Abstract
Leaf rust, caused by Puccinia hordei Otth, is an important disease of barley (Hordeum vulgare L.) in many areas of the world. The appearance of new virulent races necessitates the identification of new resistance genes in barley. Screening of spring barley landraces from former Yugoslavia led to the identification of an accession (MBR1012) carrying resistance to the most widespread virulent leaf rust pathotypes in Europe. Ninety-one doubled haploid lines derived from a cross between landrace MBR1012 and the susceptible German cultivar Scarlett were evaluated for resistance to P. hordei isolate I-80 and segregated 48 resistant : 43 susceptible (\( \chi_{1:1}^{2} = \, 0.29, \) p = 0.6), indicating a monogenic inheritance of resistance. Using simple sequence repeats (SSR) and single nucleotide polymorphism (SNP) markers, the resistance gene in MBR1012 was mapped to the telomeric region of chromosome 1HS. This gene is assigned the temporary locus designation of Rph MBR1012 until it can be unequivocally determined to be different from all previously reported resistance genes. The closest flanking markers for Rph MBR1012 are located 0.8 cM distal (SNP marker GBS546 and SSR marker GBMS187) and 6.0 cM proximal (SSR marker GMS21). The diagnostic value of the closest linked markers was assessed in a genetically diverse collection of 51 susceptible and resistant barley lines and cultivars. The SSR GBMS187 predicted the presence of Rph MBR1012 with 100% accuracy. However, this marker could not be used singly for the rapid incorporation of resistance into high yielding barley cultivars, since it detects a null allele in MBR1012. Therefore, simultaneous use of the markers closely linked to Rph MBR1012 is needed for transferring Rph MBR1012 into adapted cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf rust, caused by the biotrophic pathogen Puccinia hordei Otth, is an economically important foliar disease of barley in many temperate regions world-wide (Mathre 1997). Under experimental conditions, yield losses as high as 60% can occur in highly susceptible barley cultivars, but losses of about half that level are common in practice (Clifford 1985; Cotterill et al. 1992; Das et al. 2007). The deployment of single race-specific resistance genes alone or in combination by pyramiding (Werner et al. 2005) can be an effective and environmentally-friendly method of disease control. In cultivated barley (Hordeum vulgare ssp. vulgare) and wild barley (H. vulgare ssp. spontaneum), 19 major genes (Rph1 to Rph19) effective in the seedling stage have been described (Golegaonkar et al. 2009). These major genes are effective against different pathotypes of P. hordei, but when deployed singly have often been overcome by new pathotypes. The leaf rust resistance genes Rph7, Rph15, and Rph16 are still effective in Europe (Niks et al. 2000; Perovic et al. 2004), but are potentially vulnerable because pathotypes with virulence for Rph7 are known in Israel (Golan et al. 1978), Morocco (Parlevliet 1981) and USA (Steffenson et al. 1993). Therefore, it may be just a matter of time until these resistance genes are overcome in Europe in the same way as Rph3 and Rph10 (Dreiseitl 1990; Fetch et al. 1998). It appears that the frequency of mutation in asexually reproducing populations of P. hordei is sufficient to overcome many resistance genes in barley (Cromey and Viljanen Rollinson 1995). Thus, the economic impact of leaf rust on barley production and the ability of the fungus to generate new virulent pathotypes in a short period of time highlights the importance of searching for new resistance genes and, in parallel, for the development of tools for their efficient deployment in barley breeding programs.
Within the primary gene-pool of barley, which includes cultivars, landraces and wild barley accessions (H. vulgare ssp. spontaneum), the latter two are of particular importance regarding breeding for disease resistance. Incorporation of new leaf rust resistance genes from landraces was reported only for the genes Rph3 and Rph7 (Walther and Lehmann 1980; Jin et al. 1995), while a much greater number (Rph2, Rph10, Rph11, Rph12, Rph13, Rph15 and Rph16) was identified in the wild progenitor H. vulgare ssp. spontaneum (Franckowiak et al. 1996; Ivandic et al. 1998).
