Abstract
Genetic mapping in ornamental plant species has lagged behind crop plants from other sectors of agriculture. Here, we describe the generation of a genetic linkage map for the important herbaceous ornamental crop petunia and the identification of QTL for several key crop timing and quality traits, including plant development rate, days to flower and flower number. An F2 population derived from a cross between the progenitor species of cultivated petunia, P. integrifolia × P. axillaris, exhibited transgressive segregation for a broad panel of crop timing and quality traits. A genetic linkage map comprised of 75 simple sequence repeat and six cleaved amplified polymorphic sequence markers spanning 359.1 cM across seven linkage groups was developed and utilized to identify 24 QTL for ten crop timing and quality traits. These included QTL explaining 26.3, 25.9, 26.2 and 43 % of the observed phenotypic variation for flower length, branch number, internode length and the number of flower buds on the primary shoot, respectively. These data provide a foundation for understanding the genetic control of critical traits and identify molecular markers with potential utility to facilitate gene discovery in petunia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cultivated petunia, Petunia × hybrida, is derived from a hybrid created in the mid-1800s between P. axillaris (with both self-compatible and self-incompatible genotypes) and P. integrifolia (a self-incompatible species) (all 2n = 14; Stehmann et al. 2009). Commercially, petunias are produced as an annual bedding crop, grown in greenhouses in the late winter and early spring for sale to consumers in full bloom. In cold climates like the northern USA and Europe, a major cost of petunia production is greenhouse heating (Bartok 2001). The total cost of heating for a crop of petunias depends on the heating costs per day, and the number of days required to bring the crop into flower and a marketable size. Lowering greenhouse temperatures can reduce heating costs on a per-day basis, but also reduces plant development rates, resulting in longer cropping time, and potentially higher overall heating and labor costs for crop production (Blanchard and Runkle 2011). Petunia varieties that reach flowering earlier by some combination of higher leaf initiation rate (development rate) and/or initiating flower buds after producing fewer vegetative nodes would have a shorter cropping time and be more economically and environmentally sustainable to produce. We previously determined that specific accessions of P. integrifolia and P. axillaris have faster development rates than a broad panel of P. × hybrida cultivars (Warner and Walworth 2010).
Wild Petunia species also exhibit other novel traits including unique plant architecture (Ando and Hashimoto 1993) that may be desirable to introgress into cultivated lines. The differences between wild and domesticated petunia could be due to the genetics of the specific individuals used to create the original hybrid, loss of genes during a domestication genetic bottleneck, inadvertent selection during cultivation or a combination of all three, as commonly seen during domestication (Tanksley and McCouch 1997; Meyer and Purugganan 2013). Regardless of the underlying mechanisms, wild petunia species could be useful in diversifying and improving the genetics of cultivated petunia to develop cultivars with desirable ornamental and growth traits. Interspecific hybridization between Petunia spp. is generally successful, though with varying degrees of fertility (Watanabe et al. 1996, 2001; Griesbach 2007). Several interspecific hybrid populations of petunia have been produced and evaluated (Mather and Edwardes 1943; Stuurman et al. 2004; Galliot et al. 2006; Bossolini et al. 2011), but the emphasis of these studies has been on floral traits rather than growth and development traits.
The first linkage maps of Petunia were constructed using morphological markers (Vlaming et al. 1984) which are fundamentally limited in number and restrict high-resolution mapping. These early maps, however, provided the initial framework and determined the naming of the chromosomes from one to seven. Subsequent linkage maps based on restriction fragment length polymorphisms (RFLPs) and amplified fragment length polymorphisms (AFLPs) were developed (Gerats et al. 1995; Strommer et al. 2000, 2002; Galliot et al. 2006) in both interspecific populations and crosses within P. hybrida. Though these markers are more abundant than morphological markers, these maps still lack fine resolution, and these marker types are not well suited to marker-assisted selection. More recently, linkage maps incorporating simple sequence repeat (SSR) markers have been generated for two interspecific Petunia populations (Bossolini et al. 2011). SSRs are more advantageous for marker-assisted selection, as they are co-dominant and highly reproducible.
