Abstract
We have identified, genetically mapped and physically delimited the chromosomal location of a new blast resistance gene from a broad spectrum resistant genotype ‘DHR9’. The segregation analysis of an F2 progeny of a cross between a susceptible cv. ‘HPU741’ and the resistant genotype ‘DHR9’ suggested that the resistance was conditioned by a single dominant gene. A RAPD marker, OPA82000, linked to the resistance gene was identified by the linkage analysis of 109 F2 individuals. By chromosomal landing of the sequence of RAPD marker on the sequence of reference cv. Nipponbare, the gene was mapped onto rice chromosome 12. Further linkage analysis with two polymorphic simple sequence repeat (SSR) markers, RM2529 and RM1337 of chromosome 12, confirmed the chromosomal localization of the resistance gene. Based on linkage analysis of 521 susceptible F2 plants and comparative haplotype structure analysis of the parental genotypes with SSR and sequence tagged site (STS) markers developed from the Nipponbare PAC/BAC clones of chromosome 12, the resistance gene was delimited within a 2 cM interval defined by STS marker, STS5, on the telomeric side and SSR marker, RRS6 on the centromeric side. By aligning the sequences of linked markers on the sequence of cv. Nipponbare, a ~4.18 Mb cross-over cold region near the centromere of chromosome 12 was delineated as the region of blast resistance gene. In this region, six putatively expressed NBS-LRR genes were identified by surveying the equivalent genomic region of cv. Nipponbare in the TIGR Whole Genome Annotation Database (http://www.tigr.org). NBS-LRR locus, LOC_Os12g18374 situated in BAC clone OJ1115_G02 (Ac. No. AL772419) was short-listed as a potential candidate for the resistance gene identified from DHR9. The new gene was tentatively designated as Pi-42(t). The markers tightly linked to gene will facilitate marker-assisted gene pyramiding and cloning of the resistance gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.), the world’s most important cereal crop, is the primary calorie source for one-third of the world’s population (Shim et al. 2004). Among the biotic stresses, rice blast caused by Magnaporthe grisea is one of the most devastating diseases of rice throughout the world. The disease causes heavy yield losses ranging from 35 to 50% during the epidemic years (Padmavathi et al. 2005). Although chemical control of the disease is feasible, yet it remains economically impractical for resource-poor farmers and is environmentally unsafe. Deployment of resistant varieties is an effective approach to eliminate the use of pesticides and minimize crop losses due to this disease. So far, about 50 major blast resistance genes have been identified and utilized in developing resistant cultivars (Liu et al. 2005). However, blast resistance conferred by these genes is often short-lived due to the emergence of virulent races of the pathogen which negate the effect introduced resistance genes (Zeigler et al. 1995). Nonetheless, the major gene resistance to blast has been useful and should continue to be important in rice production if resistance genes are carefully selected and managed (Chen et al. 1996). Strategies that would create more durably resistant cultivars are presently focusing on the pyramiding of multiple resistance genes into susceptible cultivars (Hittalmani et al. 2000). Gene pyramiding by conventional breeding methods is difficult due to epistasis and/or masking effect of individual resistance genes and non-availability of a universal set of pathogen races for distinguishing the reactions of resistance genes.
Marker assisted selection represents a low cost and high throughput alternative to conventional phenotypic screening for pyramiding multiple resistance genes into susceptible varieties (Hittalmani et al. 2000; Pan et al. 2003).The knowledge about the precise chromosomal location of resistance genes and availability of markers that are tightly linked to them are the two essential requirements of a marker assisted breeding program. The availability of a large number of high density molecular maps of rice based on microsatellites, sequence tagged site (STS) and expressed sequence tag (EST) markers have provided a useful resource for the molecular mapping of blast resistance genes (Inoue et al. 1994; Wu et al. 2002; McCouch et al. 2002). The accessibility of the complete genome sequences of rice sub-species indica and japonica (http://rgp.dna.affrc.go.jp; http://www.genomics.org.cn) has further enabled the rice researchers to assay the genomes of different cultivars for DNA polymorphisms like single nucleotide polymorphisms (SNPs) and insertion–deletions (indels) that can be exploited for the fine mapping of the target genes (Hayashi et al. 2004; Shen et al. 2004). As a consequence, there has been a sudden spurt in the number of blast resistance genes that have been precisely localized on the rice chromosomes. To date, more than 40 blast resistance genes have been mapped in rice using a variety of molecular markers and 8 of these, namely, Pi-b, Pi-ta, Pi-9, Pi-d2, Pi-2, Pi-z t, Pi-36 and Pi-37, have been cloned (Wang et al. 1999; Bryan et al. 2000; Chen et al. 2006; Qu et al. 2006; Zhou et al. 2006; Lin et al. 2007; Liu et al. 2007a).
In the present study, we report the genetic and physical mapping of a novel blast resistance gene from a broad spectrum resistant rice genotype ‘DHR9’. The publicly available genomic resources of rice were extensively utilized in the study for generating PCR-based markers for subsequent use in marker assisted selection and map based cloning of the resistance gene.