To identify new sources of leaf rust resistance, barley germplasm from eco-geographically diverse collection sites in former Yugoslavia was evaluated (Perovic et al. 2001), as this is a region of high disease pressure (Reinhold and Sharp 1980; Boskovic and Boskovic 2009). One of the landraces from this collection (MBR1012) exhibited a strong resistant (hypersensitive) reaction to P. hordei isolate I-80 (Perovic et al. 2001), which is virulent to all known major resistance genes in the European barley gene pool, except Rph7, Rph15 and Rph16 (Weerasena et al. 2004). Therefore, the aims of this study were to investigate the genetics of leaf rust resistance in MBR1012 and develop molecular markers for this resistance gene, facilitating efficient marker-based selection procedures.
Materials and methods
Plant materials
An F1-derived doubled haploid (DH) population, produced via anther culture, comprising 91 lines of a cross between the resistant landrace MBR1012 and the susceptible German cultivar Scarlett was used for mapping. In addition, a collection of 51 barley accessions was evaluated for resistance to several leaf rust isolates to determine the diagnostic value of the closest linked molecular markers to the gene identified in MBR1012. This barley collection consisted of the original sources of described Rph genes, i.e. near-isogenic lines of the cultivar Bowman carrying the introgressed Rph genes, two lines with partial leaf rust resistance, and 10 susceptible genotypes. A set of 7 barley genotypes [MBR1012, Scarlett, Gold (Rph4); Cebada Capa (Rph7), NIL BowmanRph15 (Rph15), Hsp680 (Rph16) and L94] was used to determine the leaf rust infection patterns (see below).
Phenotyping
Five to ten plants of each DH line and the parents were inoculated with the leaf rust isolate I-80 in the greenhouse. Plants were inoculated at the seedling stage, according to Ivandic et al. (1998). The inoculated plants were placed in a growth chamber (20–22°C) under a plastic cover for 24 h to provide a moist environment for successful infection. Infection types were recorded between 10 and 12 days after inoculation according to the 0–4 scale of Levine and Cherewick (1952). The DH lines were classified based on their infection types. Plants exhibiting infection types from 0, 0nc (hypersensitive reactions with necrotic/chlorotic ‘flecks’), 1, 2-, or 0-2- were considered resistant, while those exhibiting infection types 2+, 3, 3-4 and 4 were considered susceptible. The Chi-squared test was used to assess segregation ratios for goodness of fit to expected ratios.
Additionally, a collection of 13 European leaf rust isolates (Walther 1987) was tested on the parental lines and accessions of Gold (carrying leaf rust resistance gene Rph4); Cebada Capa (Rph7), NIL Bowman-Rph15 (Rph15) Hsp680 (Rph16), and L94 (susceptible control) in order to determine whether the resistance of MBR1012 is different from previously described resistance genes. Inoculation and scoring was performed as described for isolate I-80. The leaf rust isolates were collected from different parts of Europe and selected from single spore progenies (Walther 1979, 1987). The isolates are maintained at the Julius Kuehn-Institute, Federal Research Institute for Cultivated Plants (JKI), Institute for Resistance Research and Stress Tolerance, in Quedlinburg, Germany.
Marker analyses
Genomic DNA was extracted from leaves of 14-day-old plants according to Stein et al. (2001). The concentration and quality of DNA was determined using the NanoDrop ND-100 spectrophotometer (PeQLab, Erlangen, Germany) and gel electrophoresis. All samples were adjusted to a final concentration of 20 ng/μl. For bulk segregant analysis (BSA) (Michelmore et al. 1991), equal aliquots (10 μl) of DNA from nine resistant and nine susceptible DH lines were pooled.
A total of 175 SSRs and 73 SNPs were screened for polymorphism between the parents and between the bulks. The sequences of the SSR primer pairs and amplification protocols were obtained from Struss and Plieske (1998), Ramsay et al. (2000), Maccaulay et al. (2001), Thiel et al. (2003), and Varshney et al. (2007), while the sequences of the SNP primer pairs, amplification protocol and restriction sites were obtained from Kota et al. (2008) and Perovic et al. (2007). All markers which generated polymorphic PCR products between the parental lines and between the bulks were assayed in all DH lines (Table 1).