The genetic control of many critical crop timing and production traits in petunia is poorly understood. Identification of QTL underlying these traits, and the impending availability of two Petunia spp. genomes (Sims et al. 2012), will facilitate the identification of candidate genes regulating these traits. Markers also allow breeders to characterize the linkage between traits so that individuals with a desirable linkage phase can be selected to minimize linkage drag and identify unrelated heterotic groups for the development of F1 hybrids (Lande and Thompson 1990; Mohan et al. 1997).
The objectives of this study were to: (1) generate and characterize a segregating interspecific hybrid population between the progenitor species of the cultivated petunia to evaluate the genetic variability encompassed by these species and (2) develop a genetic linkage map and identify QTL for traits effecting growth and development rate and additional morphological traits of interest. A genetic map based on an interspecific F2 population derived from a cross between P. integrifolia and P. axillaris was developed that contained 81 genetic markers and spanned approximately 359 cM. This F2 population exhibited wide phenotypic variation and facilitated the identification of 24 QTL for several important crop timing and quality traits including those associated with morphology and development rate. This study illustrates the potential for utilizing natural variation present in wild petunia species for crop improvement and gene discovery.
Materials and methods
Population development and phenotypic data
Petunia integrifolia × P. axillaris F1 individuals produced by Warner and Walworth (2010) were grown in a greenhouse at 20 °C under a 16-h photoperiod achieved by ambient light supplemented with high-pressure sodium lighting (50 µmol m−2 s−1) from 0600 to 2200 h. Due to observed self-incompatability (data not shown) in the P. integrifolia × P. axillaris F1 plants, F2 seed was generated by cross-pollinating between several different F1 individuals.
Seed of the parental species, F1 and F2 generations, were sown in 288-cell plug trays filled with soilless medium (Suremix, Michigan Grower Products Inc., Galesburg MI) and placed in a greenhouse at 23 °C under intermittent overhead mist. After 14 days, seedling trays were moved to a greenhouse at 20 °C under a 16-h photoperiod. Five weeks after seed sow, 212 P. integrifolia × P. axillaris F2 individuals, and ten seedlings each of the parental species and F1, were transplanted into 10-cm-diameter pots (450 mL) filled with the same soilless medium and moved to a greenhouse at 17 °C under a 16-h photoperiod achieved as described above, and the number of nodes (leaves >1 cm in length) on the main stem were determined.
Node counts were repeated every 7 days for 28 days. Development rate (in nodes day−1) was then calculated as the increase in node number divided by the number of days in between counts. The 28-day average development rate is reported. On the day of anthesis of the first flower on the main stem, leaf number below the first flower bud, flower diameter, flower length, number of visible flower buds on main stem, total number of visible flower buds, plant height, height to the flowering node and axillary branch number were determined for each individual. Pearson’s correlation coefficients between measured traits were calculated using SPSS (Version 22.0 for Windows; IBM, Armonk, NY). Broad-sense heritability estimates were calculated as previously described (Warner and Walworth 2010).
Molecular marker development and linkage map construction
We previously identified 591 SSRs from publically available P. axillaris EST sequences (Tychonievich et al. 2013) and 103 SSRs derived from P. × hybrida. Of those, 132 P. axillaris-derived SSRs and 33 P. × hybrida-derived SSRs were polymorphic between P. axillaris and P. integrifolia. From these, 21 SSRs derived from P. × hybrida and 92 SSRs derived from P. axillaris were used to genotype 164 individuals from the F2 population. PCR conditions, visualization and marker scoring were as described by Tychonievich et al. (2013). In addition to the SSRs, the population was genotyped with eleven cleaved amplified polymorphic sequence (CAPS) markers that were resolved with restriction enzymes as previously described by Bossolini et al. (2011).
A genetic linkage map was generated with JoinMap 4.0 (Van Ooijen 2006) using the Kosambi mapping function (Kosambi 1944). Linkage parameters were set to a recombination fraction of 0.30, logarithmic odds (LOD) score of 3.0 and error detection ratio of five percent. Markers with more than fifty missing individuals were removed. Linkage group (chromosome) number assignment was inferred from previous mapping studies (Strommer et al. 2000, 2009; Bossolini et al. 2011).