Materials and methods
Mapping population and blast phenotyping
An F2 population derived from a cross between blast resistant genotype ‘DHR9’ and susceptible genotype ‘HPU741’ was used in the study. The blast fungus isolate, DSN37-1, which is virulent on HPU741 and avirulent on DHR9 was used for phenotyping of the F2 population for blast resistance. A total of 747 F2 seedlings were spray inoculated with the culture of M. grisea strain DSN37-1. Preparation of inoculum and disease scoring was done as described previously (Rathour et al. 2004). The number of resistant and susceptible seedlings was counted and the data subjected to Chi-square analysis to test the goodness of fit to Mendelian ratios. The individual seedlings showing highly resistant (0–1 score) or susceptible (4–5 score) reactions were transferred to pots for further DNA analysis and to obtain single plant progenies. About 25–30 progeny seedlings from individual resistant F2 plants were inoculated with blast to deduce their allelic composition at the resistance locus. An additional set of nearly 3,000 F2 seedlings was inoculated with the blast culture and susceptible seedlings were transferred to pots. A total of 460 surviving susceptible plants were used for the fine mapping of the resistance gene employing recessive class analysis (Zhang et al. 1994).
Identification of RAPD markers linked to the resistance gene
Genomic DNA of parents and individual F2 plants was isolated using the CTAB method (Murray and Thompson 1980). The bulked segregant analysis (BSA; Michelmore et al. 1991) combined with recessive class analysis (RCA; Zhang et al. 1994) were used to identify the markers linked to resistance gene(s). A total of 158 random 10-mer primers (Operon Technologies Inc., Alameda, CA) were used for the polymorphism survey of the parental genotypes ‘HPU 741’ and ‘DHR9. Polymorphic markers were used to screen resistant and susceptible DNA bulks to identify markers linked to the resistance gene. The markers differentiating resistant and susceptible bulks were used for the linkage analysis of an F2 population comprising of 48 resistant and 61 susceptible plants. The linkage distance (d) in cM (centi Morgan) between the blast resistance gene and the RAPD marker was computed using the Kosambi function (Kosambi 1944). Specific RAPD band linked to the resistance gene was excised from 1.4% agarose gel and cloned into pGEM-T Easy vector (Promega, USA). Positive clones were identified by colony PCR and sequenced in ABI Prism 3100 automated DNA sequencer (Applied Biosystems, Foster City, CA) using dye terminator chemistry. Chromosomal location of the RAPD marker was deduced by projecting its sequence on the genome sequence of rice cv. ‘Nipponbare’ released by the International Rice Genome Sequencing Project (IRGSP) (http://rgp.dna.affrc.go.jp) using BLASTN progamme of NCBI (http://www.ncbi.nlm.nih.gov/BLAST).
Confirmation of chromosomal location of blast resistance locus
To confirm the chromosomal location of the R gene, 31 simple sequence repeat (SSR) markers derived from the sequences around the RAPD marker were selected from the published SSR map of rice (McCouch et al. 2002; http://www.gramene.org) and the primers were custom synthesized from IDT-USA. The primer sequences and annealing temperature for these markers were adopted from the web site of the International Rice Microsatellite Initiative (IRMI; http://www.gramene.org). DNA amplification was carried out in a 25 μl reaction volume containing 20 ng template DNA, 0.2 mM of each dNTP, 0.2 μM of each primer, 1.5 mM MgCl2, 1 × PCR buffer (10 mM Tris–HCl, 50 mM KCl, pH 8.3) and 1 unit Taq polymerase (MBI, Fermentas). PCR amplification was carried out in a thermocycler (Biometra, Germany) using the following temperature profile: initial denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 1 min, 55°C for 1 min, 72°C for 2 min and a final extension at 72°C for 5 min followed by rapid cooling to 4°C. The PCR products were resolved in 4% metaphor gel and visualized by ethidium bromide staining. The polymorphic SSRs were tested for their possible linkage to blast resistance gene by BSA approach as described earlier.
Development of new markers and high resolution mapping of the resistance gene
Initial mapping with RAPD marker evinced the genetic interval spanning 47.1–62.1 cM on the chromosome 12 to be the region for the blast resistance locus. Therefore, to perform fine scale mapping of the R gene, a set of 127 SSRs and 54 sequence tagged site (STS) markers was developed from the PAC/BAC clones spanning 29.2–72.2 cM on the integrated genetic-physical map of rice cv. ‘Nipponbare’ generated by the International Rice Genome Sequencing Project (IRGSP) (http://rgp.dna.affrc.go.jp). For developing SSRs, SSR motifs were identified among the selected BAC/PAC clones using simple sequence repeat identification tool (SSRIT) (http://gramene.org) and primers were designed from the sequences flanking the SSR loci using primer 3.0 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The uniqueness of each primer was confirmed by BLAST search against the complete rice genome sequence database. The procedure for STS development was essentially similar to that described for SSRs, except that random sequences within PAC/BACs were targeted for developing flanking primers. The amplicons from STS markers were resolved in 4% metaphor gel to detect length polymorphisms between the parental genotypes.