PCR was performed in a volume of 10 μl, containing 1 μl of 10 × buffer, 1 μl of 25 mM MgCl2, 0.2 μl of each 10 mM dNTPs, forward primer (1.0 pmol/μl) and reverse primer (10.0 pmol/μl), 0.08 μl 5U Hot FIREPol®DNA polymerase (Solis BioDyne, Tartu, Estonia), 6.12 μl HPLC gradient grade water (Carl Roth, Karlsruhe, Germany) and 1 μl template DNA. For SSR amplification, M13 tailed forward primers were used, so that 0.1 μl of M13 primer (10.0 pmol/μl) (5′-CACGACGTTGTAAAACGAC-3′) labelled with 5′ fluorescent dyes was added to the reaction mix (Boutin-Ganache et al. 2001). DNA amplification was performed in a Gene Amp® PCR System 9700 (Applied Biosystems, Darmstadt, Germany). The following PCR conditions were used for all primers: 94°C for 5 min followed by a touchdown PCR (−0.5°C/cycle) with 12 cycles of 30 s at 94°C, 30 s at 62°C, 30 s at 72°C; and then 35 cycles with 30 s at 94°C, 30 s at 56°C, 30 s at 72°C, and a final extension at 72°C for 10 min. For marker GBMS187, the second amplification phase was extended to 50 cycles, while the other parameters remained the same. Detection of allele sizes for the SSR marker was conducted using a capillary electrophoresis ABI PRISM® 3100 genetic analyzer (Applied Biosystems) or a CEQ™ 8000 Genetic Analysis System (Beckman). SNP markers (Kota et al. 2008) were amplified in a volume of 20 μl, containing 2.0 μl of 10 × PCR buffer with MgCl2 (25 mM), 0.4 μl of each 10 mM dNTPs, 0.5 μl forward primer (1.0 pmol/μl) and reverse primer (10.0 pmol/μl), 0.16 μl 5U Taq DNA-polymerase (Qiagen, Hilden, Germany), 6.12 μl HPLC gradient grade water and 2.0 μl template DNA using the same PCR conditions as described for the SSRs. The applied SNP markers were converted to cleaved amplified polymorphic sequences (CAPS) markers by digesting PCR products with corresponding restriction endonucleases. A restriction site for the GBS546 SNP marker was previously described by Kota et al. (2008). The other two markers were firstly sequenced to detect SNPs and then converted into CAPS markers by the use of the SNP2CAPS tool (http://pgrc.ipk-gatersleben.de/snp2caps/; Thiel et al. 2004). The PCR products were digested with a set of three restriction endonucleases to generate CAPS. Three enzymes, HpyCH4IV, HhaI and SsilI, were used according to the manufacturer’s instructions (New England Biolabs) and performed in a total volume of 20 μl with 10 μl PCR-product, 2 μl of the relevant NE Buffer, 7.9 μl HPLC gradient grade water and 0.1 μl enzyme. The cleaved DNA fragments were separated on 1.8% agarose gels as described previously. Conversion of restriction fragment length polymorphism (RFLP) marker GBR218 into PCR-based marker QBS2 (QBS2f 5′-AGCTGAATCCAACCCAACAC-3′ and QBSr 5′-AGTCGCAGAGCCACAAGTTC-3′) was performed according to Perovic et al. (2007).
Map construction
The genetic map was constructed using JoinMap 4.0 (van Ooijen 2006) applying the Kosambi function (Kosambi 1944). Only markers with a LOD score of 3 were integrated into the map.