QTL analysis was performed using composite interval mapping (CIM) of the QTL Cartographer v2.5 software (Wang et al. 2012) and confirmed utilizing the CIM function of R/qtl package version 1.21-2 (Broman et al. 2003). For QTL Cartographer, model 6 forward–backward stepwise regression was used, and five markers were selected automatically as cofactors. A walk speed of 2 cM and empirically derived LOD thresholds at a 0.05 probability based on 1,000 permutations were used to identify QTL. Other parameters were used in default settings. For R/qtl, QTL genotype probability was calculated using the calc.genoprob function with a step size of 1 cM and Kosambi’s mapping function. The cim function was then used with five marker covariates and a window size of 20 with Kosambi's mapping function. The results of these two analyses were very similar (Supplemental Fig. S1), and therefore, the results from QTL Cartographer are presented. The QTL map was generated using MapChart software (Voorrips 2002).
Results
Phenotypic data
The P. integrifolia × P. axillaris population showed a wide range of variation including transgressive segregation for all traits measured (Fig. 1), even for traits for which the parental species values were similar, such as the number of nodes below the first flower (Fig. 1c) and the number of axillary branches (Fig. 1d). For the critical crop timing traits of node number below the first flower and development rate, the population showed a threefold and fourfold range of values, respectively. Direct measurement of days to flower was less variable, with less than a twofold range of values. In addition to the measured traits, we observed ca. ten individuals with irregular white variegation on the leaves (data not shown), and individuals with deformed growth and extreme dwarfism which did not survive to flowering and were removed from the study.
Frequency distributions for crop timing and quality traits a development rate, b days to anthesis of the first flower, c number of nodes below the first flower, d number of axillary branches, e number of flower buds on the main stem, f total number of flower buds when the first flower opened, g average internode length and h plant height to the flowering node for a P. integrifolia × P. axillaris F2 population
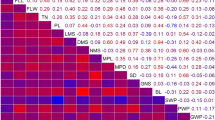
Among crop timing traits, development rate was strongly and positively correlated with node number below the first flower, but negatively correlated with days to flower (Table 1; Supplemental Fig. S2), while node number below the first flower was correlated positively with days to flower. There were also correlations between crop timing traits and crop quality traits. Development rate, node number below the first flower and days to flower were all correlated positively with total flower bud number but uncorrelated with flower bud number on the primary shoot. The growth-related traits flower diameter, flower length, plant height, internode length and axillary branch length were all correlated positively with each other, with the exception of flower diameter and flower length, which were uncorrelated (Table 1). Broad-sense heritability estimates were high for most traits (Table 2), with the exception of days to anthesis (H 2 = 0.402).
Linkage map construction
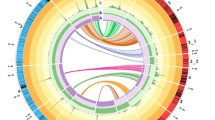
A linkage map of 81 markers on seven linkage groups spanning 359.1 cM was developed (Suplemental Table S1; Fig. 2). The average linkage group was 51.3 cM long, while the longest was 73.5 cM (chromosome 2), and the shortest 33.6 cM (chromosome 1). There was an average of 4.78 cM between markers. Chromosomes 4, 6 and 7 showed segregation ratios significantly distorted from the expected 1:2:1 ratio for a F2 hybrid population. On all three chromosomes, all markers showing Chi-squared values significantly deviated from the expected segregation ratios were due to a lack of markers homozygous for P. integrifolia alleles, and a corresponding excess of markers heterozygous or homozygous for P. axillaris alleles. Distorted segregation ratios have previously been observed in interspecific hybrid Petunia maps (Bossolini et al. 2011).