Delineation of the physical location of the blast resistance locus and identification of candidate resistance gene(s)
The physical region spanning the blast resistance locus was delineated by landing the sequences of flanking markers on the sequence of reference cv. Nipponbare using BLASTN search. Candidate resistance gene(s) were identified by searching the equivalent genomic region of cv. Nipponbare for NBS-LRR (nucleotide binding site and leucine rich repeat) genes using the TIGR Whole Genome Annotation Database of cv. Nipponbare (http://rice.plantbiology.msu.edu). To identify putatively functional candidates, the expression evidence in the form of expressed sequence tags (ESTs) and full-length cDNA (Fl-cDNA) was ascertained for each candidate gene by searching the Gene Expression Evidence Search (http://rice.plantbiology.msu.edu/locus_expression evidence.shtml) and Rice Gene Expression Anatomy Viewer (http://rice.plantbiology.msu.edu/dnav.shtml) databases of TIGR.
Development of sequence tagged site markers from the candidate resistance genes
Primers pairs for candidate resistance genes identified in the target region were designed using Primer 3.0 software. The markers amplifying monomorphic amplicons among parental genotypes were converted into cleaved amplified polymorphic sequence (CAPS) markers by digesting the amplified products with a panel of restriction enzymes. The enzymes for restriction analysis were selected on the basis of in silico restriction analysis of the candidate resistance gene sequences using restriction analysis tool of TAIR (http://www.arabidopsis.org/tools/). The cleaved products were separated on 2.0% agarose gel using 1 × TBE.
Linkage analysis
The polymorphic markers distinguishing resistant and susceptible bulks were used for the linkage analysis of an F2 population comprising 521 highly susceptible plants of cross HPU 741 × DHR9. Linkage analysis of the marker and blast resistance genotypes of F2 individuals was done to detect the markers closely linked to resistance gene. Linkage analysis was performed with MAPMAKER/EXP version 3.0b (Lander et al. 1987) and the recombination frequency was converted to map distance expressed in cM (centi Morgan) using the Kosambi function (Kosambi 1944).
Results
Genetic analysis of blast resistance
Seven hundred and forty-seven F2 plants derived from the cross of HPU741 with blast resistant genotype DHR9 were inoculated with blast pathogen. The segregation of resistant and susceptible plants fitted to 3R:1S ratio, indicating that the resistance in DHR9 is controlled by a single dominant gene (Table 1). As per the convention for naming blast resistance genes, the new gene was tentatively designated as Pi-42(t), as the gene symbols up to Pi-41(t) have already been assigned to the blast resistance genes identified elsewhere.
Identification of RAPD markers linked to resistance gene
Of the 158 random primers used for the polymorphism survey of HPU741 and DHR9, 32 generated polymorphic patterns between the parental genotypes and one, OPA-8, differentiated resistant and susceptible bulks as well. The random primer OPA-8 amplified a 2,000 bp DNA fragment in the resistant bulk, which exhibited complete linkage with the resistance gene in a F2 population of 109 individuals. The sequence of RAPD marker (OPA-82000) exhibited high homology with two overlapping BAC clones OSJNBa0024B03 (Ac. No. AL731883; E-value: 0.00) and OSJNBa0037L20 (Ac. No AL731785; E-value: 0.00) located at 51.5 cM on the physical map of rice chromosome 12 released by IRGSP. On the basis of these results, the R-gene segregating in F2 population of cross HPU741/DHR9 could be localized to rice chromosome 12.
To confirm the chromosomal location of blast resistance gene, 31 SSR markers spanning the genetic interval 38.1–75.8 cM on chromosome 12 were adopted from the IRMI SSR map for the polymorphism survey of parental genotypes. Two markers, RM2529 and RM1337, positioned at 47.6 and 51.5 cM, respectively on the SSR map detected polymorphism between the parental genotypes as well as the resistant and susceptible bulks. These results confirmed the localization of the resistance gene to chromosome 12.
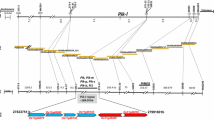
To perform high resolution mapping of the resistance gene, a total of 184 sequence tagged markers comprising 127 SSRs and 54 STSs were developed from the BAC/PAC clones spanning 29.2–72.2 cM on the physical map of rice released by IRGSP (supplemental tables S1 and S2). Based on polymorphism survey of parental genotypes with these markers, a total of 14 co-dominant single locus polymorphic markers comprising 12 SSRs and two STSs were generated and used in genetic mapping. Eight of these co-dominant markers namely, RRS6, RRS33, RRS44, RRS51, RRS60, RRS63, RRS123 and STS5 (Table 2), were also polymorphic between the resistant and susceptible bulks, indicating their association with the resistance gene. To perform high resolution mapping of the resistance locus, eight markers showing positive polymorphism in BSA were used for the linkage analysis of 521 highly susceptible F2 plants. Among the 521 F2 plants analyzed, 93 single and 3 double crossover recombinants were detected between RRS123 and the resistance gene. Similarly, 12 single and 11 double crossover recombination events were detected between RRS33 and the resistance locus (Table 3). A total of 11 double cross recombinants were detected between resistance gene and STS5. A perfect linkage was observed between co-segregating markers RRS44, RRS51, RR60, RRS63 and RRS6 and the resistance gene.