Results
Phenotypic analysis
The infection type of the susceptible parent Scarlett was 2-3, whereas that of the resistant parent MBR1012 was 0-2-. The 91 DH lines derived from the cross of the resistant landrace MBR1012 and the susceptible German cultivar Scarlett segregated 48 resistant: 43 susceptible (χ² = 0.29, p = 0.6). This segregation pattern fits well to the expected 1 resistant : 1 susceptible ratio, indicating that leaf rust resistance against isolate I-80 in MBR1012 is inherited in a monogenic manner.
Linkage analysis
The parents and the two bulks were analysed with a set of 248 co-dominant molecular markers, which were evenly distributed along the seven chromosomes of barley. Of these, 89 SSR (51%) and 21 SNP markers (29%) were polymorphic. Polymorphisms between the resistant and susceptible bulk were detected on chromosome 1H. Overall, out of the 32 SSR and 11 SNP markers localized on chromosome 1H, 14 and 3 were polymorphic, respectively. Based on these markers, a final genetic map of 119 cM of chromosome 1H was constructed (Fig. 1). The leaf rust resistance gene in MBR1012 was mapped in the telomeric region of the short arm of chromosome 1H. The closest linked markers are GBMS187 and GBS546, which map 0.8 cM distal to the resistance gene. On the proximal side, the closest marker identified is GMS21, which maps 6.0 cM from the resistance locus. The allele sizes and the restriction patterns of all mapped markers from chromosome 1H are given in Table 1.
Leaf rust infection patterns
The seven barley accessions displayed very different reaction patterns in response to the collection of 13 European leaf rust isolates (Table 2). Susceptible L94 and Gold carrying Rph4 were highly susceptible to the entire collection of leaf rust isolates, while susceptible parental cultivar Scarlet exhibited resistance to 12 isolates and was only susceptible to isolate I-80. Although Cebada Capa (Rph7), H.sp.680 (Rph16) and MBR1012 turned out to be resistant to all isolates, their reaction patterns varied from 0c (chlorosis), 0n (necrosis) to 0-2-, respectively.
Diagnostic value of closest markers
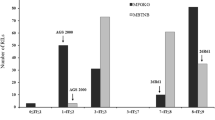
To assess the diagnostic value of the four closest linked markers to the leaf rust resistance gene in MBR1012, a set of 51 barley accessions was assayed. This barley collection consisted of the original sources of the described Rph genes, near-isogenic lines of cultivar Bowman with introgressed Rph genes, two lines with partial leaf rust resistance, and 10 susceptible lines with no known Rph genes (Table 3). The number of detected alleles per marker in this analysed collection varied from three alleles for GBS546 and six alleles for GMS21 to nine and eleven alleles for QBS2 and GBMS187, respectively. Restriction pattern B of SNP marker GBS546 (Fig. 2), characteristic of the resistant parent MBR1012, was observed for ten other genotypes. Similarly, for the closest proximal marker SSR GMS21, ten genotypes showed the same allele pattern as the resistant parent MBR1012. Accuracies in prediction of Rph MBR1012 were 74.5% for QBS2, 78.5% for GMS21, 80.5% for GBS546 and 100% for GBMS187. Therefore, we conclude that QBS2, GBS546 and GMS21 have no diagnostic value for tagging the resistance gene described in MBR1012, but could be used for the transfer of the resistance gene into elite cultivars. The closely linked marker GBMS187 (Fig. 3) detected a null allele in the resistant parent MBR1012, whereas in the other genotypes examined eight different alleles were identified. Although the null allele of the GBMS187 in the parent MBR1012 is unique in the germplasms investigated, the use of this marker in breeding is of limited value due to its dominant mode of inheritance.
Discussion
The gene pool of cultivated barley is largely depleted of major resistance genes for many fungal and viral pathogens (Graner et al. 2000a). The collection of spring barley landraces from the former Yugoslavia that was used in this study is a rich resource of important disease resistances (Perovic et al. 2001). Although the diversity present in this barley collection represents a good base for the transfer of many important traits into elite cultivars, it is seldom used in breeding programs. To enhance the use of genetic diversity from this collection, the leaf rust resistance in line MBR1012 was evaluated in detail.