Genetic linkage map of a P. integrifolia × P. axillaris F2 population and locations of QTL for development rate (DR), days to flower (DTA), flower diameter (FD), flower length (FL), plant height (HGT), branch number (BR), branch length (BL), nodes below the first flower (NBF), internode length (INT), flower bud number on the primary shoot (FBP) and total flower bud number (FB)
QTL analysis
QTL were identified for all measured crop timing and quality traits except for days to flower (Fig. 2; Table 3; Supplemental Fig S1.). Three QTL for development rate, identified on chromosome 1, 2 and 5, cumulatively explained 34 % of the observed variation. Petunia axillaris contributed the favorable alleles for development rate on chromosomes 1 and 2, while P. integrifolia contributed the favorable allele of the greatest overall effect [18 % variation explained (VE)], located on chromosome 5. Two QTL were identified for the number of nodes below the first flower, with the QTL on chromosome 4 (NBF4.1) explaining 21 % of the variation.
Several major QTL (>25 % VE) were identified for traits relating to crop quality (Table 3), including flower length (FL4.1), branch length (BL6.1), branch number (BR1.1), internode length (INT5.1) and a QTL for the number of flower buds on the primary shoot (FBP6.1) explaining 43 % of the observed variation. For those QTL, P. axillaris contributed the favorable alleles for flower length, branch number and number of flower buds on the primary shoot, and P. integrifolia contributed favorable alleles for branch length and internode length (shorter internodes are desirable).
Discussion
Utilization of genetic mapping in ornamental crops for traits of horticultural importance has lagged behind other crop sectors, with most effort confined to rose (Debener and Mattiesch 1999; Dugo et al. 2005) and Lilium (Abe et al. 2002; Shahin et al. 2011). While there have been some genetic mapping efforts in petunia, these studies have largely focused on self-incompatibility (Wang et al. 2003) and traits related to pollination syndrome, including flower size, nectar volume and floral scent production (Stuurman et al. 2004; Galliot et al. 2006; Klahre et al. 2011). Here, we report the first QTL identified for several important crop timing and quality traits for petunia, utilizing an interspecific hybrid population derived from the progenitor species of cultivated petunia.
Unlike many food crops, which have been under artificial selection for thousands of years and have a set of the ‘domestication syndrome’ traits critical for successful use as a crop (Hammer 1984), most ornamental crops, like petunia (dating to ca. the 1830’s), have been more recently domesticated. The close relationship between domesticated and wild individuals reduces the chances of significant negative linkage, and wild relatives of petunia have been successfully used to introgress novel traits into petunia (Griesbach 2007). Similarly, unlike food crops, where breeders often have to generate varieties that fit a narrow range of flavor and processing requirements, novelty is highly desirable for ornamental crops and is often a significant focus of breeding programs. Therefore, desirable alleles identified in this population could be introgressed into elite petunia breeding lines.
All measured traits exhibited population distributions that suggest these traits are under polygenic control, and a high degree of transgressive segregation was observed (Fig. 1). Our previous results identified that the P. integrifolia and P. axillaris accessions used to generate the F2 population described herein exhibited faster development rates than a panel of grandiflora-type P. × hybrida cultivars (Warner and Walworth, 2010). Twenty-two of the F2 population plants had faster development rates than the faster parent (P. axillaris; Fig. 1a), suggesting further improvement over a number of existing commercial cultivars. There were several correlations among the crop timing traits development rate, node number below the first flower and days to flower (Table 2). Development rate was strongly correlated negatively with days to flower. These traits were not significantly correlated in the interspecific hybrid F2 populations P. exserta × P. axillaris, P. × hybrida × P. axillaris or P. axillaris × P. integrifolia (the reciprocal cross of the population described here), although the population sizes were considerably smaller (Warner and Walworth 2010). Node number below the first flower and days to flower were correlated positively, similar to results for the P. axillaris × P. integrifolia population (Warner and Walworth 2010).