As many of the recombinants detected by RRS123 and RRS33 and RRS33 and STS5 were same, these markers were deduced to lie on the same side of the gene towards the telomeric end of the short arm of chromosome 12. We did not observe any recombination breakpoints towards the centeromeric side as all the markers developed from this side exhibited complete linkage with the resistance gene in spite of the fact that these markers were physically distributed across a 2.87 Mb interval spanning from position 9369532–12248913 bp on the physical map of rice (Table 2). Nevertheless, we could delimit the centromeric side of the resistance locus by comparison of the haplotype structure between ‘HPU741’ and ‘DHR9’ with a set of 111 markers (comprising 65 SSRs, 39 STS and 7 NBS-LRR gene derived markers) developed from the genetic region beyond RRS6, extending from position 51.8 to 72.2 cM towards the long arm of chromosome 12. None of these markers detected polymorphism between HPU741 and DHR9 (data not shown), thus leaving RRS6 to define the centromeric side of the resistance locus.
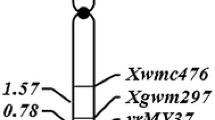
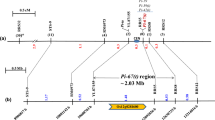
Linkage map showing the placement of linked markers relative to resistance gene is shown in the Fig. 1a. The resistance gene was mapped within a 2 cM genetic interval flanked by STS5 on telomeric side and cosegregating markers RRS6, RRS44, RRS51, RRS60, and RRS63 on centromeric side. Since the genetic mapping was performed by sequence-tagged markers, the physical location of the resistance locus could be ascertained by chromosome landing of the primer sequences of STS5 and RRS6 (most distal marker on the centromeric side) on the genome sequence of rice cv. Nipponbare. Based on primer landing, a ~4.18 Mb region covered by 41 overlapping PAC/BAC clones and spanning the position 8073819–12248913 near the centromere of chromosome 12 was delineated as the region of blast resistance locus (Fig. 1b). This region seems to be a recombination-suppressed region as no recombination breakpoints were detected in the 2.87 Mb interval (9369532–12248913 bp) between RRS44 and RRS6 in spite of the presence of six polymorphic markers in the region.
Genetic and physical maps of blast resistance locus Pi-42(t) on rice chromosome 12. A Genetic map based on segregation analysis of 521 F2 susceptible plants of cross HPU 741/DHR-9. The map distances (cM) are shown on the left side and are reported in Kosmabi units. CEN centromere, tentative map location of centromere was deduced from the TIGR database. S and L Short and long arm, respectively. B Physical map of Pi-42(t) locus constructed by e-landing of eight gene linked markers on the reference sequence of cv. Nipponbare released by IRGSP (International Rice Genome Sequencing Project). C Six putative NBS-LRR genes identified in the Pi-42(t) region by searching the equivalent region of reference cv. Nipponbare using TIGR Whole Genome Annotation Database (http://rice.plantbiology.msu.edu). *The expressed candidates are those for which the expression support in the form of ESTs or FL-cDNA is available
The search of TIGR Whole Genome Annotation Database of cv. Nipponbare (http://www.tigr.org) in the interval defined by flanking markers STS5 and RRS6 revealed the presence of 632 predicted genes, of which six, namely, LOC_Os12g17090, -17410, -17430, -17480, -18360 and -18374 were identified as having NBS-LRR structure (Fig. 1c). On the premise that majority of characterized R genes and seven of the eight cloned blast resistance genes belong to NBS-LRR class, the six NBS-LRR genes were considered to be the likeliest candidates for Pi-42(t). Of the six candidates, four, namely, LOC_Os12g17090 (a stripe rust resistance gene Yr13 homolog), LOC_Os12g17430, LOC_Os12g 18360 (a blast resistance gene Pi-ta homolog) and LOC_Os12g18374 most likely represent the functional genes because expression evidence in the form of expressed sequence tags (ESTs) and full-length cDNA (FL-cDNA) was detected for these genes by searching Gene Expression Evidence Search (http://rice.plantbiology.msu.edu/locus_expressionevidence.shtml) and Rice Gene Expression Anatomy Viewer (http://rice.plantbiology.msu.edu/dnav.shtml) databases of TIGR. While matching ESTs exist in database for the four putatively expressed genes, FL-cDNA transcripts could be detected for all but LOC_Os12g17430.
