid DNA was extracted from randomly picked white colonies and plasmids containing the cellobiohydrolase genes were confirmed by restriction digestion analysis and DNA sequencing.
Transformation of S. cerevisiae H158 with cellobiohydrolases genes
The cellobiohydrolase genes cbhI and cbhII were digested from the pMD18-T vectors with restriction enzymes KpnI/SphI and BamHI/SphI, respectively. Then they were ligated into recognition sites downstream of the GAL1 promoter of pYES2, employing E. coli DH5α competent cells. The resulting plasmids (pYES2-cbhI and pYES2-cbhII) were characterized by double enzymatic digestion. Plasmid DNA was extracted from randomly picked colonies and the insert size and orientation were confirmed by sequencing. Recombinant plasmids pYES2-cbhI and pYES2-cbhII in the correct orientation were transformed into S. cerevisiae H158 by the lithium acetate method, as described by Krautwurst et al. [11], yielding transgenic yeast strains Sc-cbhI and Sc-cbhII.
Northern blot analysis
The transformants Sc-cbhI and Sc-cbhII were cultured in SC-U medium containing 2% galactose for 48 h, respectively. Total RNA was extracted using a Yeast RNA minikit (Watson Biotechnologies, China). The extracted RNA was separated on a 1.2% agarose gel containing 1.5% formaldehyde and blotted onto a Nylon membrane. The cellobiohydrolases genes (cbhI and cbhII) digested with KpnI/SphI and BamHI/SphI from plasmids (pYES2-cbhI and pYES2-cbhII) were labeled with digoxigenin as probes, respectively. Probes for hybridization were prepared by the random primer extension method.
Characterization of recombinant cellobiohydrolases CBHI and CBHI in S. cerevisiae
To induce expression of cellobiohydrolase genes, the transgenic yeast strains Sc-cbhI and Sc-cbhII were grown on minimal medium SC-U [12]. Isolated colonies were used to inoculate 200 ml of minimal medium with 10 mg l−1 adenine and 2% raffinose and were grown at 30°C for 72 h. Yeast cells were then used to inoculate 50 ml SC-U medium containing 2% galactose and were cultured at 30°C for 48 h. The suspension was collected by centrifugation for identifying enzyme activity.
Enzymatic activity was determined according to a modification of the Miller procedure [13]. Enzyme activity was measured colorimetrically using CMC-Na as substrate. The reaction mixture, consisting of 0.5 ml CMC-Na (0.1%, w/v) and 0.5 ml enzyme solution, was incubated at 50°C in a water bath for 0.5 h. The reaction was stopped by adding 0.5 ml dinitrosalicylic acid and the reaction mixture was immediately boiled for 5 min. The reducing sugars released as enzyme activity were measured at 540 nm after cooling. One unit of enzymatic activity was defined as the amount of enzyme which produced 1 μmol min−1 reducing sugars. To determine optimal of pH and temperature, enzyme assays were performed using CMC-Na as substrate.
Results
Cloning of cellobiohydrolase genes cbhI and cbhII
The cDNA coding sequences of cbhI and cbhII genes were isolated using RT-PCR protocol. The full-length sequence of a 1746-bp gene encoding CBHI and a 1697-bp gene encoding CBHII were obtained. The open reading frame of the cbhI gene consisted of 1,542 bp, of which the G + C content was 58.59% and the open reading frame of cbhII gene consisted of 1,413 bp, of which the G + C content was 55.22%. They consisted of 514 amino acids residues with a predicted molecular mass of 53.96 kDa and 471 amino acids residues with a predicted molecular mass of 49.55 kDa, respectively. The cDNA sequences of cbhI and cbhII genes were deposited into GenBank database with accession numbers of AY368686 and AY368688.
Analysis of protein family and conserved region
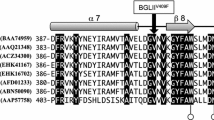
Pfam analysis found that cellobiohydrolase CBHI from Trichoderma viride AS3.3711 belongs to glycoside hydrolase family 7 (Fig. 1a). These enzymes were formerly known as cellulase family C. Cellobiohydrolase CBHII from T. viride CICC 13038 is member of glycoside hydrolase family 6 (Fig. 1b). These enzymes were formerly known as cellulase family B. Cellulase family C and B play a role in the conversion of cellulose to glucose by cutting the disaccharide cellobiose from the nonreducing end of the cellulose polymer chain.