The total map distance reported here (359.1 cM) is similar to the 404.6 cM map generated for a P. axillaris × P. inflata (until recently, classified as a subspecies of P. integrifolia) BC1 population using AFLP markers (Galliot et al. 2006). Linkage maps of petunia have consistently resulted in short linkage groups due to a low frequency of recombination, though the severity of the effect varies by population and chromosome (Strommer et al. 2002). A test cross population generated by crossing an F1 hybrid between two P. axillaris subspecies to an inbred P. × hybrida line produced a genetic linkage map with a total map distance of only 2.4 cM (Galliot et al. 2006). Ten Hoopen et al. (1996) used four independent T-DNA insertion lines, each with an insertion mapping to <1 cM from the flavanol synthase gene Fl on chromosome 2, and florescence in situ hybridization (FISH) to provide physical evidence of suppressed recombination in petunia. Despite the tight genetic linkage between the T-DNAs and Fl, the four insertions physically mapped to locations spanning ca. two-thirds of chromosome 2. The Rm1 (recombination modulator 1) locus affects recombination rates in petunia (Cornu et al. 1989; Robert et al. 1991). The issue of suppressed recombination is a significant problem for breeders. A breeder working with a low recombining population faces increased linkage drag, as large sections of a chromosome are inherited together, making it more difficult to introgress useful traits from wild relatives without also introducing potentially undesirable traits into elite varieties.
Several QTL that could be very useful for gene discovery in petunia were identified in this study. The only trait presented here that has previously been mapped in Petunia is flower diameter. Utilizing a P. axillaris × P. inflata BC1 population, Galliot et al. (2006) identified QTL for flower diameter on chromosomes 2, 3, 4 and 5, while the current study identified QTL on chromosomes 1, 2 and 3 (Table 3). At least one QTL was identified for each of the traits evaluated (Table 3; Fig. 2) except days to anthesis, and QTL explaining >25 % of the observed variation were identified for several plant quality traits. Of particular note are a QTL on chromosome 6 which explains 42 % of the variation of number of flower buds on the main stem and a QTL explaining 25 % of the variation in branch number on chromosome 1 which together could be used to select for more floriferous, highly branched individuals.
Three QTL for development rate were identified, though these three loci collectively explained only 34 % of the observed variation, with DR5.1 having the largest effect (18 % VE). Therefore, it seems likely that multiple loci of small effect combine to control this trait. Several genes impacting development rate have been identified in other species. Mutations in PLASTOCHRON1 and PLASTOCHRON2 in rice (Miyoshi et al. 2004; Kawakatsu et al. 2006), TERMINAL EAR1 in maize (Veit et al. 1998) and ALTERED MERISTEM PROGRAM1 (Helliwell et al. 2001) in arabidopsis increase the leaf initiation rate, while mutations in PHYTOCHROME B (Reed et al. 1993) and SERRATE (Prigge and Wagner 2001) decrease leaf initiation rate. The influence of naturally occurring allelic differences at these loci on development rate has not been evaluated. In addition, QTL for several traits (development rate, flower diameter, flower length and branch number) co-localized on chromosome 1 around a CAPS marker for the isopentenyl transferase gene SHO, with a QTL for flower bud number also overlapping this marker. This gene encodes an enzyme catalyzing cytokinin biosynthesis in petunia, and was originally identified in an activation-tagged line exhibiting increased lateral shoot production (Zubko et al. 2002). This region may exert an influence on these growth and development traits through a general impact on cytokinin synthesis. Additional work is needed to fine map this region to determine whether SHO or other genes in the region are responsible for these effects and also to determine whether known regulators of development rate co-localize with the identified QTL.
This study has identified variation in key quality and timing traits in an important ornamental crop species. However, the QTL described here were identified from a single, non-replicated population. Future efforts will focus on validation and refinement of these QTL in an F7 population of recombinant inbred lines (RILs) that are derived from the F2 population described herein. The use of the RIL population will facilitate replicated trials under different environments to determine the robustness of the QTL and ascertain their utility for improvement of petunia quality traits.