To narrow down the putative locus for the resistance gene identified from DHR9, a total of 11 sequence tagged sequence (STS) markers were developed from the open reading frames of the candidate NBS-LRR resistance genes (supplemental table S3). All the candidate resistance gene derived STS markers (CRG-STSs) amplified expected sized PCR amplicons but failed to reveal polymorphism between the parental genotypes. The monomorphic CRG-STS markers were converted into cleaved amplified polymorphic sequence (CAPS) markers by digesting the PCR products with a set of restriction enzymes. Restriction analysis of all the CRG-STS markers, except those derived from LOC_Os12g18374, revealed identical profiles in the parental genotypes despite the fact that 11–23 restriction enzymes were assayed per locus. CRG-STS markers, CRG6-1 and CRG 6-2 developed from locus LOC_Os12g18374 located in BAC clone OJ1115_G02 (AL772419) revealed cleavage polymorphism between the parents, when digested with restriction enzymes HpaII and TaqI, respectively. Testing of 23 recombinants detected at RRS33 and STS5 marker loci with CAPS marker CRG6-1/HpaII detected no recombinant i.e. the marker co-segregated with the resistance locus (Fig. 2). These results indicate that the NBS-LRR gene, LOC_Os12g18374, situated in BAC clone OJ1115_G02 is a potential candidate for the resistance gene identified in the present study.
Previously, four blast resistance genes, namely, Pi-ta (=Pi-4(t)), Pi-ta 2, Pi-20 and Pi-39(t) have been localized to recombination suppressed region near the centromere of chromosome 12 (Rybka et al. 1997; Li et al. 2008; Liu et al. 2007b). To determine whether the resistance gene identified from ‘DHR9’ is identical to one of these genes or is a new gene, monogenic blast differentials harboring blast resistance genes Pi-ta (=Pi-4(t)), Pi-ta 2 and Pi-20 were tested for their reaction to blast isolate ‘DSN37-1’. The monogenic lines harboring genes Pi-ta (=Pi-4) and Pi-20 were susceptible, while those with Pi-ta 2 showed resistant reaction (Table 4). These results indicate that the resistance gene identified from ‘DHR9’ is different from Pi-ta and Pi-20 genes. The detailed haplotyping of DHR9 and Pi-ta 2 harboring monogenic lines with Pi-42(t) linked markers, STS5, RRS44, RRS51, RRS60, RRS63, RRS6 and CRG 6-1 indicated that ‘DHR9’ and Pi-ta 2 lines do not share identical haplotypes at the region of the resistance locus (Fig. 3). These results suggest that the resistance gene identified from ‘DHR9’ is also different from Pi-ta 2. To differentiate Pi-42(t) from the Pi-39(t) gene identified from rice genotype ‘Q15’, three markers, 39M11, 39M12 and 39M6, which cosegregate with Pi-39(t) (Liu et al. 2007b), were used for the polymorphism survey of ‘HPU741’ and ‘DHR9’. These markers amplified identical alleles in both the parental genotypes (Fig. 4) thus suggesting that Pi-39(t) homologue in ‘DHR9’ is probably different from the Pi-39(t) allele of Q15.
Comparison of haplotype structure between DHR9 and Pi-ta 2 monogenic lines at the region of Pi-42(t) locus. The amplicons of STS marker CRG6-1 were digested with HpaII to reveal cleavage polymorphisms and were resolved in 2% agarose gel. The amplicons from other PCR markers were resolved on 4% metaphor. Note that DHR9 exhibits allele types different from Pi-ta 2 monogenic lines IRBLta2-Pi and IRBLta2-Re
Analysis of allele types in HPU741 and DHR9 for markers linked to gene Pi-39(t) identified from rice genotype ‘Q15’. The amplicons from marker 39M6 were digested with HinfI to reveal cleavage polymorphisms and were resolved in 2% agarose gel. The amplicons from other two markers were resolved on 4% metaphor gel. Note that HPU741 and DHR9 share identical alleles at all loci
Discussion
In this study, efforts were made to study the genetics of broad spectrum blast resistance in genotype ‘DHR9’ and to precisely map the blast resistance gene on the physical map of rice. A multi-pronged strategy involving a combination of bulked segregant analysis (BSA), recessive class analysis (RCA) and electronic chromosome landing was used to establish a high-resolution map of the Pi-42(t) gene identified in ‘DHR9’. These approaches have many merits for use in gene mapping: (1) BSA provides a rapid and technically simple means for identifying candidate markers in the target region (Michelmore et al. 1991), (2) RCA doubles the efficiency of detecting recombinants between the dominant gene and candidate markers as two chromosomes are probed per F2 plant using the homo-recessive progenies and (3) The use of electronic chromosome landing (e-Landing) enables in silico construction of physical map of the target locus by aligning the sequences of the linked markers on the reference sequence of rice genome. This approach obviates the need for constructing time consuming and costly artificial chromosome library of the resistance donor.