Multiple sequence alignments of cellulose-binding domain (CBD) showed that CBD of CBHI enzyme is at C-terminal and CBD of CBHII is at N-terminal. As it is shown in the Fig. 2, there are four conserved cysteines in this type of CBD domain, all involved in disulphide bonds.
Expression of two cellobiohydrolases genes in S. cerevisiae
The mature cbhI and cbhII genes were amplified and inserted to the yeast expression vector pYES2. After verification of correct insertion, the selected plasmids were introduced into S. cerevisiae 158. The transformants were screened on SC-U medium. The presence of the recombinant cbhI and cbhII genes in the transformants was confirmed by genomic PCR with recognition primers. Positive clones yielded an approximately 1,700 bp DNA product which was the predicted size of the fusion gene.
To demonstrate the transcripts of cellobiohydrolase genes cbhI and cbhII in transformants Sc-cbhI and Sc-cbhII can be inhibited by glucose and induced by galactose, northern blot analysis were carried (Fig. 3). Using cbhI and cbhII genes as probes, no hybridization signals were observed when expression of cbhI and cbhII genes were inhibited by glucose. However a single band of 1.8 kb was observed when expression of cbhI and cbhII genes in transformant Sc-cbhI and Sc-cbhII were induced by galactose.
The Northern blot analysis of total RNAs from the Sc-cbhI and Sc-cbhII transformants. a, c Total RNA from transformant Sc-cbhI and Sc-cbhII; b, d Northern blot analysis of cbhI and cbhII expression. Left in every subfigure: gene expression in transformant was inhibited by glucose; right in every subfigure: gene expression in transformant was induced by galactose
Analysis of enzymatic characterization
The transformed yeast cells Sc-cbhI and Sc-cbhII were cultured in minimal medium with 2% raffinose as a carbon source and then used to inoculate rich medium containing 2% galactose, where induction of the gene took place (Fig. 4). From these data, it is apparent that enzyme activity was present in cells grown in raffinose; and a substantial increase was produced after 24 h growth using galactose as carbon source. At 60 and 36 h, the maximal enzyme activity of recombinant CBHI and CBHII reached 0.03 and 0.089 units ml−1, respectively. The effect of different pH and temperature on enzymatic activity was determined with CMC-Na as a substrate. Buffers containing 0.1 M citric acid and 0.2 M Na2HPO4 in different proportions [14] were used to achieve the desired pH. The result showed that the recombined enzymes had an optimal activity at pH 5.8 and 5.0, respectively (Fig. 5). The temperature profiles of recombinant CBHI and CBHII were determined at different temperatures (30–90°C). The optimal temperatures of recombinant enzymes CBHI and CBHII were at 60 and 70°C, respectively (Fig. 6).
Discussion
Cellobiohydrolase genes cbhI and cbhII were isolated from two strains T. viride and successfully expressed in yeast S. cerevisiae. BlastP analysis indicated that the amino acid sequence of CBHI and CBHII contained putative conserved domains of glycosyl hydrolase family 7 and 6. BlastX searches revealed high levels of homology between CBHI gene sequence and other CBHI gene sequence from different fungi: AB021656 (T. viride, 90% identity), X53931 (T. viride, 89% identity), X69976 (T. konni, 99% identity), X59054 (Penicilium janthinellum, 75% identity) and AF223252 (T. harzianum, 77% identity). For cbhII gene, sequence comparison showed that cbhII gene is similarity with other CBHII gene from T. viride (DQ86499, 99% identity), T. koningii (DQ660372, 91% identity), T. parceramosum (AY761091, 90% identity), M55080 (T. reesei, 99% identity).
CBHI contains three conserved carboxylate residues (Glu233, Asp235 and Glu238) that are implicated as the catalytic residues, based on the studies of T. reesei cellobiohydrolase I [15]. The active site of CBHII is located at the C-terminal end of a parallel beta barrel, in an enclosed tunnel through which the cellulose threads. Two aspartic acid residues, located in the centre of the tunnel are the probable catalytic residues PUBMED:2377893.
The microbial degradation of cellulose and xylans requires several types of enzymes such as endoglucanases, cellobiohydrolases (exoglucanases), or xylanases [16]. Structurally, cellulases and xylanases generally consist of a catalytic domain joined to a CBD by a short linker sequence rich in proline and/or hydroxy-amino acids. The CBD of a number of fungal cellulases has been shown to consist of 36 amino acid residues, and it is found either at the N-terminal or at the C-terminal extremity of the enzymes. There are four conserved cysteines in this type of CBD domain, all involved in disulphide bonds (Fig. 2).