References
Abe H, Nakano M, Nakatsuka A, Nakayama M, Koshioka M, Yamagashi M (2002) Genetic analysis of floral anthocyanin pigmentation traits in Asiatic hybrid lily using molecular linkage maps. Theor Appl Genet 105:1175–1182
Ando T, Hashimoto G (1993) Two new species of Petunia (Solanaceae) from southern Brazil. Bot J Linn Soc 111:265–280
Bartok JW (2001) Energy conservation for commercial greenhouses, 2001 rev. Natural Resource, Agriculture, and Engineering Service, Ithaca
Blanchard MG, Runkle ES (2011) The influence of day and night temperature fluctuations on growth and flowering of annual bedding plants and greenhouse heating cost predictions. J Am Soc Hortic Sci 46:599–603
Bossolini E, Klahre U, Brandenburg A, Reinhardt D, Kuhlemeier C (2011) High resolution linkage maps of the model organism Petunia reveal substantial synteny decay with the related genome of tomato. Genome 54:327–340
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Cornu A, Farcy E, Mousset C (1989) A genetic basis for variations in meiotic recombination in Petunia hybrida. Genome 32:46–53
Debener T, Mattiesch L (1999) Construction of a genetic linkage map for roses using RAPD and AFLP markers. Theor Appl Genet 99:891–899
Dugo ML, Satovic Z, Millán T, Cubero JI, Rubiales D, Cabrera A, Torres AM (2005) Genetic mapping of QTLs controlling horticultural traits in diploid roses. Theor Appl Genet 111:511–520
Galliot C, Hoballah M, Kuhlemeier C, Stuurman J (2006) Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta 225:203–212
Gerats T, Keukeleire P, Deblaere R, Montagu M, Zethof J (1995) Amplified fragment length polymorphism (AFLP) mapping in Petunia; a fast and reliable method for obtaining a genetic map. Acta Hortic 420:58–61
Griesbach RJ (2007) Petunia. In: Anderson NO (ed) Flower breeding and genetics. Springer, Dordrecht, pp 301–336
Hammer K (1984) Das Domestikationssyndrom. Die Kulturpflanze 32:11–34
Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudury A (2001) The arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell 13:2115–2125
Kawakatsu T, Itoh J-I, Miyoshi K, Kurata N, Alvarez N, Veit B, Nagato Y (2006) PLASTOCHRON2 regulates leaf initiation and maturation in rice. Plant Cell 18:612–625
Klahre U, Gurba A, Hermann K, Saxenhofer M, Bossolini E, Guerin PAM, Kuhlemeier C (2011) Pollinator choice in petunia depends on two major genetic loci for floral scent production. Curr Biol 21:730–739
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124:743–756
Mather K, Edwardes PMJ (1943) Specific differences in Petunia: III. Flower colour and genetic isolation. J Genet 45:243–260
Meyer RS, Purugganan MD (2013) Evolution of crop species: genetics of domestication and diversification. Nat Rev Genet 14:840–852
Miyoshi K, Ahn B-O, Kawakatsu T, Ito Y, Itoh J-I, Nagato Y, Kurata N (2004) PLASTOCHRON1, a timekeeper of leaf initiation in rice, encodes cytochrome P450. PNAS 101:875–880
Mohan M, Nair S, Bhagwat A, Krishna TG, Yano M, Bhatia CR, Sasaki T (1997) Genome mapping, molecular markers and marker-assisted selection in crop plants. Mol Breed 3:87–103
Prigge MJ, Wagner DR (2001) The arabidopsis SERRATE gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13:1263–1280
Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5:147–157
Robert N, Farcy E, Cornu A (1991) Genetic control of meiotic recombination in Petunia hybrida: dosage effect of gene Rm1 on segments Hf1-Lg1 and An2-Rt; role of modifiers. Genome 34:515–523
Shahin A, Arens P, van Heusden AW, van der Linden G, van Kaauwen M, Schouten HJ, van de Weg WE, Visser RGF, van Tuyl JM (2011) Genetic mapping in Lilium: mapping of major genes and quantitative trait loci for several ornamental traits and disease resistances. Plant Breed 130:372–382