Based on linkage analysis of 521 susceptible F2 plants of cross HPU741/DHR9 with eight candidate markers, the resistance gene was mapped close to the centromere on the short arm of chromosome 12. The gene was localized within a 2 cM genetic interval flanked by STS5 on telomeric side and cosegregating markers RRS6, RRS44, RSS51, RRS60 and RRS63 on the centromeric side. Chromosome landing of the linked markers on the sequence of cv. Nipponbare, evinced ~4.18 Mb region spanning from position 8073819 to 12248913 near the centromere of chromosome 12 as the region of blast resistance locus.
Previously, four blast resistance genes, namely Pi-ta (=Pi-4(t)), Pi-ta 2, Pi-20 and Pi-39(t), have been localized on the same chromosomal region (Rybka et al. 1997; Li et al. 2008, Liu et al. 2007b), suggesting the existence of R-gene cluster at the pericentromeric region on the short arm of chromosome 12. In the rice genome, at least four other clusters of blast resistance genes have been identified on chromosomes 6, 8, 9 and 11 (Monosi et al. 2004). Our results, based on the susceptibility of monogenic differential lines harboring Pi-ta and Pi-20 genes and comparative analysis of haplotype structure at the resistance locus between ‘DHR9’ and Pi-ta 2 monogenic lines, suggest that the blast resistance gene identified from ‘DHR9’ is not identical to Pi-ta, Pi-20 and Pi-ta 2. It was not feasible to directly compare the identity of Pi-42(t) with that of Pi-39(t) identified by Liu et al. (2007b) due to non-availability of Pi-39(t) donor line ‘Q15’. At the same time, observance of identical alleles in ‘DHR9’ and ‘HPU741’ at the Pi-39(t) linked marker loci, 39M11, 39M12 and 39M6, is suggestive of divergent genealogies of the two resistance loci.
The Pi-42(t) gene identified from ‘DHR9’ is inferred to be embedded in a highly recombination suppressed region as suggested by the clustering of six markers that are otherwise physically distributed across the 2.87 Mb interval (9369532–12248913 bp). We could not detect recombinants in this region in spite of the fact that 1,042 gametes were analyzed with these markers. Similarly, the physical/genetic (P/G) distance ratio in the interval defined by markers STS5 and RRS44 (8073819–9369351 bp) was also higher than the average P/G ratio of 260–280 Kb/cM estimated in rice (Wu and Tanksley 1993). In consonance to present study, suppression of recombination near the pericentromeric region on the short arm of chromosome 12 has earlier been reported during the mapping of Pi-ta, Pita- 2 and Pi-20(t) genes (Rybka et al. 1997; Nakamura et al. 1997; Li et al. 2008). Various factors including lack of sequence homology in the parental genotypes, and abundance of repetitive sequences in the centromeric and pericentromeric regions have been reported to be responsible for suppressing recombination near the centromeres (Wu et al. 2003). The annotation of sequence of cv. Nipponbare has revealed abundance of repetitive sequences at centromere and pericentromeric regions of rice chromosome 12 (The Rice Chromosomes 11 and 12 Sequencing Consortia), thereby supporting the occurrence of reduced recombination in these regions. Contrary to the findings of present study and earlier reports regarding highly suppressed recombination near the centromeric region of chromosome 12, Liu et al. (2007b) observed high recombination rates (P/G ratio ranging from 160 to 730 Kb/cM) in the pericentromeric region on the short arm of chromosome 12, notwithstanding that the parents involved in developing mapping population in their study belonged to different sub-species of rice. Ostensibly, the reduced recombination observed in the regions around the centromeres is not a universal phenomenon and besides sequence homology other factors might play role in determining recombination rates in these regions.