The recombinant CBHI enzyme showed acidity stability, as it retained >70% activity of its maximal activity at pH 3.8–7.0. But recombinant CBHII enzyme only had a sharp stability, as it retained activity at pH 4.6–5.8. The two recombinant enzymes also showed interesting thermal stability properties since 60% of its maximal activity remained at 40–90°C. These properties make them useful for conversion of cellulose to ethanol.
In summary, the two cellobiohydrolase genes cbhI and cbhII were successfully isolated from T. viride (AS3.3711 and CICC13038) using RT-PCR protocol. The two genes contain the conserved catalytic amino acid residues that have been reported in other fungal cellulases. Two enzymes were successfully expressed and secreted in yeast S. cerevisiae and they were secreted in an active form. Enzymatic properties of the two recombinant enzymes were also determined. Due to their temperature and high acidity stabilities, transgenic yeast strains Sc-cbhI and Sc-cbhII might be useful for renewable fuels industries.
References
Slade R, Bauen A, Shah N (2009) The commercial performance of cellulosic ethanol supply-chains in Europe. Biotechnol Biofuels 2:3
Carere CR, Sparling R, Cicek N, Levin DB (2008) Third generation biofuels via direct cellulose fermentation. Int J Mol Sci 9:1342–1360
Fischer CR, Klein-Marcuschamer D, Stephanopoulos G (2008) Selection and optimization of microbial hosts for biofuels production. Metab Eng 10:295–304
Lau MW, Dale BE (2009) Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A (LNH-ST). Proc Natl Acad Sci USA 2009(106):1368–1373
Reddy N, Yang Y (2009) Natural cellulose fibers from soybean straw. Bioresour Technol 100(14):3593–3598
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Kanokratana P, Chantasingh D, Champreda V, Tanapongpipat S, Pootanakit K, Eurwilaichitr L (2008) Identification and expression of cellobiohydrolase (CBHI) gene from an endophytic fungus, Fusicoccum sp. (BCC4124) in Pichia pastoris. Protein Expr Purif 58:148–148
Haan RD, Mcbride JE, La Grange DC, Lynd LR, Van Zyl WH (2007) Functional expression of cellobiohydrolases in Saccharomyces cerevisiae towards one-step conversion of cellulose to ethanol. Enzym Microb Technol 40:1291–1299
Teeri TT (1997) Crystalline cellulose degradation: new insight into the function of cello-biohydrolases. Trends Biotechnol 15:160–167
Clarke AJ (1997) Biodegradation of cellulose. In: Enzymology and biotechnology. Technomic Publishing Company Inc., Lancaster
Krautwurst H, Bazaes S, González FD, Jabalquinto AM, Frey PA, Cardemil E (1998) The strongly conserved lysine 256 of Saccharomyces cerevisiae phosphoenolpyruvate carboxykinase is essential for phosphoryl transfer. Biochemistry 37:6295–6302
Adams A, Gottschling DE, Kaiser CA, Stearns T (1998) Methods in yeast genetics: a Cold Spring Harbor course manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Miller GL (1959) Using dinitrosalicylic acid for determination of reducing sugar. Anal Chem 31:426–428
Dawson R, Elliott D, Elliott W, Jones K (1986) Data for biochemical research, 3rd edn. Clarendon Press, Oxford
Stahlberg J, Divne C, Koivula A, Piens K, Claeyssens M, Teeri TT, Jones TA (1996) Activity studies and crystal structures of catalytically deficient mutants of cellobiohydrolase I from Trichoderma reesei. J Mol Biol 264:337–349
Gilkes NR, Henrissat B, Kilburn DG, Miller RC Jr, Warren RA (1991) Domains in microbial beta-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev 55:303–315
Acknowledgments
This research was funded by the project of the Natural Scientific Research Innovation Foundation in Harbin Institute of Technology (HIT.NSRIF.2008-18) and the Natural Science Foundation of Heilongjiang Province, China (Grant No. C200609).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J., Liu, B., Liu, Z. et al. Cloning of two cellobiohydrolase genes from Trichoderma viride and heterogenous expression in yeast Saccharomyces cerevisiae . Mol Biol Rep 37, 2135–2140 (2010). https://doi.org/10.1007/s11033-009-9683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9683-3