Leaf rust resistance in landrace MBR1012 is conferred by a major gene, which exhibits a hypersensitive reaction to many of the most widespread virulent European P. hordei isolates and is located on the distal portion of the short arm of chromosome 1H.
A linkage map was constructed for chromosome 1H. In general, the order of markers in this genetic map was in agreement with previously published maps (Stein et al. 2007; Varshney et al. 2007; Kota et al. 2008). Only slight deviations in the marker order among the different maps were observed and they may be explained by the fact that the maps of Varshney et al. (2007) and Stein et al. (2007) are consensus maps constructed by the integration of four independent genetic maps of barley. In order to convert the RFLP marker GBR218 that is mapped to the most telomeric region of chromosome 1H according to Stein et al. (2007), specific primers were designed for amplification and sequencing. Generally, RFLP markers are very robust markers, but their analysis is expensive and laborious. Here, we converted the GBR marker into an easy-to-use PCR marker. The newly developed PCR marker QBS2 directly detects an insertion/deletion polymorphism of 33 bp between the parents (Table 1), was locus-specific and showed the same map location as the corresponding RFLP marker.
The leaf rust resistance locus Rph MBR1012 was mapped in a 6.8-cM interval between the markers GBMS187/GBS546 and GMS21. GBMS187 and GBS546 co-segregate and are the closest linked markers (0.8 cM distal) to Rph MBR1012. Because of this genetic position, it was assumed that they may be useful as diagnostic molecular markers in breeding programs employing marker-assisted selection (MAS). Although they co-segregated, an assessment of germplasm revealed different accuracies in the prediction of the MBR1012 resistance gene, viz. 80.5% for GBS546 and 100% for GBMS187. It is important to note that GBMS187 (Fig. 3) is a dominant marker in our population; hence, it may not be the best marker for MAS of this leaf rust resistance gene. Since we were able to convert SNP markers into CAPS markers, an easy-to-use PCR MAS scheme can be conducted in combination with flanking markers GBS546/GBMS187 and GMS21.
Other dominant leaf rust resistance genes have been mapped to nearly all barley chromosomes (see overview by Golegaonkar et al. 2009). The genes Rph7 and Rph10 were mapped to chromosome 3H (Feuerstein et al. 1990; Graner et al. 2000b; Zhong et al. 2003), Rph2 to chromosome 5HS (Borovkova et al. 1997), Rph3 to 7HL (Jin et al. 1993), Rph11 to 6HL (Feuerstein et al. 1990) and Rph15 and Rph16 to 2H (Weerasena et al. 2004). Only the dominant gene Rph4, derived from cultivar Gold, was localized on chromosome 1HS through its linkage (~17 cM) with the powdery mildew resistance locus Mla (McDaniel and Hathcock 1969). However, this chromosome 1H assignment is probably incorrect, based on comprehensive SNP data from cultivar Bowman and the corresponding near-isogenic line carrying Rph4 (Arnis Druka, personal communication). The most likely location of Rph4 according to this information is chromosome 2H. This, coupled with the fact that the leaf rust resistance gene in MBR1012 exhibits a completely different resistance spectrum from Rph4 to the common European leaf rust isolates (including I-80, Table 2), provides strong evidence that the two genes are different. Before we can unequivocally determine that the leaf rust resistance gene in MBR1012 is different from all other previously described genes in barley, a provisional locus designation of Rph MBR1012 is being assigned.
Rph MBR1012 provides effective resistance against leaf rust isolate I-80, which is capable of overcoming all common leaf rust resistance genes in the European barley gene pool, except for Rph7, Rph15 and Rph16. While Rph7 has been incorporated into many European barley cultivars, there are no reports to our knowledge on the incorporation of Rph15 and Rph16, which are derived from H. vulgare ssp. spontaneum. In contrast to genes derived from wild barley where linkage drag can be a serious problem, the resistance gene Rph MBR1012 is derived from a landrace and may be integrated into adapted cultivars much faster, thereby broadening the genetic base of leaf rust resistance. Although further research on this gene is needed, the results of this work open the opportunity to broaden the genetic base of resistance to P. hordei in barley and provide breeding programs with genetic markers that will facilitate the faster incorporation of this resistance into elite cultivars.