Sims TL, Gomez AB, Delledonne M, Gerats T, Mitrick J, Mueller L, Pezzotti M, Quattrocchio F, Yang B (2012). Sequencing and comparison of the genomes of Petunia inflata and Petunia axillaris. In: Plant and Animal Genome XX Conference (abstract) https://pag.confex.com/pag/xx/webprogram/Paper1786.html. Accessed 26 Oct 2014
Stehmann JR, Lorenz-Lemke AP, Freitas LB, Semir J (2009) The genus Petunia. In: Gerats T, Strommer J (eds) Petunia evolutionary, developmental and physiological genetics, Springer, New York, pp 1–28. doi:10.1007/978-0-387-84796-2
Strommer J, Gerats AGM, Sanago M, Molnar SJ (2000) A gene-based RFLP map of petunia. Theor Appl Genet 100:899–905
Strommer J, Peters J, Zethof J, de Keukeleire P, Gerats T (2002) AFLP maps of Petunia hybrida: building maps when markers cluster. Theor Appl Genet 105:1000–1009
Strommer J, Peters JL, Gerats T (2009) Genetic recombination and mapping in Petunia. In: Gerats T, Strommer J (eds) Petunia. Springer, New York, pp 325–341
Stuurman J, Hoballah ME, Broger L, Moore J, Basten C, Kuhlemeier C (2004) Dissection of floral pollination syndromes in petunia. Genetics 168:1585–1599
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Ten Hoopen R, Robbins TP, Fransz PF, Montijn BM, Oud O, Gerats AGM, Nanninga N (1996) Localization of T-DNA insertions in petunia by fluorescence in situ hybridization: physical evidence for suppression of recombination. Plant Cell 8:823–830
Tychonievich J, Wangchu L, Barry C, Warner RM (2013) Utilizing wild species for marker-assisted selection of crop timing and quality traits in Petunia. Acta Hortic 1000:465–469
Van Ooijen JW (2006) JoinMap® 4, Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, Netherlands
Veit B, Briggs SP, Schmidt RJ, Yanofsky MF, Hake S (1998) Regulation of leaf initiation by the terminal ear 1 gene of maize. Nature 393:166–168
Vlaming P, Gerats AGM, Wiering H, Wijsman HJW, Cornu A, Farcy E, Maizonnier D (1984) Petunia hybrida: a short description of the action of 91 genes, their origin and their map location. Plant Mol Biol Rep 2:21–42
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang Y, Wang X, McCubbin AG, Kao T (2003) Genetic mapping and molecular characterization of the self-incompatibility (S) locus in Petunia inflata. Plant Mol Biol 53:565–580
Wang S, Basten CJ, Zeng Z-B (2012) Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm. Accessed 22 Oct 2014
Warner RM, Walworth AE (2010) Quantitative inheritance of crop timing traits in interspecific hybrid Petunia populations and interactions with crop quality parameters. J Hered 101:308–316
Watanabe H, Ando T, Iida S, Suzuki A, Buto K-I, Tsukamoto T, Hashimoto G, Marchesi E (1996) Cross compatibility of Petunia cultivars and P. axillaris with native taxa of Petunia in relation to their chromosome number. J Jpn Soc Hortic Sci 65:625–634
Watanabe H, Ando T, Tsukamoto T, Hashimoto G, Marchesi E (2001) Cross-compatibility of Petunia exserta with other Petunia taxa. J Jpn Soc Hortic Sci 70:33–40
Zubko E, Adams CJ, Macháèková I, Malbeck J, Scollan C, Meyer P (2002) Activation tagging identifies a gene from Petunia hybrida responsible for the production of active cytokinins in plants. Plant J 29:797–808
Acknowledgments
We thank Mike Olrich for technical assistance. This research was supported by the US Dept. of Agriculture (USDA) Specialty Crop Block Grant Program and the USDA Specialty Crop Research Initiative (award 2011-01508). R. M .W. and C. S. B. are supported in part by Michigan AgBioResearch and through USDA National Institute of Food and Agriculture, Hatch project numbers MICL02121 and MICL02265.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vallejo, V.A., Tychonievich, J., Lin, WK. et al. Identification of QTL for crop timing and quality traits in an interspecific Petunia population. Mol Breeding 35, 2 (2015). https://doi.org/10.1007/s11032-015-0218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0218-4