Genes encoding proteins with nucleotide-binding site (NBS) and C-terminal leucine rich repeats (LRR) represent the largest class of R-genes in plants (Martin et al. 2003). The discovery of more than 500 NBS-LRR genes in the genome sequence of rice cv. Nipponbare (Zhou et al. 2004) and several reports on co-localization between the NBS-LRR genes and blast resistance loci identified through genetic analysis (Sallaud et al. 2003; Wisser et al. 2005) suggest that NBS-LRR genes are potential candidates for the genes conferring resistance to M. grisea. This perception has been substantiated with the findings that the seven of the eight blast resistance genes cloned till date viz., Pi-b, Pi-ta, Pi-9, Pi-2, Pi-z t, Pi-36 and Pi-37 encode proteins with NBS and LRR motifs (Wang et al. 1999; Bryan et al. 2000; Qu et al. 2006; Zhou et al. 2006; Lin et al. 2007; Liu et al. 2007a). In the present study, six NBS-LRR genes were identified in the genomic region encompassing Pi-42(t) locus by surveying the corresponding genomic region of cv. Nipponbare. Comparative analysis of the genomic structure of ‘DHR9’ and ‘HPU741’ at the six putative NBS-LRR genes revealed identical haplotypes in these genotypes for five of the six predicted NBS-LRR genes. The divergent haplotypic structure observed in the NBS-LRR gene, LOC_Os12g18374, located in BAC clone OJ1115_G02 (Ac. no.AL772419), makes it a potential candidate for the resistance gene identified from ‘DHR9’. The two gene models for LOC_Os12g18374 are identical to the full-length cDNAs J090090I18 (Ac. no. AK289025) and J023026M23 (Ac. no. AK072326), respectively. While J090090I18 originated from a cDNA library established from embryo’s after pollination, J023026M23 was identified in a flower cDNA library (http://cdna01.dna.affrc.go.jp/cDNA/). The ESTs matching LOC_Os12g18374 have also been identified in leaf and flower cDNA libraries (http://rice.plantbiology.msu.edu/dnav.shtml). Thus the expression pattern of LOC_Os12g18374 conforms to that expected of a gene mediating protection to blast pathogen which primarily infects leaf and floral tissues (panicles) of the rice plant. However, the final confirmation regarding the functionality of candidate gene requires complementation analysis of susceptible genotype with the full length gene amplified from DHR9. Coincidentally, the BAC clone OJ1115_G02 (Ac. no.AL772419) is also reported to harbor a potential candidate for the blast resistance gene Pi-39(t) identified from rice genotype Q15 (Liu et al. 2007b). This indicates that Pi-39(t) and Pi-42(t) occupy syntenic positions in rice genome and may be truly allelic to each other. Further characterization of resistance spectra of these two putatively allelic genes using geographically diverse blast isolates and sequence comparisons of functional members of both resistance loci will offer insight into the molecular mechanism underlying the evolution of novel blast resistance specificities.
The rice genotype DHR9 exhibits a broad spectrum resistance, showing resistance to blast isolates collected from different regions of north-western Himalayas (Gaur 2004). The blast resistance genes Pi-ta, Pi-ta 2, Pi-20 and Pi-39(t), that co-localize with Pi-42(t) at the pericentromeric region of chromosome 12 have also been reported to exhibit broad spectrum blast resistance (Jia et al. 2002; Li et al. 2008; Liu et al. 2007b). All these resistance genes including Pi-42(t) are embedded in a recombination-suppressed region where several NBS-LRR genes are clustered together. Therefore, it is likely that the broad spectrum resistance exhibited by theses genes may be due to the action of several NBS-LRR genes that can recognize AVR gene encoded ligands of multiple blast races. In the present study, six markers, RRS44, RRS51, RRS60, RRS63, RRS6 and CRG 6-1 cosegregating with the Pi-42(t) were identified. The existence of six polymorphic markers and the decreased recombination near the centromere will ensure the precise and speedy transfer of Pi-42(t) gene and other tightly linked resistance gene homologues from ‘DHR9’ into susceptible breeding lines for achieving a broad spectrum blast resistance. The delineation of physical position of blast resistance locus and short listing of one of the NBS-LRR genes as potential candidate gene has also laid the foundation for the positional cloning of Pi-42(t).
References
Bryan GT, Wu KS, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance genes Pi-ta. Plant Cell 12:2033–2045
Chen DH, Zeigler RS, Ahn SW (1996) Phenotypic characterization of the rice blast resistance gene Pi-2(t). Plant Dis 80:52–56
Chen XW, Shang JJ, Chen DX, Lei CL, Zou Y, Zhai WX, Liu GZ, Xu JC, Ling ZZ, Cao G, Ma BT, Wang YP, Zhao XF, Li SG, Zhu LH (2006) A B-lectin receptor kinase gene conferring rice blast resistance. Plant J 46:794–804
Gaur VS (2004) Development and characterization of doubled haploids involving an effective blast resistance gene in rice (Oryza sativa L.) M.Sc. Thesis, CSKHPKV, Palampur, India
Hayashi K, Hashimoto N, Daigen M, Ashikawa I (2004) Development of PCR based SNP markers for rice blast. Theor Appl Genet 108:1212–1220
Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N (2000) Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor Appl Genet 100:1121–1128
Inoue T, Zhong HS, Miyao A, Ashikawa I, Monna L, Fukuoka S, Miyadera N, Nagamura Y, Kurata K, Sasaki T, Minobe Y (1994) Sequence-tagged sites (STSs) as standard landmarks in the rice genome. Theor Appl Genet 89:728–734