References
Borovkova IG, Jin Y, Steffenson BJ, Kilian A, Blake TK, Kleinhofs A (1997) Identification and mapping of a rust resistance gene in barley line Q21861. Genome 40:236–241. doi:10.1139/g97-033
Borovkova IG, Jin Y, Steffenson BJ (1998) Chromosomal location and genetic relationship of leaf rust resistance genes Rph9 and Rph12 in barley. Phytopathology 88:76–80. doi: 10.1094/PHYTO.1998.88.1.76
Boskovic J, Boskovic M (2009) Durable resistance to Puccinia triticina by accumulation of resistance genes. Genetika-Belgrade 41:353–378. doi:10.2298/GENSR0903355B
Boutin-Ganache I, Raposo M, Raymond M, Deschepper CF (2001) M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele sizing methods. Biotechniques 31:24–28
Brunner S, Keller B, Feuillet C (2000) Molecular mapping of Rph7.g leaf rust resistance gene in barley (Hordeum vulgare L.). Theor Appl Genet 101:783–788. doi:10.1007/s001220051544
Clifford BC (1985) Barley leaf rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts. Diseases, distribution, epidemiology, and control, vol II. Academic Press, New York, pp 173–205
Cotterill PJ, Rees RG, Platz GJ, Dill-Macky R (1992) Effects of leaf rust on selected Australian barley. Aust J Exp Agric 32:747–751. doi:10.1071/EA9920747
Cromey MG, Viljanen Rollinson SLH (1995) Virulence of Puccinia hordei on barley in New Zealand from 1990 to 1993. N Z J Crop Hort Sci 23:115–119
Das MK, Griffey CA, Baldwin RE, Waldenmaier CM, Vaughn ME, Price AM, Brooks WS (2007) Host resistance and fungicide control of leaf rust (Puccinia hordei) in barley (Hordeum vulgare) and effects on grain yield and yield components. Crop Prot 26:1422–1430. doi:10.1016/j.cropro2006.12.003
Dreiseitl A (1990) Overcoming the resistance of barley conferred by gene Pa3 against leaf rust (Puccinia hordei Otth). Genet Sel 26:159–160
Fetch TG, Steffenson BJ, Jin Y (1998) Worldwide virulence of P. hordei on barley. Phytopathology 88(Suppl):28–34
Feuerstein U, Brown AHD, Burdon JJ (1990) Linkage of rust resistance genes from wild barley (Hordeum spontaneum) with isozyme markers. Plant Breed 104:318–324. doi:0523.1990tb00442-x
Franckowiak JD, Jin Y, Steffenson BJ (1996) Recommended allele symbols for leaf rust resistance genes in barley. Barley Genet Newsl 27:36–44
Golan T, Anikster Y, Moseman JG, Wahl I (1978) A new virulent strain of Puccinia hordei. Euphytica 27:185–189. doi:10.1007/BF00039134
Golegaonkar PG, Karaoglu H, Park RF (2009) Molecular mapping of leaf rust resistance gene Rph14 in Hordeum vulgare. Theor Appl Genet 119:1281–1288. doi:10.1007/s00122-009-1132-0
Graner A, Michalek W, Streng S (2000a) Molecular mapping of genes conferring resistance to viral and fungal pathogens. In: Proc. VIIIth Int. Barley Genetics Symp., Adelaide, Australia, vol I, pp 45–52
Graner A, Streng S, Drescher A, Jin Y, Borovkova I, Steffenson BJ (2000b) Molecular mapping of the leaf rust resistance gene Rph7 in barley. Plant Breed 119:389–392. doi:10.1111/j1439-0523,1990tb00442.x
Ivandic V, Walther U, Graner A (1998) Molecular mapping of a new gene in wild barley conferring complete resistance to leaf rust (Puccinia hordei Otth). Theor Appl Genet 97:1235–1239. doi:10.1007/s001220051015
Jin Y, Statler GD, Franckowiak JD, Steffenson BJ (1993) Linkage between leaf rust resistance genes and morphological markers in barley. Phytopathology 83:230–233. doi:10.1094/Phyto-83-230
Jin Y, Steffenson BJ, Bockelman HE (1995) Evolution of cultivated and wild barley for resistance to pathotypes of Puccinia hordei with wide virulence. Genet Resour Crop Evol 42:1–6. doi:10.1007/BF02310678
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kota R, Varshney RK, Prasad M, Zhang H, Stein N, Graner A (2008) EST-derived single nucleotide polymorphism markers for assembling genetic and physical maps of the barley genome. Funct Integr Genomics 8:223–233. doi:10.1007/s10142-007-0060-9
Levine MN, Cherewick WJ (1952) Studies on dwarf leaf rust of barley. US Dept Agr Tech Bull 1056:1–17
Li JZ, Sjakste TG, Röder MS, Ganal MW (2003) Development and genetic mapping of 127 new microsatellite markers in barley. Theor Appl Genet 107:1021–1027. doi:10.1007/s00122-003-1345-6
Maccaulay M, Ramsay L, Powell W, Waugh R (2001) A representative, highly informative ‘genotyping set’ of barley SSR’s. Theor Appl Genet 102:801–809. doi:10.1007/s001220000487
Mammadov JA, Zwonitzer JC, Biyashev RM, Griffey CA, Jin Y, Steffenson BJ, Saghai Maroof MA (2003) Molecular mapping of leaf rust resistance gene Rph5 in barley. Crop Sci 43:388–393
Mathre DE (1997) Compendium of Barley Diseases, 2nd edn. American Phytopathological Society Press, St. Paul
McDaniel ME, Hathcock BR (1969) Linkage of Pa 4 and Ml a loci in barley. Crop Sci 9:822. doi:10.2135/cropsci1969.0011183X000900060047
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Niks RE, Walther U, Jaiser H, Martinez F, Rubiales D, Anderson O, Flath K, Gymer P, Heinrichs F, Jonsson R, Kuntze L, Rasmussen M, Richter E (2000) Resistance against barley leaf rust (Puccinia hordei) in West European spring barley germplasm. Agronomie 20:769–782. doi:10.1051/agro:2000174
Parlevliet JE (1981) Race-non-specific disease resistance. In: Jenkyn JF, Plumb RT (eds) Strategies for the control of cereal disease. Blackwell Scientific Publishers, Oxford, pp 47–54
Perovic D, Przulj N, Milovanovic M, Prodanovic S, Perovic J, Kophanke D, Ordon F, Graner A (2001) Characterisation of spring barley genetic resources in Yugoslavia. In: Proceedings of a symposium dedicated to the 100th birthday of Rudolf Mansfeld, Band 22, pp 301–306
Perovic D, Stein N, Zhang H, Drescher A, Prasad M, Kota R, Kopahnke D, Graner A (2004) An integrated approach for comparative mapping in rice and barley with special reference to Rph16. Funct Integr Genomics 4:74–83. doi:10.1007/s10142-003-0100-z
Perovic D, Tiffin P, Douchkov D, Bäumlein H, Graner A (2007) An integrated approach for comparative analysis of multigene families with special reference to the barley nicotianamine synthase genes. Funct Integr Genomics 7:169–179. doi:10.1007/s10142-006-0040-5
Ramsay L, Macaulay M, degli Ivanissevich S, McLean K, Cardle L, Fuller J, Edwards KJ, Tuvesson S, Morgante M, Massari A, Maestri E, Marmiroli N, Sjakste T, Ganal M, Powell W, Waugh R (2000) A simple sequence repeat-based linkage map of barley. Genetics 156:1997–2005
Reinhold M., Sharp EL (1980) Some virulence types of Puccinia hordei from semi-arid environments. In: Proceedings of 5th European and Mediterranean Cereal Rusts Conference, Bari, Italy, pp 147–148
Roane CW, Starling TM (1967) Inheritance of reaction to Puccinia hordei in barley. II Gene symbols for loci in differential cultivars. Phytopathology 57:66–68