Jia Y, Wang Z, Singh P (2002) Development of dominant rice blast Pi-ta resistance gene markers. Crop Sci 42:2145–2149
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green SP, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newbury L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genome 1:174–181
Li W, Lei C, Cheng Z, Jia Y, Huang D, Wang J, Wang J, Zhang X, Su N, Guo X, Zhai H, Wan J (2008) Identification of SSR markers for a broad-spectrum blast resistance gene Pi20(t) for marker-assisted breeding. Mol Breed 22:141–149
Lin F, Chen S, Que Z, Wang L, Liu X, Pan Q (2007) The blast resistance gene Pi37 encodes a nucleotide binding site—leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177:1871–1880
Liu XQ, Wang L, Chen S, Lin F, Pan QH (2005) Genetic and physical mapping of Pi36(t), a novel rice blast resistance gene located on rice chromosome 8. Mol Genet Genomics 274:394–401
Liu X, Lin F, Wang L, Pan Q (2007a) The in silico map-based cloning of Pi36, a rice coiled-coil-nucleotide-binding site-leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 176:2541–2549
Liu X, Yang Q, Lin F, Hua L, Wang C, Wang L, Pan Q (2007b) Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol Genet Genomics 278:403–410
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54:23–61
McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, Fu BY, Maghirang R, Li ZK, Xing YZ, Zhang QF, Kono I, Yano M, Fjellstrom R, Declerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Michelmore RW, Paran I, Kessel RV (1991) Identification of markers linked to disease resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genome regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Monosi B, Wisser RJ, Pennill L, Hulbert SH (2004) Full-genome analysis of resistance gene homologues in rice. Theor Appl Genet 109:1434–1447
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nakamura S, Asakawa S, Ohmido N, Fukui K, Shimizu N, Kawasaki S (1997) Construction of an 800-kb contig in the near-centromeric region of the rice blast resistance gene Pi-ta 2 using a highly representative rice BAC library. Mol Genet Genomics 254:611–620
Padmavathi G, Ram T, Satyanarayana K, Mishra B (2005) Identification of blast (M. grisea) resistance genes in rice. Curr Sci 88:628–630
Pan QH, Hu ZD, Tanisaka T, Wang L (2003) Fine mapping of the blast resistance gene Pi15, linked to Pii, on rice chromosome 9. Acta Bot Sin 45:871–877
Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172:1901–1914
Rathour R, Singh BM, Sharma TR (2004) Population structure of Magnaporthe grisea from north western Himalayas and its implications for blast resistance breeding of rice. J Phytopathol 152:304–312
Rybka K, Miyamoto M, Ando I, Saito A, Kawasaki S (1997) High resolution mapping of the indica-derived rice blast resistance genes II. Pi-ta 2 and Pi-ta and a consideration of their origin. Mol Plant Microbe Interact 10:517–524
Sallaud C, Lorieux M, Roumen E, Tharreau D, Berruyer R, Svestasarani P, Garsmeur A, Notteghem JL (2003) Identification of five new blast resistance genes in the highly blast resistant rice variety IR64 using a QTL mapping strategy. Theor Appl Genet 106:794–803
Shen YL, Jiang H, Jiang PJ, Zhang ZB, Xi B, He YY, Wang G, Qian L, Li X, Yu QB, Liu HJ, Chen DH, Gao JH, Huang H, Shi TL, Yang ZH (2004) Development of genome wide DNA polymorphism database for map based cloning of rice genes. Plant Physiol 135:1198–1205
Shim KS, Cho SK, Jeung JU et al (2004) Identification of fungal (M. grisea) stress-induced genes in wild rice (O. minuta). Plant Cell Rep 22:599–607
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19:55–64
Wisser RJ, Sun Q, Hulbert SH, Kresovich S, Nelson RJ (2005) Identification and characterization of regions of the rice genome associated with broad-spectrum, quantitative disease resistance. Genetics 169:2277–2293
Wu KS, Tanksley SD (1993) PFGE analysis of the rice genome: estimation of fragment sizes, organization of repetitive sequences and relationships between genetic and physical distances. Plant Mol Biol 23:243–254
Wu J, Maehara T, Shimokawa T et al (2002) A comprehensive rice transcript map containing 6591 expressed sequence tag sites. Plant Cell 14:525–535
Wu J, Mizuno H, Tsugane MH et al (2003) Physical maps and recombination frequency of six rice chromosomes. Plant J 36:720–730
Zeigler RS, Cuoc LX, Scott RP, Bernardo MA, Chen DH, Valent B, Nelson RJ (1995) The relationship between lineage and virulence in Pyricularia grisea in the Philippines. Phytopathology 85:443–451
Zhang Q, Shen BZ, Dai XK, Mei MH, Saghai Maroof MA, Li ZB (1994) Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc Natl Acad Sci USA 91:8675–8679
Zhou T, Wang Y, Chen JQ, Araki H, Jing Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Genet Genomics 271:402–415
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact 19:1216–1228
Acknowledgments
The authors gratefully acknowledge the financial support (No. 102/IFD/SAN/2558/2004-05) received from the Department of Biotechnology, Govt. of India. The authors also wish to thank Dr. Y. Fukuta, International Rice Research Institute, Philippines, for providing the seeds of rice monogenic lines.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kumar, P., Pathania, S., Katoch, P. et al. Genetic and physical mapping of blast resistance gene Pi-42(t) on the short arm of rice chromosome 12. Mol Breeding 25, 217–228 (2010). https://doi.org/10.1007/s11032-009-9320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-009-9320-9