Steffenson BJ, Jin Y, Griffey CA (1993) Pathotypes of Puccinia hordei with virulence for the barley leaf rust resistance gene Rph7 in the United States. Plant Dis 77:867–869
Stein N, Herren G, Keller B (2001) A new DNA extraction method for high-throughput marker in a large-genome species such as Triticum aestivum. Plant Breed 120:354–356. doi:10.1046/j.1439-0523.2001.00615.x
Stein N, Prasad M, Scholz U, Thiel T, Zhang H, Wolf M, Kota R, Varshney K, Perovic D, Grosse I, Graner A (2007) A 1,000-loci transcript map of the barley genome: new anchoring points for integrative grass genomics. Theor Appl Genet 114:823–839. doi:10.1007/s00122-006-0480-2
Struss D, Plieske J (1998) The use of microsatellite markers for detection of genetic diversity in barley populations. Theor Appl Genet 97:308–315. doi:10.1007/s001220050900
Sun Y, Neate S (2007) Genetics and molecular mapping of Rph13, a gene conferring resistance to leaf rust in barley. Phytopathology 97:S112–S112
Thiel T, Michalek W, Varshney RK, Graner A (2003) Exploiting EST databases for the development and characterization of gene derived SSR markers in barley. Theor Appl Genet 103:411–422. doi:10.1007/s00122-002-1031-0
Thiel T, Kota R, Grosse I, Stein N, Graner A (2004) SNP2CAPS: a SNP and INDEL analysis tool for CAPS marker development. Nucleic Acids Res. doi:10.1093/nar/gnh006
Van Ooijen JW (2006) Join Map®4.0 software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, The Netherlands
Varshney RK, Marcel TC, Ramsay L, Russell J, Röder MS, Stein N, Waugh R, Langridge P, Niks RE, Graner A (2007) A high density barley microsatellite consensus map with 775 SSR loci. Theor Appl Genet 114:1091–1103. doi:10.1007/s00122-007-0503-7
Walther U (1979) Virulence and resistance—the situation in Puccinia hordei West. Archiv fur Zuchtungsforschung 9:49–54
Walther U (1987) Inheritance of resistance of Puccinia hordei Otth in the spring barley variety Trumpf. Cereal Rusts Bull 15:20–26
Walther U, Lehmann CO (1980) Resistenzeigenschaften im Gersten- und Weizensortiment Gatersleben. 24. Prüfung von Sommer- und Wintergersten auf Verhalten gegenüber Zwergrost (Puccinia hordei Otth.). Kulturpflanze 28:227–228
Weerasena JS, Steffenson BJ, Falk AB (2004) Conversion of an amplified fragment length polymorphism marker into a co-dominant marker in mapping of the Rph15 gene conferring resistance to barley leaf rust, Puccinia hordei Otth. Theor Appl Genet 108:712–719. doi:10.1007/s00122-003-1470-2
Werner K, Friedt W, Ordon F (2005) Strategies for pyramiding resistance genes against the barley yellow mosaic virus complex (BaMMV, BaYMV, BaYMV2). Mol Breed 16:45–55. doi:10.1007/s1132-005-3445-2
Zhong S, Effertz RJ, Jin Y, Franckowiak JD, Steffenson BJ (2003) Molecular mapping of the leaf rust resistance gene Rph6 in barley and its linkage relationships with Rph5 and Rph7. Phytopathology 93:604–609. doi:10.1094/Phytho.2003.93.5.604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
König, J., Kopahnke, D., Steffenson, B.J. et al. Genetic mapping of a leaf rust resistance gene in the former Yugoslavian barley landrace MBR1012. Mol Breeding 30, 1253–1264 (2012). https://doi.org/10.1007/s11032-012-9712-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-012-9712-0